Published online Sep 28, 2025. doi: 10.3748/wjg.v31.i36.110210
Revised: June 30, 2025
Accepted: August 27, 2025
Published online: September 28, 2025
Processing time: 110 Days and 22.6 Hours
Celiac disease (CD) is a chronic inflammatory disease that affects multiple systems in genetically predisposed individuals. The only known treatment for CD is adherence to a gluten-free diet. Gluten has been found to exert deleterious immune-inflammatory effects beyond the small bowel, involving several genetic, cellular, and paracellular mechanisms in the context of chronic inflammation, leading to colorectal carcinoma (CRC) in CD patients. Several neoplasms, inc
To critically evaluate and synthesize existing evidence on the association between CD and CRC to encourage early-stage detection through lower gastrointestinal screening in CD patients and suggest individual-specific management strategies.
The Scopus, Web of Science, and PubMed databases were searched via Medical Subject Headings words related to the criteria pertinent to CD and colon cancer/neoplasm, with a focus on pathophysiological mechanisms, clinical presentations, and outcomes reviewed.
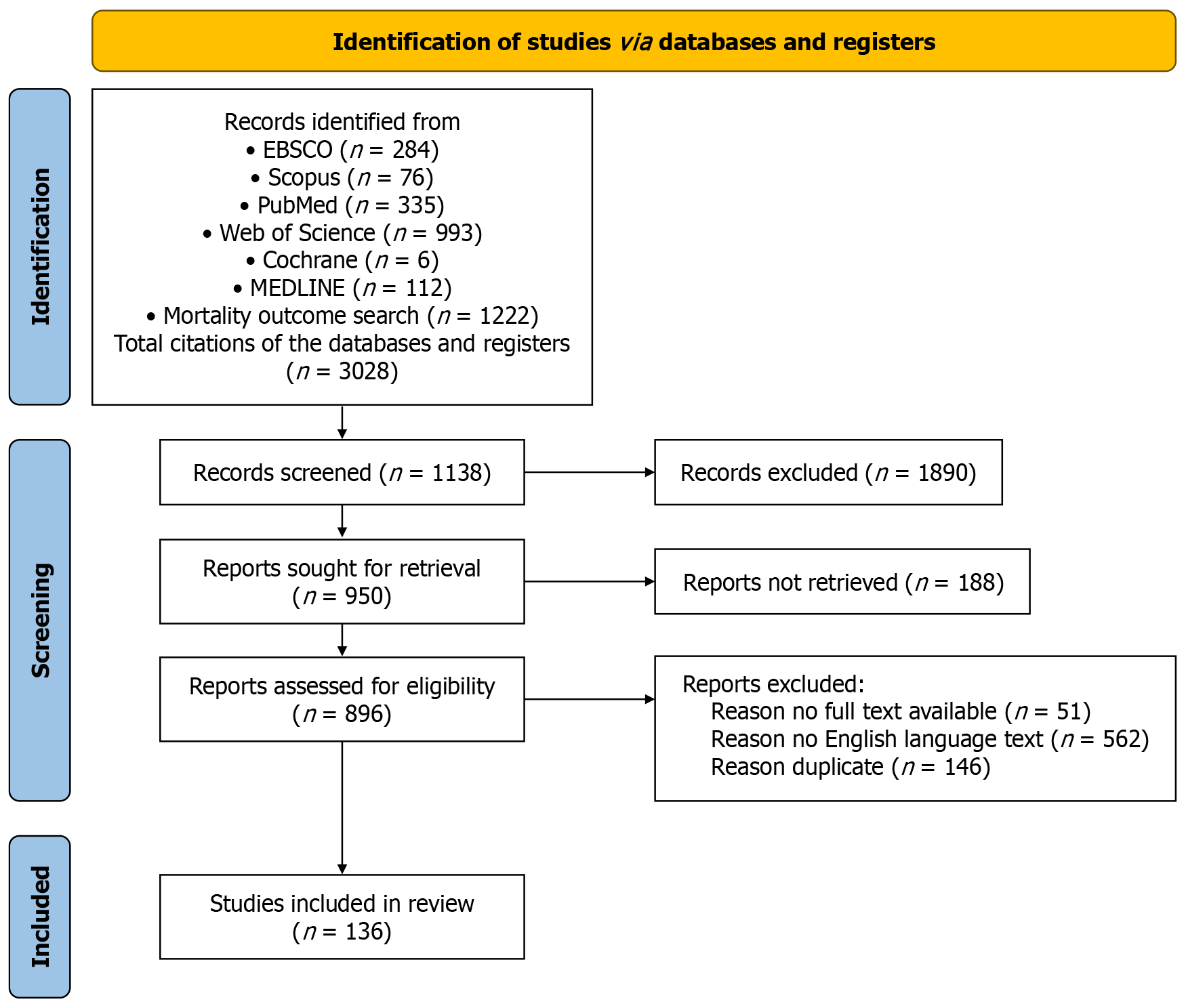
A total of 3028 citations related to CD and neoplasia were initially identified. Following a critical review and exclusions, 136 citations were suitable for inclusion in this study. Despite its low incidence, a clinically significant asso
A low incidence of colon lymphoma and adenocarcinoma has been revealed. The clinical presentation of colon lymphoma and adenocarcinoma is indolent and nonspecific, with late presentation in the form of adhesions and perforation. A modest but statistically significant increase in CRC risk among CD patients was noted. Several overlapping factors, including individual variability, genetic and environmental factors, diagnostic delays and duration of gluten exposure, compliance with a gluten-free diet, lack of educational awareness, and complex immune-inflammatory interactions, were found to contribute to the overall incidence of CRC in CD patients. However, the true incidence may be underestimated due to the iceberg phenomenon, where limited clinical suspicion, poor screening, and underreporting may mask the underlying burden. This study highlights the need for increased clinical awareness and early screening, especially in noncompliant patients.
Core Tip: Celiac disease (CD) involves proinflammatory mechanisms that predispose untreated patients who adhere to a gluten-free diet to the development of neoplastic complications. Gluten peptides have known direct effects on several levels of cell structure, and they increase proinflammatory potential and oncogenesis. Neoplastic complications of the colorectum are considered rare among neoplasms induced by CD. Intestinal lymphoma is a recognized and well-known long-term complication of CD; however, little is known about its role in colorectal cancer. The unfavorable outcomes related to colorectal lymphoma and adenocarcinoma associated with CD require further evaluation to inform possible screening initiatives.
- Citation: Khayyat YM. Colonic neoplasia and celiac disease: A systematic review. World J Gastroenterol 2025; 31(36): 110210
- URL: https://www.wjgnet.com/1007-9327/full/v31/i36/110210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i36.110210
Celiac disease (CD) is an immune-mediated disease with several gastrointestinal and extra gastrointestinal manifestations triggered by gluten intake in susceptible individuals with the histocompatibility molecules human leukocyte antigen (HLA)-A1 and HLA-A8[1]. Gluten is recognized by CD4 HLA-DQ1 and HLA-DQ8 cells through a heterogeneous group of γ/δ T-cell receptors (TCRs) in the lamina propria[2]. In contrast, noncoeliac wheat allergy is an entity with a different presentation of wheat-related intolerance that poses no immunological sequelae, with no antibody response against the gluten. The characteristic microscopic RNA signature hsa-miR-30e-5p distinguishes noncoeliac wheat allergy from CD, as observed in CD3+ intraepithelial lymphocytes (IELs) and CD45+ immunocytes in the duodenal lamina propria[3,4].
With a global incidence of 1% for CD, interest in pathogenesis and its complications, especially neoplastic complications, is increasing. Small bowel neoplasia is thought to be the prevailing pathology and includes adenocarcinoma and lymphoma (due to chronic intestinal inflammation) in addition to carcinoids and leiomyosarcoma[5]. Notably, intestinal lymphoma is an aggressive neoplasm associated with CD; colon neoplasms are less commonly reported neoplasms in CD[6]. The rarity of colorectal carcinoma (CRC) in confirmed CD patients could be explained by possible immune-mediated protective effects within the colon[7]. CD triggers a surge of IELs, which results in increased tumor immune surveillance and inhibits epithelial malignancies in the colon at the cost of chronic inflammation[8]. Additionally, malabsorption associated with CD leads to decreased dietary fat- and fat-soluble carcinogen intake, which could also explain the possible protective mechanisms in the colon[8]. More specifically, the colon has far less direct gluten exposure. Its mucosal immune system, which is supported by gut-associated lymphoid tissue and a robust microbiome, provides stronger surveillance and protection[9]. Considering these factors, a lower threshold for large bowel investigation is needed in patients with established CD to avoid delayed diagnosis and under recognition of neoplasia.
The Scopus, Web of Science, and PubMed databases were searched via Medical Subject Headings words related to the criteria pertinent to CD and colon cancer/neoplasm, with a focus on pathophysiological mechanisms and clinical presentations, and the literature was reviewed.
A total of 3028 citations related to CD and neoplasia were initially identified. Following a critical review and exclusions, 136 citations were suitable for inclusion in this study (Figure 1 as shown in PRISMA flow diagram). Despite its low incidence, a clinically significant association was found between CRC and CD that could impact the overall patient survival rate, early screening investigations are advocated with consideration of individual-specific decisions. Further longitudinal studies of global nature will help in structuring a precise recommendation.
Within the dynamic milieu of the gastrointestinal tract (GIT), several key factors operate in concert to maintain the integrity of the intestinal barrier during periods of chronic inflammation. These factors include cytoskeletal proteins[10], cellular factors[11], microbial factors[12], and immune cells[13,14], all of which play critical roles in maintaining barrier function (Table 1).
| Factor | Type | Role in barrier protection | Notes | Ref. |
| KIF | Cytoskeletal protein | Maintains cytoskeletal structures. Keratin 7 and 20 upregulation leads to crypt widening, while keratin 8 downregulation is observed during inflammation | KIFs are influenced by stress and inflammation. Types 1 (acidic) and 2 (basic) are noted | Helenius et al[10] |
| Paneth cell granules | Cellular component | Synthesizes and releases antimicrobial peptides to maintain the microbial barrier | Low zinc concentration is associated with granule depletion | Kelly et al[11] |
| Microbiome environment | Microbial component | Influences the risk of certain diseases, including celiac disease; enteroviral infections are linked to increased disease risk | The intestinal microbiome’s composition and health have systemic effects | Clinton et al[12] |
| γδ T cells | Immune cells | Reside in the intestinal wall; overactivation and control by IL-15 are crucial for gut homeostasis | Elevated IL-15 expression disrupts gut hemostasis | Suzuki et al[13] |
| IEL | Immune cells | γ/δ CD3+ and CD8+ IELs are increased in the duodenum of celiac patients, indicating a protective or reactive role | The distribution of IELs varies between the duodenum and colon in celiac patients | [14] |
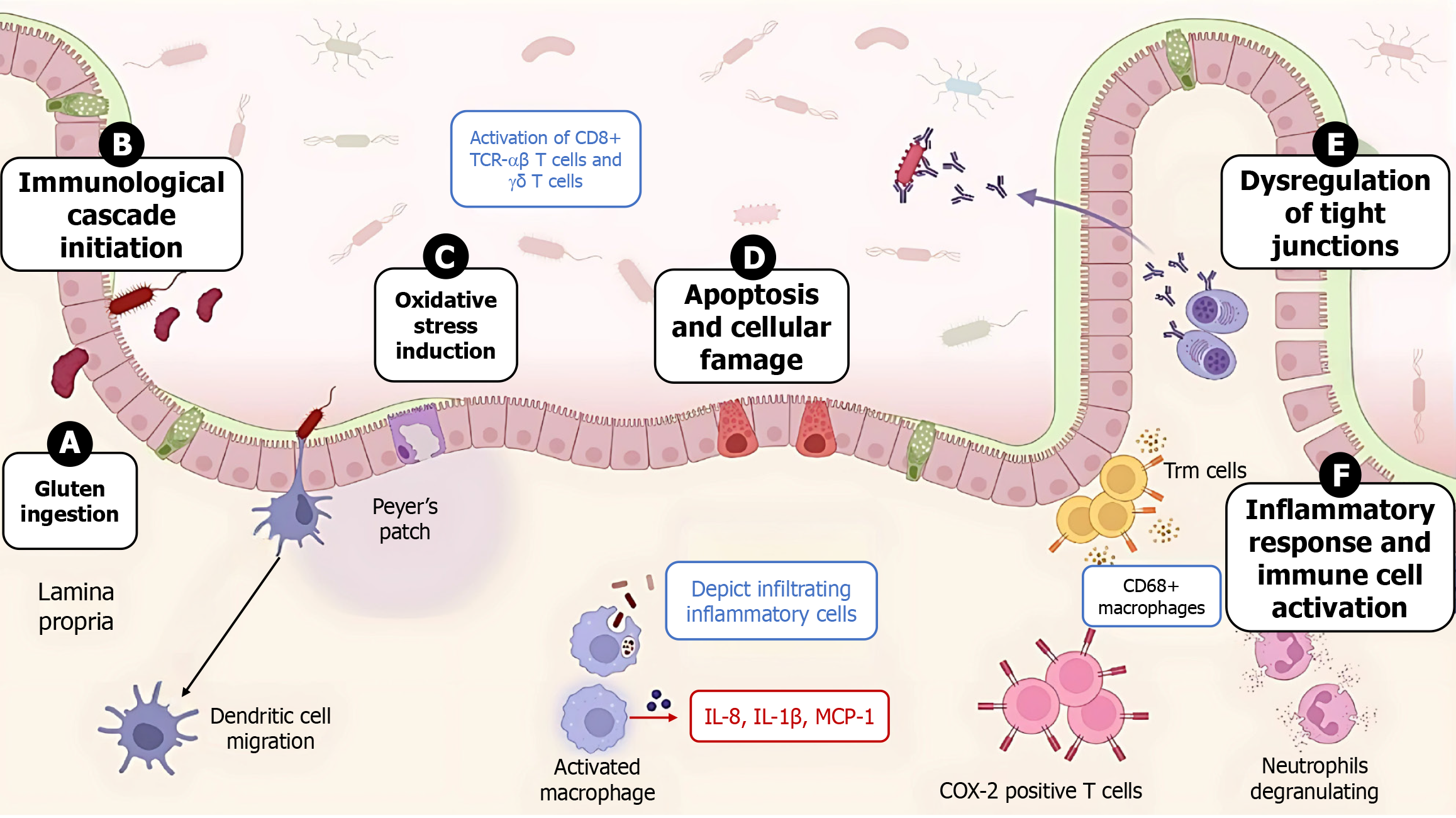
Gliadin peptides exert systemic and local effects. Among those that affect the bowel wall are the induction of oxidative stress, the disruption of the intestinal barrier, and damage to the intestinal mucosa[15] (Figure 2). The deleterious effects occur at the onset of the initial humoral reaction with the recruitment of CD8+ αβ and γδ T cells expressing TCRs, which interact with the natural killer group 2, member D ligand on enterocytes, a class Ib molecule, and the major histocompatibility complex (MHC) class 1 chain-related genes A and B, which act as activating receptors. In contrast, gluten-free diet (GFD)-adherent CD patients exhibit CD8αα TCR γδ IELs that express the inhibitory natural killer cell receptor group II member A receptor[2].
Glutathione enzymes (glutathione reductase, glutathione S-transferase, and reduced glutathione peroxidase) that combat reactive oxygen species (ROS) markedly decreased after treatment with gliadin peptides[16]. Furthermore, a high prevalence of proapoptotic signals is observed in immune-mediated diseases such as CD[17]. The expression of MHC class 1 chain-related genes A and B, which is recognized by the natural killer group 2, member D receptor on enterocytes and mediates apoptosis, is greater in untreated CD patients than in control subjects, and these genes are broadly expressed in CD7+, CD20+, CD138+, and CD68+, as is the mouse monoclonal antibody HAM56[18].
Compared with data on outcomes in other malignancies, such as lymphoma, little is known about the outcomes of patients with colorectal cancer and strict adherence to a GFD. Available evidence is limited by differing methods of assessment of adherence and inherent recall bias within the studies reviewed. Additionally, factors such as inability to reverse prior gluten exposure during study assessment, inability to comply strictly with a GFD and the absence of a noninvasive assessment method for a GFD are factors used to assess long-term effects decisively[19]. The physiological basis attributed to a lower risk of colon cancer in CD patient’s adherent decisively thought to be a balanced healthy diet and the limitation of high-calorie food items with a lean body mass index as well as a decreased fat absorption capability, hence attenuating most of the main inflammatory risk factors involved in gastrointestinal tumorigenesis[20]. Conversely, patients who are not adherent to a gluten diet would theoretically be protected from CRC due to impaired absorption of fat- and fat-soluble agents[8]. Patient-specific risk modifiers, including older age at CD diagnosis, prolonged untreated disease, and nonadherence to a GFD, likely exacerbate inflammation-driven epigenetic changes and genomic instability, increasing CRC susceptibility[20,21]. These factors likely explain the variable risks of CRC in CD patients. Conversely, early diagnosis and strict adherence to a GFD may maintain mucosal barrier integrity and mitigate this risk, as found in a study by Pereyra et al[22]. Low GFD adherence increased adenoma risk nearly sevenfold. Regarding precancerous lesions of CRC, such as adenoma and advanced neoplasia, low adherence to a GFD is significantly associated with adenoma (odds ratio = 6.78, confidence interval: 1.39-33.20) according to multivariate analysis, as reported by Pereyra et al[22], when adherence was evaluated by a Biagi score of 0-1. For CRC, long-term studies investigating the association between CD diagnosis and the development of new diagnoses of CRC are rare, and a case series by Volta et al[23], who adhered to a strict GFD based on a food diary assessment, eventually developed CRC. Additionally, Marafini et al[24] reported that late CD diagnosis and delayed treatment increase overall cancer risk.
The influence of gluten exposure is discussed in a multilayered classification to understand the toxic effects of gluten that target several body compositions based on observations at de novo and cell culture levels, starting from the gene level to the organ level.
At the gene level, hypermethylation induces high-frequency microsatellite instability (MSI) and deficient mismatch repair (MMR), as shown by the loss of expression of the repair genes human mutL homolog 1 (MLH1) and human mutS homolog 2 in CD. CD-associated CRC is distinct from Lynch syndrome. Although MMR deficiency and MSI are common in both CD-associated CRC and Lynch syndrome, in CD, they arise largely from age- or inflammation-driven epigenetic silencing (MLH1 promotes hypermethylation) rather than germline mutations[25,26]. This silencing adds microsatellite mutations to genes [transforming growth factor-beta (TGF-β) receptor type II, B-cell lymphoma (BCL) 2-associated X protein, and insulin-like growth factor 2 receptor], promoting malignant transformation[27]. Furthermore, genomic instability at the tumor protein 53 gene locus, which acts as a tumor-suppressor gene with a specific short tandem repeat in untreated patients, is observed with a high frequency in CD[20].
Although the significant risk of CRC in patients with CD has been explored, the countera-association, i.e., whether CRC patients have a higher incidence or altered immune profiles suggestive of latent or undiagnosed CD, has not been studied in detail in the literature. Some studies suggest the potential underdiagnosis of CD in patients with CRC due to overlapping symptoms and masking by cancer-associated immunosuppression. However, evidence is limited, warranting exploration of shared immunopathogenic pathways. Hence, genome-wide and biopsy-based studies are needed to determine whether the CRC-induced tumor microenvironment (TME) can mimic CD-like mucosal changes.
Several complex and integrated mechanisms have been described to explain the association between CD and CRC at the molecular level. CD results in the overexpression of interleukin-15 (IL-15) in intestinal epithelial cells, which then activates the Janus kinase/signal transducer and activator of transcription 3/5 and phosphatidylinositol 3-kinase/protein kinase B signaling pathways, leading to the overexpression of BCL-2 and the survival of genetically damaged epithelial cells, thus promoting tumorigenesis[28]. Moreover, IL-15 suppresses TGF-β/Sma and Mad related protein 3, which is responsible for epithelial cell homeostasis and anti-proliferative signaling[29]. Chronic inflammation in CD activates nuclear factor-κB (NF-κB), which upregulates cyclooxygenase-2 (COX-2), a proinflammatory and procarcinogenic enzyme that contributes to overall DNA damage, angiogenesis, and immune evasion[30].
Studies have shown that chronic epithelial turnover and chronic inflammation in CD may promote Wnt pathway dysregulation, which is central to CRC tumorigenesis, leading to beta-catenin accumulation and unchecked proliferation[31]. As discussed previously, MSI and MMR play key roles in CRC tumorigenesis by increasing mutation rates in oncogenes. Additionally, elevated IL-6 Levels in active CD led to the activation of signal transduction and activation of transcription 3 (an oncogene present in several gastrointestinal cancers), which supports survival, angiogenesis, and the proliferation of intestinal cells[32]. These diverse signaling mechanisms highlight overlapping pathways in the TME in the context of chronic inflammation leading to colorectal tumorigenesis in CD patients.
At the cell level, activated lymphocytes lead to respiratory bursts and the release of ROS, affecting the prooxidant-antioxidant balance. There is evidence to suggest that gliadin has a direct effect on cytoplasmic mitochondria, when mitochondria are disrupted and cell viability is reduced, as observed in Caco cell lines exposed to gliadin, increased mitochondrial biogenesis and a reduced mitochondrial antioxidant response occur. Gliadin peptide-driven dendritic cells (ijCD80/83/83/HLA-DR) via mitogen-activated protein kinases and NF-κB signaling lead to elevated cytokines (IL-6, IL-8, TNF-α) and enhanced T-cell priming[33]. Gliadin also promotes NF-κB-mediated TNF-α and IL-8 release from HLA-DQ2+ monocytes and macrophages[34]. Moreover, gliadin interacts with C-X-C chemokine receptor 3 on enterocytes and peripheral blood mononuclear cells, driving zonulin-mediated permeability and IL-8 secretion[35].
These proinflammatory cytokines alter the TME, promoting the maturation of antigen-presenting cells (enhancing the T helper 1 cells response), supporting CD8+ cell cytotoxic activity, and interferon secretion, creating a cytokine-rich environment that can promote antitumor immune surveillance and may cause immunosuppression in the long-term[36,37]. This colorectal TME critically governs patient prognosis and hence response to immunotherapy[38]. Notably, the number of apoptotic cells increases when cells are treated with peptide-treated gliadin[21,39]. Changes in cell viability of 20% to 80% observed in LoVo cell lines with increasing concentrations of peptic tryptic digested peptide from bread wheat gliadin; cell damage, as shown by the presence of autophagic vacuoles and intracytoplasmic lipid-like droplets, also occurs[40].
Interestingly, the properties of gluten change in response to chemical and enzymatic activities. In the non-heat-treated state, acid undergoes deamidation at a temperature of (90 °C) for 3 hours and renders more soluble and less immunoreactive in human colon LoVo cell lines than under the conditions of enzymatic changes in response to microbial transglutaminase, which leads to increased gluten immunoreactivity[41].
Treatment of experimental cell lines with gluten-specific immunodominant peptides (p56-88 and p57-68) results in reduced cell growth and inhibition of tissue transglutaminase (tTG). This effect was abolished by the addition of antibodies against tTG to CaCo2 cells. No inhibitory effect on cell growth or tTG activity was observed with the addition of the deamidated peptide p57-68 (E65). In contrast, inhibition persists with the other peptide, p69-82 (formerly nontoxic), which is produced through the substitution of glutamine 72 with glutamic acid[22,42].
Altered angiogenesis is a factor that is involved in tumorigenesis in CD, in which a regulatory single-nucleotide polymorphism in TNF-superfamily13 (rs11552708) is associated with CD. The expression of proangiogenic factors (G protein subunit alpha 13, TGF-α, v-erb-b2 receptor tyrosine kinase 2, and soluble guanylyl cyclase 2 is downregulated, while the expression of transglutaminase 2 and promyelocytic leukemia protein is upregulated, and the tTG antigen was shown to increase transglutaminase 2 expression[23,43]. Electron microscopy analysis of the sigmoid mucosa in CD patients revealed an abnormal microvillus that was irregular, short, and sparse, with fine granular material within its borders and abundant round structures within the microvillus borders of the principal cells[23,44].
At the paracellular level, gliadin alters the expression of tight junction components (actin, zonula occludens-1, and occludin), as demonstrated in a 3-dimensional LoVo cell culture system, after treatment with enzymatically digested gliadin[45,46]. Notably, in areas of partial or total villous atrophy of the small intestine, several blisters within the subepithelium contain inflammatory COX-2 cells, indicating the presence of CD3+ and CD68+ T-cell subtypes[25,26].
Microbiota consists of bacteria, viruses, and fungi that interact in a state of symbiosis, maintain bowel barrier integrity, and serve as a defense mechanism. Several health disorders have recently been attributed to dysregulation of microbiota fingerprints in the gut that surprisingly affect gut health and several other organ systems[47]. The gut virome responds in a dynamic state and diversely to the type of dietary intake or restrictions, such as a GFD[20,48]. CrAssphage is a phage taxon among the human virome that responds to dietary manipulation and is actively studied in response to different diets[49]. Metagenomic studies have shown that viral infections with enteroviruses early in life play a role in early/subclinical CD state[50]. The microbiota, especially the virome, interact with intestinal immune cells in delicate balance. Disturbance in the interplay between mononuclear phagocytes and innate lymphoid cells (ILCs), as well as the adaptive immune system, results in intestinal inflammatory states and impaired immunity against enteric infections[51].
To understand the relationship between colon cancer, the microbiota and ILCs, which increase the release of inflammatory mediators from ILCs, constitute a unique family of innate immune cells, including natural killer cells, which differentiate from the bone marrow. IL-22 secreted by ILC-3 cells plays a role in controlling epithelial cell proliferation and tumorigenesis. Intestinal dysbiosis leads to the release of IL-22 and IL-17 from ILC-3 cells, and the proportion of ILC-3s significantly decreases in colon cancer with increasing inflammatory activity of T helper 17 cells in the intestine. Notably, ILC-3s express the antigen-presenting factor MHC-II, which halts intestinal inflammation by limiting the activity of microbiota-specific T helper 17 cells in an MHC-II-dependent manner[52]. Direct microbial tumorigenesis effects are observed with Escherichia coli, Bacteroides fragilis, Epsilonproteobacteria, and Proteobacteria, with cancer-promoting effects via the production of colicin, Bacteroides fragilis toxin, and lethal cell-tumescent toxins[53,54]. These mechanisms are involved in the induction of ROS that led to damage to host DNA[55,56].
Gluten has been shown to exert several effects on various organs. Gluten challenge increases humoral and cellular responses, with increasing numbers of CD3+ and CD25+ cells, and the mRNA levels of the proinflammatory cytokines IL-8 and IL-1β and the C-C chemokine monocyte chemotactic protein-1 significantly increase as early as 2 hours following challenge; however, the levels of tumor necrosis factors (TNF-α and β1) remain unchanged[57]. In the rectum of CD patients, most of the physiologically normal T-cell population is composed of α/β subtypes and contains a preponderance of γ/δ T cells. Furthermore, a rectal gluten challenge with Frazer fraction III (a mixture of gliadin and glutenin peptides that is used as a source of toxic gliadin peptides for experiments) markedly increased the number of CD3+ IELs but did not change γ/δ T lymphocyte receptor expression levels[58]. In the colon, examination of a sigmoid organ culture of CD patients on a gluten diet revealed the presence of endomysial (epithelial membrane antigen-immunoglobulin A and immunoglobulin G) and tTG antibodies. Therefore, based on the observed trophic effect of a deleterious gluten diet, Catassi et al[59] proposed that a strict GFD could prevent this subset of aggressive lymphoma.
Chronic inflammation of the colon in patients with CD is traditionally associated with microscopic colitis (lymphocytic colitis (20%-27%) and microscopic gastritis)[60-63], and the incidence of lymphocytic colitis in refractory CD (RCD) has been reported to range widely from 0%-75%[64]. In clinical practice, CD is managed as a malabsorptive condition of the small bowel, and it is rare to perform lower endoscopy and biopsy for the investigation or surveillance of a pathology from that perspective, except for cases involving lower GIT bleeding[65], or to investigate new-onset diarrhea not explained by GFD adherence. Hence, the need to investigate or screen the colon for neoplasia, particularly among CD patients, has yet to be further explored in the context of its related neoplastic risk. Unusual associations between CD and related conditions should be considered a coexistent malignancy. A report of a lymphocytic colitis patient with monomorphic epitheliotropic intestinal T-cell lymphoma, formerly known as type 2 enteropathy-associated T-cell lymphoma (EATL), detected in a multifocal area of the GIT in a case series from East Asia; therefore, consideration to perform multiple biopsies from the GIT are needed[66].
CD is associated with an increased risk of various neoplastic conditions, including EATL and colorectal cancer[60-62]. In this section, the link between CD and the development of neoplasia is explored, particularly in terms of colonic inflammation mechanisms, carcinogenesis processes, and genetic factors.
Chronic inflammation in patients with CD can induce carcinogenesis via several mechanisms. Increased expression of tumor promoters (inflammatory cytokines) through tumor-associated lymphocytes and macrophages, induction of COX-2, and loss of immune tolerance are caused by genetic and epigenetic shifts[65,67]. The explanation for this intolerance lies in the autoimmune nature of CD, with chronic activation of IELs and heightened cytokine expression (IL-15, interferon-γ). This chronic inflammatory state, a key component of the TME, drives DNA damage and induces epithelial stress, epigenetic changes, and genomic instability, mimicking mechanisms implicated in CRC in other autoimmune conditions [e.g., systemic lupus erythematosus (SLE) and Sjogren’s disease]. However, this robust immune surveillance may lead to effective clearance of emerging tumor cells, as evidenced by studies indicating a lower incidence of certain neoplasms in autoimmune conditions [i.e., SLE, rheumatoid arthritis (RA), and psoriasis] due to enhanced CD8+ T-cell-driven antitumor surveillance[68-71].
In contrast, other studies suggest that chronic antigen exposure and exhausted CD8+ IELs may lead to immune escape mechanisms, allowing tumor growth despite active immunity, and persistent IL-15 signaling has been linked with the downregulation of MHC1, leading to poor immune anticancer surveillance[72]. Recently, an umbrella review revealed that while many autoimmune conditions favor malignant transformation, RA is associated with a lower CRC risk[73]. Similarly, a review on female-specific cancer revealed a detrimental breast and ovarian cancer risk in patients with known autoimmune conditions (i.e., RA, SLE), suggesting enhanced antitumor surveillance mechanisms[74,75]. Given the dual role of immune responses in both promoting and suppressing tumorigenesis, individualized risk stratification is essential. Further longitudinal studies are needed to clarify context-specific immune outcomes in CD-associated CRC. Of note, NF-κB acts as an intracellular transducer and tumor promoter with TNF and IL-1, which are involved in CD immunological reactions and act as potent activators[65].
EATL originates from α/β rather than γ/δ T cells, which express TCRs, as well as common neoplastic cells, which are CD3+, CD4, CD8-, and CD103-[59]. An analysis of a case series of gluten enteropathy patients revealed dense nonspecific infiltration of lymphocytes at the lamina propria of the colon[76]. The presence of aberrant IELs and a chronic inflammatory state in the gastric and colonic mucosa of RCD-II patients indicate an active lymphangiogenesis process[77,78].
A common signature observed for a monoclonal phenotypically abnormal IEL population exists in reticulum cell sarcoma along the GIT in the stomach, duodenum, jejunum, and colon. This group of RCD patients has a greater number of IELs with CD3+CD8- cells than CD patients do, regardless of whether they are GFD adherent or nonadherent; these independent findings were attributed to infiltration by circulating T cells but supported by TCR-γ clonality and histological findings[64]. The pathogenesis of type 1 EATL is related to the activation of IELs and is associated with the downregulation of TCR CD3 expression, the loss of CD8 expression, and TCR gene rearrangement[79]. The characteristic TCR proteins associated with CD and EATL are CD45R0, CD3, CD8, and CD56[80,81]. Compared with solitary CRC, synchronous CRC has a different immune cell composition, with IELs more frequently on the surface epithelium than on the crypt epithelium, which is indicative of different functions of immune-related processes at the gene transcription level[82].
Conflicting risk exists regarding the risk of colorectal cancer and CD. Long-term follow-up studies have revealed an association between CD and Hodgkin lymphoma risk [risk ratio (RR) = 6.9] over 11.2 years of follow-up; for patients with colon cancer, the adjusted RR was 1.23 (P = 0.6), and for patients with rectal cancer, the adjusted RR was 1.04 (P = 0.857)[83]. Conversely, other reports have shown a decreased risk of CRC (RR = 0.7) after the diagnosis of CD[84]. A recent review by Pelizzaro et al[27] revealed variability in the risk of malignancy, with an increased risk of neoplasia associated with a long disease duration beyond 10 years [standardized incidence ratio (SIR) = 1.3] and up to 15 years after diagnosis (SIR = 0.92). Interestingly, the diagnosis of colon cancer occurred soon after CD diagnosis (within the pre diagnostic period), at less than 2 years (SIR = 2), and at more than 40 years (hazard ratio = 2.47)[85].
HLA-DQ2/DQ8 are essential for CD diagnosis when ambiguity exists in clinical and histopathological findings. Although they have insignificant predictive value for colon cancer risk alone, studies suggest that chronic immune activation in genetically predisposed individuals may contribute to chronic inflammation (mucosal injury), adding to a tumor-promoting microenvironment[86]. More recent genome-wide association studies have indicated that many non-HLA variants (e.g., IL-2 and IL-21) are associated with the severity of the inflammatory response and cancer progression[87]. Untreated CD, especially when the diagnosis is delayed (prolonged exposure to gluten), is associated with extensive periods of mucosal inflammation, which may trigger MMR and genomic instability[88]. Therefore, more neoplastic complications are observed in poorly compliant patients with a GFD[89].
Although not specifically linked to CD, genetic and environmental risk assessment in combination with genetic polymorphisms and biochemical markers (folate levels) enhances CRC risk in the general population, which mandates earlier colonoscopy, lifestyle interventions, and targeted surveillance[90]. More specifically, advanced marsh classification suggests a greater neoplastic risk associated with persistent villous atrophy despite adherence to a GFD. Future biomarkers might include elevated IL-5, TGF-β, and MLH1 methylation for neoplastic transformation[91]. Incorporating these factors into individualized monitoring, potentially combining genetic profiling, serologic testing, and endoscopic surveillance, could help CRC prevention strategies in CD patients. Risk-based assessment with early colonoscopy screening and immune profiling may emerge as future screening standards.
It is used to study pathological masses of suspected neoplasms in CD patients and to characterize types of lymphoma with certain characteristic immunophenotypes that are associated with patient outcome. Considering RCD is a potential stage of disease where neoplastic complications may develop, understanding these types of lymphoma and their immunophenotypes would help to predict those patients with CD disease who develop neoplastic complications. Common types of lymphoma in CD with reported involvement of the colon are Burkitt lymphoma, which expresses CD19, CD2, and CD10 and is B cell lymphoma 6 or BCL-6 positive, and follicular lymphoma, which accounts for less than 7% of gastrointestinal-related lymphomas, with rare involvement of the colon and BCL-2 and CD20 positivity.
EATL or mature T-cell lymphoma, natural killer/T-cell lymphoma with rare involvement of the colon, is characterized by CD3, CD7, and CD103 positivity, in addition to variable reactivity to CD8, CD30 and TCR-β. Specifically, EATL-II expresses CD8+, CD3+, and TCR-β, and the co-expression of CD56 positivity. However, information regarding the prognostic nature of these types of lesions requires invasive biopsy. Combination of clinical parameters and biopsies could be a promising tool to predict the nature of prognosis in patients with RCD. The use of information from patients with RCD and symptoms of malabsorption and findings of anemia (low serum iron, B12 and folate), low electrolytes (Na, K, Cl, and zinc), elevated international normalized ratio and hypoalbuminemia applied in a structured malabsorption score (ranging from 0-8) with a cutoff of ≥ 3 is useful for identifying high-risk patients with RCD who would benefit from further evaluation to identify occult neoplasms, especially lymphoma. Colorectal cancer is a rare neoplasm in patients with RCD for which there is no available literature to investigate and link immunophenotyping to RCD patients.
The development of lymphoma in CD patients is a significant milestone complication of the disease secondary to the chronic immune-inflammatory response in GIT. Ancient studies of malignancy and CD in 1974 described lymphoma as reticular cell sarcoma of the GIT, mostly in the stomach, small intestine, and lymph nodes[92]. The intestine is an extra nodal site where the immune-inflammatory reaction occurs at the local lymphoreticular tissue of “gut-associated lymphoid tissue”[93]. Most lymphomas have a T-cell origin (96%), with BCL accounting for less than 5% of lymphomas[94], and BCL can present in multifocal areas such as the colon, stomach, and small bowel[95].
EATL and CD share similar IEL haplotypes, clinical presentations, and histological findings[96]. Predominantly, type 1 EATL accounts for 80%-90% of CD cases, whereas type 2 EATL is a sporadic disease and accounts for the remaining 10%-20% of cases[97]. Risk factors for EATL include inflammatory bowel disease, CD, immunosuppressive states, and infectious etiologies such as Epstein-Barr virus and human T-lymphotropic virus[98]. An alteration in chromosome 9, q33-34, is a hallmark of EATL that occurs in 70% of patients, and poor prognosis is associated with the presence of more than 3 chromosomal gains[99]. Advanced age at the time of diagnosis of CD (60 years) is more commonly associated with small bowel neoplasia than colonic lesions such as colon adenoma[100]. EATL was the most frequently associated malignancy (RR = 35.8), followed by small intestine and duodenal adenocarcinoma (RR = 14.4 and RR = 10.2, respectively). The contributing risk factors are male sex, the classic form of CD, advanced age at diagnosis, delayed diagnosis, untreated CD, persistent villous atrophy, and HLA-DQ2 homozygosity[101]. Several case reports of EATL commonly presented with abdominal pain and tumor perforation into the peritoneum with the development of peritonitis[102,103].
The described pathologies have different types of presentations of solitary or multiple lymphomas, such as non-B, non-T lymphoma[94], marginal cell lymphoma[103], and aggressive Burkitt-like lymphoma, which are histologically characterized as high-grade BCL[104]. Aggressive disease has been reported in patients as young as 29 years of age with metastatic colon type 1 EATL[97]. The disease survival and prognosis of EATL decrease with duration, with 1- and 5-year failure-free survival rates of 19.4% and 3.2%, respectively, and actuarial 1- and 5-year survival rates of 38.7% and 19.7%, respectively[105].
The importance of colorectal adenoma and adenocarcinoma in patients with CD is of interest for further advancements in screening strategies for CD patients at risk of malignancy[61]. Although CD is commonly associated with small bowel lymphoma, risk and screening assessments for colorectal neoplasia remain a point of debate among researchers. The controversies surrounding the association between CD and colorectal neoplasia are presented in Table 2 for colon adenomas and Table 3 for colon adenocarcinoma.
| Ref. | Year | Country | Design | Celiac disease cases | Colon adenoma cases | Survival and mortality findings |
| Pereyra et al[22] | 20131 | Argentina | Multicenter, retrospective case–control study | 118 | Polyps: 24; adenoma: 18; advanced colonic lesions: 3 | On multivariate analysis, there was increased risk of colonic adenoma observed in CD patient aged ≥ 75 years old. Polyps, OR = 6.59 (CI: 1.64-26.57), P = 0.008; adenoma, OR = 8.12 (CI: 1.83-35.94), P = 0.005; advanced colonic lesions, OR = 15.14 (CI: 1.23-186.67), P = 0.03 |
| Lasa et al[107] | 20181 | Argentina | Hospital-based retrospective case control study | 57 | Adenoma: 27; left sided adenoma: 21; right sided adenoma: 10; advanced adenoma: 6 | Adenoma, OR = 2.31 (CI: 1.18-4.53), P = 0.01; left sided adenoma, OR = 2.69 (CI: 1.29-5.61), P = 0.006; right sided adenoma, OR = 1.18 (CI: 0.5-2.76), P = 0.7; advanced adenoma, OR = 1.27 (CI: 0.43-3.71), P = 0.6 |
| Lasa et al[109] | 20182 | Argentina | Systemic review and meta-analysis | 367 | No increased prevalence of colorectal adenomas in CD patients, compared with controls [OR = 0.94 (CI: 0.65-1.38), P = 0.76], and no significant difference was observed when assessing the prevalence of advanced adenomas [OR = 0.97 (CI: 0.48-1.97)], no significant heterogeneity was observed (I2 = 26%) | |
| González et al[110] | 20122 | Argentina | Hospital-based retrospective case control study | 178 | Polyps: 9; adenoma: 7; advanced colonic lesions: 2 | No significant association between CD and colorectal adenoma among CD patients and control; polyps: OR = 1.48 (CI: 0.59-3.73), adenoma: OR = 1.89 (CI: 0.66-5.42), advanced colonic lesions: OR = 1.34 (CI: 0.26-7.05) |
| Lebwohl et al[108] | 20101 | United States | Hospital-based retrospective case control study | 180 | 23 | On multivariate analysis, age (OR per year 1.04, 95%CI: 1.02-1.07) and male gender (OR = 2.33, 95%CI: 1.36-3.98) are associated with adenomas; no significant association between CD and adenoma detection (OR = 0.75, 95%CI: 0.41-1.34), P = 0.33 |
| Ref. | Year | Country | Study design | Celiac disease cases | Cancer cases | Survival and mortality findings |
| Askling et al[111] | 20021 | Sweden | Population-based prospective cohort study | 11019 | 26 | Compared to the general population: Increased risk of colon cancer: SIR = 1.9 (95%CI: 1.2-2.8); similar risk of rectum cancer: SIR = 0.8 (95%CI: 0.3-1.6) |
| Elfström et al[112] | 20121 | Sweden | Nationwide retrospective cohort study (biopsy based) | Total CD patients: 28882 | First year (colon: 49, rectum: 14); after the first year (colon: 88, rectum: 30) | For colon cancer, in the CD total group, the HR was 7.94 (5.21-12.12) at the first year of FU and 1.10 (0.87-1.39) after the first year of FU. For rectum cancer, in the CD total group, the HR was 2.57 (1.36-4.86) at the first year of FU and 0.58 (0.4-0.85) after the first year of FU. In the CD with active inflammation group for colon cancer, the HR was 9.02 (6.08-13.4) at the first year of FU and 1.01 (0.76-1.34) after the first year of FU; for rectum cancer, the HR was 3.49 (1.45-8.43) at the first year of FU and 0.83 (0.55-1.27) after the first year of FU. In the latent CD group for colon cancer, the HR was 4.03 (1.26-12.9) at the first year of FU and 0.39 (0.09-1.62) after the first year of FU; for rectum cancer, the HR was 13.6 (4.04-45.7) at the first year |
| CD with inflammation (marsh I-II) 12860 | At first year (colon: 60, rectum: 8); after the first year (colon: 57, rectum: 25) | |||||
| Latent CD 3705 | First year (colon: 4, rectum: 6); after first year (colon: 2) | |||||
| Ilus et al[113] | 20141 | Finland | Population-based prospective cohort study | 32439 | 133 colon cancers, 51 rectal cancers | Increased risk of colon cancer: SIR = 1.35 (95%CI: 1.13-1.58); no increased risk of rectum cancer: SIR = 0.82 (95%CI: 0.61-1.07) |
| Onwuzo et al[6] | 20231 | United States | Retrospective multicenter | 1890 | 82880 | Increased risk of colon cancer, OR of CRC in CD 10.18 (95%CI: 9.72-10.65), P < 0.001). On multivariate regression analysis: OR of CRC in: Males 1.49 (95%CI: 1.36-1.63); African Americans 1.51 (95%CI: 1.35-1.68); type 2 diabetes mellitus 2.71 (95%CI: 2.66-2.76); smokers 2.49 (95%CI: 2.44-2.54); obese individuals 2.21 (95%CI: 2.17-2.25); alcoholics 1.72 (95%CI: 1.66-1.78) |
| Viljamaa et al[114] | 20062 | Finland | Population-based prospective cohort study | 781 | 4 | No increased risk of colon and rectum cancer: SIR = 1.1 (95%CI: 0.3-2.8) |
| Silano et al[115] | 20072 | Italy | Hospital-based prospective cohort study | 1968 | 7 | No increased risk of colon cancer: SIR = 1.1 (95%CI: 0.68-1.56), P < 0.001 |
| Goldacre et al[83] | 20082 | United States | Hospital-based retrospective cohort study | 1997 | 11 colon cancer, 4 rectum cancer | No increased risk (excluding cases occurred within the first year after CD diagnosis). Colon: Adjusted RR = 1.23 (95%CI: 0.61-2.20); rectum: Adjusted RR = 1.04 (95%CI: 0.28-2.67) |
| Lebwohl et al[116] | 20222 | Sweden | Population-based cohort study | 47241 | 448 | No increased risk of colorectal cancer: HR = 1.06 (95%CI: 0.96-1.18) |
| Landgren et al[117] | 20112 | United States | Hospital-based retrospective cohort study | 11 colon cancer, 9 rectum cancer | No increased risk: Colon: Adjusted RR = 0.85 (95%CI: 0.47-1.54); rectum: Adjusted RR = 1.29 (95%CI: 0.67-2.48) | |
| Grainge et al[19] | 20122 | United Kingdom | Population-based retrospective cohort study | 435 | 6 | No increased risk of colorectal cancer: SIR = 1.17 (95%CI: 0.43-2.54) |
| Volta et al[23] | 20142 | Italy | Multicenter retrospective cohort study | 1757 | 6 | Decreased risk of colon carcinoma compared to the general population: SIR = 0.29 (95%CI: 0.07-0.45) |
| van Gils et al[84] | 20182 | Netherlands | Population-based retrospective case control study | 349 | 105 | Increased risk during the first year after CD diagnosis [RR = 5.1 (95%CI: 3.1-8.3)], then decreased risk afterward period [RR = 0.7 (95%CI: 0.5-0.9, P < 0.001)]; sensitivity analyses showed decreased risk for colorectal carcinoma after CD diagnosis [RR = 0.7 (95%CI: 0.5-0.9), P = 0.02] |
| Haider et al[118] | 20242 | United States | Population-based study | 529842 | 1752 | No significant association, OR = 0.99 (0.93-1.06), P = 0.7087 |
Increased risk of development of colon adenoma in CD reported[106], and with advanced age of CD patients especially male patients[22,107], while no increased risk detected between CD and development of colonic adenomas[108,109]. For colonic adenocarcinoma studies showed increased risk of development in patients with CD[6,110-112], while other studies showed no increased risk shown in the studies cited[19,23,83,84,113-118]. Non-adenomatous dysplasia has been reported on CD, in which rectal neuroendocrine polyps (well-differentiated grade 1) have been described[119].
There is no consensus or recommended approach for the surveillance, screening or early diagnosis of colorectal neoplasia in patients with CD. Our suggested approaches for the management of neoplasia in CD patients are as follows.
Initial clinical evaluation: Upon the diagnosis of CD, a detailed clinical evaluation, including medical history and physical examination, should be performed. Changes in bowel habits in otherwise GFD-adherent patients should not be dismissed as coexistent irritable bowel syndrome. Abnormal physical findings, especially abdominal tenderness or masses should prompt further evaluation via computed tomography or magnetic resonance imaging of the abdomen to investigate potentially growing masses, such as lymphoma or carcinoma, that present as abdominal adhesions and obstruction[120].
Diagnostic investigations: Serology and biopsy are necessary to confirm the diagnosis of CD. Biopsies are critical for detecting conditions that coexist with CD, such as lymphoma[121]. While general blood tests are of limited diagnostic utility for early lymphoma diagnosis, serum albumin level measurement might be helpful, and a low serum albumin level in elderly CD patients has been significantly associated with lymphoma[121].
Gastrointestinal evaluation: Colonoscopy is essential for ruling out microscopic colitis (including lymphocytic colitis) and for identifying dysplastic lesions in the colon, and it is particularly useful for investigating diarrhea that is not managed with a GFD in CD patients. Iron deficiency anemia is a critical warning sign, especially in patients aged older than 45 years, and should prompt investigation[63,121-123]. No pathognomonic endoscopic findings for lymphoma exist, but findings of mucosal thickening, masses with circumferential ulcerations, and edematous mucosa might suggest an underlying lymphoma[124].
Radiological findings: Radiological examinations are useful but not exclusive for the diagnosis of suspected intestinal and/or colonic lymphoma. Possible findings are polypoid masses, circumferential bowel thickening, and lymphadenopathy[125-127]. Nuclear imaging with positron emission tomography is performed to identify monomorphic epitheliotropic intestinal T-cell lymphoma, which typically manifests as endometriotic lesions[128-130].
Genetic testing and biomarkers: MMR gene testing and MSI analysis via the identification of MLH1, MutS homolog 2, MutS homolog 6, and post meiotic segregation increased 2 methylation or mutation (PMS2) can reveal the MSI-high status, guiding immune checkpoint inhibitor therapy, which is highly effective in colon adenocarcinomas with high neoantigen loads[131]. Additionally, elevated fecal calprotectin levels are a noninvasive marker of colonic inflammation. Evidently, meta-analysis revealed 5-fold greater odds of elevated fecal calprotectin levels than in controls are significant for chronic inflammation[132]. Moreover, circulating tumor DNA and epigenetic markers [assays targeting methylated genes, i.e., Septin9 (SEPT9) and vimentin (VIM)] are emerging tools as adjuncts for risk stratification in CD patients with borderline screening indications[133]. Emerging evidence supports the clinical utility of circulating tumor DNA methylation assays targeting SEPT9, VIM and related genes for early CRC detection[133]. Moreover, a large-scale panel including SDC2, SEPT9, and VIM, applied to fecal and tissue samples, achieved over 91% sensitivity and 100% specificity, underscoring its diagnostic accuracy in early-stage CRC[134].
Targeted and immune-based therapies: Immune-based and targeted therapies are transforming the management of CD-associated neoplasia. For MSI-high/deficient MMR colorectal adenocarcinoma, pembrolizumab demonstrated efficacy in KEYNOTE177 study, a significantly longer progression-free survival was achieved (16.5 vs 8.2 months; hazard ratio = 0.60) and improved 5-year overall survival compared with chemotherapy[135]. Furthermore, in previously treated patients in KEYNOTE164 trial showed a 33%-35% objective response rate and prolonged survival[136]. Together, these developments underscore the benefit of genetic and molecular profiling to guide precision immunotherapy for high-risk individuals within the CD patient population.
Current knowledge of the incidence and prevalence of colonic neoplasia is based on retrospective studies with limited longitudinal follow-up, mostly involving patients with a duration of follow-up of up to 10 years, and are prone to selection and reporting biases. Since CD is a life-long illness for which management depends on adherence to a GFD, several other key factors also need to be accounted for, including factors that may increase dysplasia in the intestine and demographic and genetic backgrounds that limit the generalization of these recommendations, as most data are derived from Western populations. The underdiagnosis of CD and inconsistent colorectal screening practices may result in underestimation of the incidence of neoplasms. Variations in diagnostic criteria and a lack of detailed molecular profiling in CD-associated colorectal tumors further limit the ability to draw definitive conclusions. Long prospective studies with standardized protocols are needed to clarify the true nature of this association.
Strategies are needed for the early diagnosis and detection of large bowel and small bowel lymphoma in CD patients. High-performance stool tests for detecting neoplasms, including lymphoma and adenocarcinoma, in CD patients also need to be developed. Notably, enrolling CD patients in a regular screening program for intestinal neoplasia via less invasive or invasive methods should be considered. These strategies should be feasible, less invasive, and appropriate for the management and follow-up of CD patients. Furthermore, biotechnology and artificial intelligence are needed to incorporate data from biological samples to accurately detect abnormal lymphocytes and identify abnormal clones. Genetic testing for at-risk alleles in CD is considered among the possible targeted tests; hence, the identification of patients who may be at risk of developing EATL is a necessary future investigational target.
Accumulating evidence suggests that gluten exposure has a deleterious effect both locally and systemically. The mechanisms of gluten damage to the bowel, including small bowel changes with villous atrophy, have been proven to extend beyond the small bowel and involve genetic, cellular, and paracellular mechanisms. Although colonic lymphoma is rare, it has a poor prognosis because of its late and nonspecific clinical presentation. A GFD is a mandatory management strategy, and evidence shows that strict adherence is a therapeutic and preventative clinical outcome. A search for gastrointestinal lymphoma is warranted in patients with CD with symptoms of abdominal pain, weight loss, anemia, and hypoalbuminemia not otherwise explained.
| 1. | Stokes PL, Asquith P, Holmes GK, Mackintosh P, Cooke WT. Histocompatibility antigens associated with adult coeliac disease. Lancet. 1972;2:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 196] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Saurer L, Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy. 2009;64:505-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Clemente E, Efthymakis K, Carletti E, Capone V, Sperduti S, Bologna G, Marchisio M, Di Nicola M, Neri M, Sallese M. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PLoS One. 2019;14:e0226478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Carroccio A, Giannone G, Mansueto P, Soresi M, La Blasca F, Fayer F, Iacobucci R, Porcasi R, Catalano T, Geraci G, Arini A, D'Alcamo A, Villanacci V, Florena AM. Duodenal and Rectal Mucosa Inflammation in Patients With Non-celiac Wheat Sensitivity. Clin Gastroenterol Hepatol. 2019;17:682-690.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Ryan JC. Premalignant conditions of the small intestine. Semin Gastrointest Dis. 1996;7:88-93. [PubMed] |
| 6. | Onwuzo S, Boustany A, Saleh M, Gupta R, Onwuzo C, Mascarenhas Monteiro J, Lawrence F, Emeshiobi C, Odu J, Asaad I. Increased Risk of Colorectal Cancer in Patients With Celiac Disease: A Population-Based Study. Cureus. 2023;15:e36964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Freeman HJ. Colorectal Cancer in Biopsy-defined Celiac Disease Seen over 30 Years: Rare, Even in Elderly Adults. Int J Celiac Dis. 2021;9:93-95. [DOI] [Full Text] |
| 8. | Wang M, Yu M, Kong WJ, Cui M, Gao F. Association between intestinal neoplasms and celiac disease: A review. World J Gastrointest Oncol. 2021;13:1017-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 9. | Rampertab SD, Forde KA, Green PH. Small bowel neoplasia in coeliac disease. Gut. 2003;52:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Helenius TO, Antman CA, Asghar MN, Nyström JH, Toivola DM. Keratins Are Altered in Intestinal Disease-Related Stress Responses. Cells. 2016;5:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kelly P, Feakins R, Domizio P, Murphy J, Bevins C, Wilson J, McPhail G, Poulsom R, Dhaliwal W. Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin Exp Immunol. 2004;135:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Clinton NA, Hameed SA, Agyei EK, Jacob JC, Oyebanji VO, Jabea CE. Crosstalk between the Intestinal Virome and Other Components of the Microbiota, and Its Effect on Intestinal Mucosal Response and Diseases. J Immunol Res. 2022;2022:7883945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Suzuki T, Hayman L, Kilbey A, Edwards J, Coffelt SB. Gut γδ T cells as guardians, disruptors, and instigators of cancer. Immunol Rev. 2020;298:198-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Abstracts from USCAP 2020: Gastrointestinal Pathology (612-832). Lab Invest. 2020;100:624-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Orlando A, Chimienti G, Pesce V, Fracasso F, Lezza AMS, Russo F. An In Vitro Study on Mitochondrial Compensatory Response Induced by Gliadin Peptides in Caco-2 Cells. Int J Mol Sci. 2019;20:1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Dolfini E, Elli L, Dasdia T, Bufardeci B, Colleoni MP, Costa B, Floriani I, Falini ML, Guerrieri N, Forlani F, Bardella MT. In vitro cytotoxic effect of bread wheat gliadin on the LoVo human adenocarcinoma cell line. Toxicol In Vitro. 2002;16:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Ciccocioppo R, Di Sabatino A, Gasbarrini G, Corazza GR. Apoptosis and gastrointestinal tract. Ital J Gastroenterol Hepatol. 1999;31:162-172. [PubMed] |
| 18. | Allegretti YL, Bondar C, Guzman L, Cueto Rua E, Chopita N, Fuertes M, Zwirner NW, Chirdo FG. Broad MICA/B expression in the small bowel mucosa: a link between cellular stress and celiac disease. PLoS One. 2013;8:e73658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Grainge MJ, West J, Solaymani-Dodaran M, Card TR, Logan RF. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2012;35:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Fundia AF, Cottliar AS, La Motta G, Crivelli A, Gómez JC, Slavutsky IR, Larripa IB. Analysis of genomic instability in adult-onset celiac disease patients by microsatellite instability and loss of heterozygosis. Eur J Gastroenterol Hepatol. 2008;20:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Ivanova M, Bottiglieri L, Sajjadi E, Venetis K, Fusco N. Malignancies in Patients with Celiac Disease: Diagnostic Challenges and Molecular Advances. Genes (Basel). 2023;14:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Pereyra L, Gonzalez R, Mohaidle A, Fischer C, Mella JM, Panigadi GN, Manazzoni D, Matoso MD, Lasa JS, Novillo A, De Paula J, Soifer L, Nadales A, Cimmino DG, Pedreira S, Boerr L. Risk of colorectal neoplasia in patients with celiac disease: a multicenter study. J Crohns Colitis. 2013;7:e672-e677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Volta U, Vincentini O, Quintarelli F, Felli C, Silano M; Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol. 2014;49:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Marafini I, Monteleone G, Stolfi C. Association Between Celiac Disease and Cancer. Int J Mol Sci. 2020;21:4155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 717] [Cited by in RCA: 730] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 26. | Kainulainen H, Rantala I, Collin P, Ruuska T, Päivärinne H, Halttunen T, Lindfors K, Kaukinen K, Mäki M. Blisters in the small intestinal mucosa of coeliac patients contain T cells positive for cyclooxygenase 2. Gut. 2002;50:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Pelizzaro F, Marsilio I, Fassan M, Piazza F, Barberio B, D'Odorico A, Savarino EV, Farinati F, Zingone F. The Risk of Malignancies in Celiac Disease-A Literature Review. Cancers (Basel). 2021;13:5288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Ettersperger J, Montcuquet N, Malamut G, Guegan N, Lopez-Lastra S, Gayraud S, Reimann C, Vidal E, Cagnard N, Villarese P, Andre-Schmutz I, Gomes Domingues R, Godinho-Silva C, Veiga-Fernandes H, Lhermitte L, Asnafi V, Macintyre E, Cellier C, Beldjord K, Di Santo JP, Cerf-Bensussan N, Meresse B. Interleukin-15-Dependent T-Cell-like Innate Intraepithelial Lymphocytes Develop in the Intestine and Transform into Lymphomas in Celiac Disease. Immunity. 2016;45:610-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M, Cosnes J, Lavergne-Slove A, Meresse B, Trinquart L, Macintyre E, Radford-Weiss I, Hermine O, Brousse N, Cerf-Bensussan N, Cellier C. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 30. | Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (1)] |
| 31. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4088] [Cited by in RCA: 4526] [Article Influence: 226.3] [Reference Citation Analysis (1)] |
| 32. | Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 931] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 33. | Palová-Jelínková L, Rozková D, Pecharová B, Bártová J, Sedivá A, Tlaskalová-Hogenová H, Spísek R, Tucková L. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J Immunol. 2005;175:7038-7045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Cinova J, Palová-Jelínková L, Smythies LE, Cerná M, Pecharová B, Dvorák M, Fruhauf P, Tlaskalová-Hogenová H, Smith PD, Tucková L. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Lammers KM, Khandelwal S, Chaudhry F, Kryszak D, Puppa EL, Casolaro V, Fasano A. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology. 2011;132:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Terrazzano G, Sica M, Gianfrani C, Mazzarella G, Maurano F, De Giulio B, de Saint-Mezard S, Zanzi D, Maiuri L, Londei M, Jabri B, Troncone R, Auricchio S, Zappacosta S, Carbone E. Gliadin regulates the NK-dendritic cell cross-talk by HLA-E surface stabilization. J Immunol. 2007;179:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 37. | Hou S, Zhao Y, Chen J, Lin Y, Qi X. Tumor-associated macrophages in colorectal cancer metastasis: molecular insights and translational perspectives. J Transl Med. 2024;22:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 38. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1615] [Article Influence: 100.9] [Reference Citation Analysis (2)] |
| 39. | Giovannini C, Sanchez M, Straface E, Scazzocchio B, Silano M, De Vincenzi M. Induction of apoptosis in caco-2 cells by wheat gliadin peptides. Toxicology. 2000;145:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Dolfini E, Elli L, Ferrero S, Braidotti P, Roncoroni L, Dasdia T, Falini ML, Forlani F, Bardella MT. Bread wheat gliadin cytotoxicity: a new three-dimensional cell model. Scand J Clin Lab Invest. 2003;63:135-141. [PubMed] |
| 41. | Berti C, Roncoroni L, Falini ML, Caramanico R, Dolfini E, Bardella MT, Elli L, Terrani C, Forlani F. Celiac-related properties of chemically and enzymatically modified gluten proteins. J Agric Food Chem. 2007;55:2482-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Sakly W, Thomas V, Quash G, El Alaoui S. A role for tissue transglutaminase in alpha-gliadin peptide cytotoxicity. Clin Exp Immunol. 2006;146:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Castellanos-Rubio A, Caja S, Irastorza I, Fernandez-Jimenez N, Plaza-Izurieta L, Vitoria JC, Maki M, Lindfors K, Bilbao JR. Angiogenesis-related gene expression analysis in celiac disease. Autoimmunity. 2012;45:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Pittman FE, Pittman JC. A light and electron microscopic study of sigmoid colonic mucosa in adult celiac disease. Scand J Gastroenterol. 1966;1:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Picarelli A, Di Tola M, Borghini R, Isonne C, Saponara A, Marino M, Casale R, Tiberti A, Pica R, Donato G, Frieri G, Corazziari E. Colonic involvement in celiac disease and possible implications of the sigmoid mucosa organ culture in its diagnosis. J Clin Immunol. 2013;33:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Elli L, Roncoroni L, Doneda L, Ciulla MM, Colombo R, Braidotti P, Bonura A, Bardella MT. Imaging analysis of the gliadin direct effect on tight junctions in an in vitro three-dimensional Lovo cell line culture system. Toxicol In Vitro. 2011;25:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Wang J, He M, Yang M, Ai X. Gut microbiota as a key regulator of intestinal mucosal immunity. Life Sci. 2024;345:122612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 48. | Tiamani K, Luo S, Schulz S, Xue J, Costa R, Khan Mirzaei M, Deng L. The role of virome in the gastrointestinal tract and beyond. FEMS Microbiol Rev. 2022;46:fuac027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Zhao F, Wang J. Another piece of puzzle for the human microbiome: the gut virome under dietary modulation. J Genet Genomics. 2024;51:983-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Lindfors K, Lin J, Lee HS, Hyöty H, Nykter M, Kurppa K, Liu E, Koletzko S, Rewers M, Hagopian W, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Petrosino JF, Lloyd RE, Agardh D; TEDDY Study Group. Metagenomics of the faecal virome indicate a cumulative effect of enterovirus and gluten amount on the risk of coeliac disease autoimmunity in genetically at risk children: the TEDDY study. Gut. 2020;69:1416-1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 51. | Metzger RN, Krug AB, Eisenächer K. Enteric Virome Sensing-Its Role in Intestinal Homeostasis and Immunity. Viruses. 2018;10:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Guo Y, Liu Y, Rui B, Lei Z, Ning X, Liu Y, Li M. Crosstalk between the gut microbiota and innate lymphoid cells in intestinal mucosal immunity. Front Immunol. 2023;14:1171680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 53. | Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 54. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1736] [Article Influence: 124.0] [Reference Citation Analysis (1)] |
| 55. | Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 885] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 56. | Putze J, Hennequin C, Nougayrède JP, Zhang W, Homburg S, Karch H, Bringer MA, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, Dobrindt U. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect Immun. 2009;77:4696-4703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 57. | Chowers Y, Marsh MN, De Grandpre L, Nyberg A, Theofilopoulos AN, Kagnoff MF. Increased proinflammatory cytokine gene expression in the colonic mucosa of coeliac disease patients in the early period after gluten challenge. Clin Exp Immunol. 1997;107:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Sturgess RP, Loft D, Kontakou M, Crowe P, Marsh MN, Ciclitira PJ. Rectal epithelial gamma/delta T-lymphocyte responses to local gluten challenge in coeliac disease. Scand J Gastroenterol. 1993;28:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 198] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 60. | Tangri V, Driman DK, Chande N. Latent lymphocytic enterocolitis associated with celiac disease manifesting after resection for colon cancer: case report and review of the literature. Can J Gastroenterol. 2008;22:771-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 61. | Dickey WJ. Celiac Disease and the Colon. Pract Gastroenterol. 2008;32:40-45. |
| 62. | Stahl E, Roda G, Dobbyn A, Hu J, Zhang Z, Westerlind H, Bonfiglio F, Raj T, Torres J, Chen A, Petras R, Pardi DS, Iuga AC, Levi GS, Cao W, Jain P, Rieder F, Gordon IO, Cho JH, D'Amato M, Harpaz N, Hao K, Colombel JF, Peter I. Collagenous Colitis Is Associated With HLA Signature and Shares Genetic Risks With Other Immune-Mediated Diseases. Gastroenterology. 2020;159:549-561.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Casella G, Villanacci V, Di-Bella C, de-Marco E, Pagni F, Drera E, Ortenzi R, Baldini V, Bassotti G. Colonoscopic findings in coeliac disease on a gluten-free diet. Rev Esp Enferm Dig. 2010;102:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Verkarre V, Asnafi V, Lecomte T, Patey Mariaud-de Serre N, Leborgne M, Grosdidier E, Le Bihan C, Macintyre E, Cellier C, Cerf-Bensussan N, Brousse N. Refractory coeliac sprue is a diffuse gastrointestinal disease. Gut. 2003;52:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Beyaert R, Beaugerie L, Van Assche G, Brochez L, Renauld JC, Viguier M, Cocquyt V, Jerusalem G, Machiels JP, Prenen H, Masson P, Louis E, De Keyser F. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer. 2013;12:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Ishibashi H, Nimura S, Kayashima Y, Takamatsu Y, Aoyagi K, Harada N, Kadowaki M, Kamio T, Sakisaka S, Takeshita M. Multiple lesions of gastrointestinal tract invasion by monomorphic epitheliotropic intestinal T-cell lymphoma, accompanied by duodenal and intestinal enteropathy-like lesions and microscopic lymphocytic proctocolitis: a case series. Diagn Pathol. 2016;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | MacFarlane AJ, Stover PJ. Convergence of genetic, nutritional and inflammatory factors in gastrointestinal cancers. Nutr Rev. 2007;65:S157-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8412] [Article Influence: 525.8] [Reference Citation Analysis (8)] |
| 69. | Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Res. 2006;66:3959-3962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Pol J, Paillet J, Plantureux C, Kroemer G. Beneficial autoimmunity and maladaptive inflammation shape epidemiological links between cancer and immune-inflammatory diseases. Oncoimmunology. 2022;11:2029299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 71. | Yu KH, Kuo CF, Huang LH, Huang WK, See LC. Cancer Risk in Patients With Inflammatory Systemic Autoimmune Rheumatic Diseases: A Nationwide Population-Based Dynamic Cohort Study in Taiwan. Medicine (Baltimore). 2016;95:e3540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol. 2015;15:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 73. | Yang P, Liu Q, Zhang H, Wu M, Zhao J, Shen G, Zhao Y. Risk relationship between six autoimmune diseases and malignancies: An umbrella review. Autoimmun Rev. 2025;24:103779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 74. | Fischer S, Meisinger C, Freuer D. Autoimmune diseases and female-specific cancer risk: A systematic review and meta-analysis. J Autoimmun. 2024;144:103187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Hemminki K, Liu X, Ji J, Försti A, Sundquist J, Sundquist K. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol Oncol. 2012;127:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Sahan A, Kahraman S, Acet E, Sezer C, Sagcan M, Sivgin H, Yilmaz A. Involvement in colon cases detected gluten enteropathy. Proceedings of Asia Pacific Digestive Week 2013/World Congress of Gastroenterology; 2013 Sept 21-24; Shanghai, China. NJ, United States: Wiley, 2013: 151-151. |
| 77. | Allgayer H, Dietrich CF. [Celiac sprue and malignancies: analysis of risks and prevention strategies]. Med Klin (Munich). 2008;103:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Ho-Yen C, Chang F, van der Walt J, Mitchell T, Ciclitira P. Recent advances in refractory coeliac disease: a review. Histopathology. 2009;54:783-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 80. | Katoh A, Ohshima K, Kanda M, Haraoka S, Sugihara M, Suzumiya J, Kawasaki C, Shimazaki K, Ikeda S, Kikuchi M. Gastrointestinal T cell lymphoma: predominant cytotoxic phenotypes, including alpha/beta, gamma/delta T cell and natural killer cells. Leuk Lymphoma. 2000;39:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Yasuoka H, Masuo T, Hashimoto K, Sato K, Okada S, Kusano M, Oishi T, Yokoo H, Kojima M, Nakazato Y, Mori M. Enteropathy-type T-cell lymphoma that was pathologically diagnosed as celiac disease. Intern Med. 2007;46:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Cereda M, Gambardella G, Benedetti L, Iannelli F, Patel D, Basso G, Guerra RF, Mourikis TP, Puccio I, Sinha S, Laghi L, Spencer J, Rodriguez-Justo M, Ciccarelli FD. Patients with genetically heterogeneous synchronous colorectal cancer carry rare damaging germline mutations in immune-related genes. Nat Commun. 2016;7:12072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, Jewell D. Cancer in patients with ulcerative colitis, Crohn's disease and coeliac disease: record linkage study. Eur J Gastroenterol Hepatol. 2008;20:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 84. | van Gils T, Nijeboer P, Overbeek LI, Hauptmann M, Castelijn DA, Bouma G, Mulder CJ, van Leeuwen FE, de Jong D. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up: Celiac disease, lymphoma and GI carcinoma. United European Gastroenterol J. 2018;6:1485-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Freeman HJ. Adult celiac disease and its malignant complications. Gut Liver. 2009;3:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 612] [Article Influence: 87.4] [Reference Citation Analysis (1)] |
| 87. | Weinberg DS, Myers RE, Keenan E, Ruth K, Sifri R, Ziring B, Ross E, Manne SL. Genetic and environmental risk assessment and colorectal cancer screening in an average-risk population: a randomized trial. Ann Intern Med. 2014;161:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Gutierrez-Achury J, Zhernakova A, Pulit SL, Trynka G, Hunt KA, Romanos J, Raychaudhuri S, van Heel DA, Wijmenga C, de Bakker PI. Fine mapping in the MHC region accounts for 18% additional genetic risk for celiac disease. Nat Genet. 2015;47:577-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 89. | Kamat N, Khidhir MA, Alashari MM, Rannug U. Microsatellite instability and loss of heterozygosity detected in middle-aged patients with sporadic colon cancer: A retrospective study. Oncol Lett. 2013;6:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 90. | Lebwohl B, Granath F, Ekbom A, Smedby KE, Murray JA, Neugut AI, Green PH, Ludvigsson JF. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013;159:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 91. | Meresse B, Ripoche J, Heyman M, Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009;2:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 92. | Thompson H. Necropsy studies on adult coeliac disease. J Clin Pathol. 1974;27:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Otto HF, Bettmann I, Weltzien JV, Gebbers JO. Primary intestinal lymphomas. Virchows Arch A Pathol Anat Histol. 1981;391:9-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Koo V, Armstrong A, Harvey C. Coeliac disease presenting with colonic lymphoma. Ulster Med J. 2002;71:136-138. [PubMed] |
| 95. | Tursi A, Inchingolo CD. Synchronous gastric and colonic MALT-lymphoma in coeliac disease: a long-term follow-up on gluten-free diet. Dig Liver Dis. 2007;39:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Zettl A, deLeeuw R, Haralambieva E, Mueller-Hermelink HK. Enteropathy-type T-cell lymphoma. Am J Clin Pathol. 2007;127:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Zhang JC, Wang Y, Wang XF, Zhang FX. Type I enteropathy-associated T-cell lymphoma in the colon of a 29-year-old patient and a brief literature review. Onco Targets Ther. 2016;9:863-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Grigg-Gutierrez NM, Estremera-Marcial R, Caceres WW, Toro DH. Primary Enteropathy-Associated T-Cell Lymphoma Type 2: An Emerging Entity? Cancer Control. 2015;22:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Zettl A, Ott G, Makulik A, Katzenberger T, Starostik P, Eichler T, Puppe B, Bentz M, Müller-Hermelink HK, Chott A. Chromosomal gains at 9q characterize enteropathy-type T-cell lymphoma. Am J Pathol. 2002;161:1635-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Freeman HJ. Neoplastic Disorders in 100 Patients with Adult Celiac Disease. Can J Gastroenterol. 1996;10:163-166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Packova B, Kohout P, Dastych M, Prokesova J, Grolich T, Kroupa R. Malignant complications of celiac disease: a case series and review of the literature. J Med Case Rep. 2022;16:460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 102. | Shet T, Karpate A, Bal M, Gupta S, Gujral S, Nair R. Primary intestinal T cell lymphomas in Indian patients--in search of enteropathic T cell lymphoma. Indian J Pathol Microbiol. 2010;53:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Freeman HJ. Free perforation due to intestinal lymphoma in biopsy-defined or suspected celiac disease. J Clin Gastroenterol. 2003;37:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Spiewak T, Chormanski D, Bader GA, Owen S. A Case of Isolated Colonic MALT Lymphoma and Synchronous Celiac Disease. Am J Gastroenterol. 2022;117:e1355-e1356. [DOI] [Full Text] |
| 105. | Ahluwalia M, Gotlieb V, Damerla V, Saif MW. Aggressive Burkitt-like lymphoma of colon in a patient with prior celiac disease. Yale J Biol Med. 2006;79:173-175. [PubMed] |
| 106. | Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 219] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 107. | Lasa J, Rausch A, Bracho LF, Altamirano J, Speisky D, de Dávila MTG, Iotti A, Zubiaurre I. Colorectal Adenoma Risk Is Increased among Recently Diagnosed Adult Celiac Disease Patients. Gastroenterol Res Pract. 2018;2018:6150145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Lebwohl B, Stavsky E, Neugut AI, Green PH. Risk of colorectal adenomas in patients with coeliac disease. Aliment Pharmacol Ther. 2010;32:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Lasa J, Rausch A, Zubiaurre I. Risk of colorectal adenomas in patients with celiac disease: a systematic review and meta-analysis. Rev Gastroenterol Mex (Engl Ed). 2018;83:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | González R, Pereyra L, Mohaidle A, Mella JM, Fischer C, Medrano MA, Vizcaino B, Hadad AR, Luna P, Cimmino DG, Pedreira SC, Boerr LA. [Celiac disease and risk of colorectal neoplasia]. Acta Gastroenterol Latinoam. 2012;42:87-91. [PubMed] |
| 111. | Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (2)] |
| 112. | Elfström P, Granath F, Ye W, Ludvigsson JF. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol. 2012;10:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 113. | Ilus T, Kaukinen K, Virta LJ, Pukkala E, Collin P. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol. 2014;109:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 115. | Silano M, Volta U, Mecchia AM, Dessì M, Di Benedetto R, De Vincenzi M; Collaborating centers of the Italian registry of the complications of coeliac disease. Delayed diagnosis of coeliac disease increases cancer risk. BMC Gastroenterol. 2007;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 116. | Lebwohl B, Green PHR, Emilsson L, Mårild K, Söderling J, Roelstraete B, Ludvigsson JF. Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2022;20:e111-e131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (4)] |
| 117. | Landgren AM, Landgren O, Gridley G, Dores GM, Linet MS, Morton LM. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011;117:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 118. | Haider MB, Al Sbihi A, Reddy SN, Green P. Prevalence of malignant neoplasms in celiac disease patients - a nationwide United States population-based study. World J Clin Oncol. 2024;15:1048-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 119. | Wang Y, Cao Y, Lebwohl B, Song M, Sun Q, Green PHR, Giovannucci EL, Willett WC, Chan AT. Gluten Intake and Risk of Digestive System Cancers in 3 Large Prospective Cohort Studies. Clin Gastroenterol Hepatol. 2022;20:1986-1996.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Çetin D, Tanrıverdi Ö, Solak Özşeker H, Özşeker B. A rare association of celiac disease and rectal neuroendocrine tumor. Clin J Gastroenterol. 2017;10:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 121. | Elseidy SA, Awad AK. Celiac disease with intra-abdominal adhesions in a 32-year-old female patient: a case report and literature review. J Surg Case Rep. 2020;2020:rjaa351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 122. | Freeman HJ. Lymphoproliferative and intestinal malignancies in 214 patients with biopsy-defined celiac disease. J Clin Gastroenterol. 2004;38:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Sundar N, Mukhtar A, Finnie IA. Ileocolonoscopic diagnosis of coeliac disease. Endoscopy. 2003;35:374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 124. | Hopper AD, Leeds JS, Hurlstone DP, Hadjivassiliou M, Drew K, Sanders DS. Are lower gastrointestinal investigations necessary in patients with coeliac disease? Eur J Gastroenterol Hepatol. 2005;17:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Yanai S, Matsumoto T, Nakamura S, Fujisawa K, Ueki T, Hirahashi M, Yao T, Iida M. Endoscopic findings of enteropathy-type T-cell lymphoma. Endoscopy. 2007;39 Suppl 1:E339-E340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Hong YS, Woo YS, Park G, Lee K, Kang SH, Lee HW, Kim ER, Hong SN, Chang DK, Kim YH, Rhee PL, Kim JJ. Endoscopic Findings of Enteropathy-Associated T-Cell Lymphoma Type II: A Case Series. Gut Liver. 2016;10:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Chen JH, Bai D, Dabhi V, Wood BL, Kussick SJ. Clinicopathologic features of primary colonic enteropathy-associated T cell lymphoma type II in an elderly Asian male with diarrhea. J Hematopathol. 2015;8:37-41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 128. | Zhu L, Wu G, Ghimire P, Xu L. CT features of peripheral T-cell lymphoma in the gastrointestinal tract in Chinese population and literature review. J Med Imaging Radiat Oncol. 2012;56:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Scholz FJ, Afnan J, Behr SC. CT findings in adult celiac disease. Radiographics. 2011;31:977-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 130. | Chan TSY, Lee E, Khong PL, Tse EWC, Kwong YL. Positron emission tomography computed tomography features of monomorphic epitheliotropic intestinal T-cell lymphoma. Hematology. 2018;23:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Nádorvári ML, Lotz G, Kulka J, Kiss A, Tímár J. Microsatellite instability and mismatch repair protein deficiency: equal predictive markers? Pathol Oncol Res. 2024;30:1611719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 132. | Ross FA, Park JH, Mansouri D, Combet E, Horgan PG, McMillan DC, Roxburgh CSD. The role of faecal calprotectin in diagnosis and staging of colorectal neoplasia: a systematic review and meta-analysis. BMC Gastroenterol. 2022;22:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 133. | Gopal P, Ahmed Z, Venkata Ravi Kant V, Rao GV, Rebala P. Circulating tumor DNA for monitoring colorectal cancer: A prospective observational study to assess the presence of methylated SEPT9 and VIM promoter genes and its role as a biomarker in colorectal cancer management. Turk J Surg. 2023;39:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 134. | Liu Y, Ming H, Xu L, Li L, Liu Q, Zhao J, Zhong C, Li H. DNA methylation analysis of the SDC2, SEPT9 and VIM genes in fecal DNA for colorectal cancer diagnosis. BMC Cancer. 2024;24:1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 135. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, Alcaide-Garcia J, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zuo Y, Fogelman D, Adelberg D, Diaz LA. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann Oncol. 2025;36:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 136. | Le DT, Diaz LA Jr, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil BH, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Élez E, Al-Batran SE, Boland PM, Cui Y, Leconte P, Marinello P, André T. Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: final analysis of KEYNOTE-164. Eur J Cancer. 2023;186:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/