Published online Sep 28, 2025. doi: 10.3748/wjg.v31.i36.109846
Revised: June 30, 2025
Accepted: August 26, 2025
Published online: September 28, 2025
Processing time: 114 Days and 5.9 Hours
Rectal neuroendocrine tumors (NETs) smaller than 10 mm and well-differentiated tumors are generally considered to have a low risk of lymph node and distant metastasis, making them suitable for endoscopic resection. In contrast, tumors ≥ 20 mm in size typically require surgical resection. However, the optimal management of intermediate-sized (10-15 mm) rectal NETs remains controversial.
To compare the clinical outcomes of endoscopic resection of rectal NETs < 1 cm and those 1-1.5 cm in size.
A retrospective study was conducted on 1056 patients with rectal NETs treated at the Samsung Medical Center between January 2005 and June 2021. After propensity score matching (1:10) for age, sex, and type of endoscopic resection, 225 patients with tumors < 1 cm in size and 27 patients with tumors 1-1.5 cm in size were analyzed.
Surgical resection was more frequent in the 1-1.5 cm group (37.2%) than in the < 1 cm group (10.7%) (P < 0.01). Endoscopic submucosal dissection was also more commonly performed in the 1-1.5 cm group (48.1% vs 18.5%, P < 0.01). Negative resection margins were achieved in 97.2% of the patients, with no significant difference between the groups (P = 0.22). No lymphovascular invasion was observed. During a median follow-up of 54 months, no recurrence occurred in the 1-1.5 cm group, while one case of metachronous recurrence was noted in the < 1 cm group (P = 1.00). There was no significant difference in recurrence-free survival (P = 0.48).
Endoscopic resection of 1-1.5 cm grade 1 rectal NETs yielded comparable outcomes to those < 1 cm in size, suggesting its feasibility as a treatment.
Core Tip: This study demonstrated that endoscopic resection for 1-1.5 cm well-differentiated rectal neuroendocrine tumors offers clinical outcomes comparable to those for tumors < 1 cm, with high rates of negative margins and no observed recurrence in the intermediate-size group. These findings support the feasibility of endoscopic treatment for 1-1.5 cm rectal neuroendocrine tumors, address a key area of clinical uncertainty, and potentially expand the role of minimally invasive management in this subgroup.
- Citation: Kim M, Kim Y, Kim JE, Hong SN, Chang DK, Kim YH, Kim ER. Long-term outcomes of endoscopic resection of 1-1.5 cm sized grade 1 rectal neuroendocrine tumor: A retrospective study. World J Gastroenterol 2025; 31(36): 109846
- URL: https://www.wjgnet.com/1007-9327/full/v31/i36/109846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i36.109846
Rectal neuroendocrine tumors (NETs) are a distinct subset of gastrointestinal neuroendocrine neoplasms. Rectal NETs have an incidence of approximately 0.17% and have shown an increasing trend in recent years[1-4]. This increase is primarily due to the expanded use of endoscopic screening for colorectal cancer and advances in endoscopic techniques. Most patients with rectal NETs are asymptomatic and are often incidentally detected during endoscopic evaluation for colorectal cancer screening or unrelated gastrointestinal symptoms. The treatment options for NETs include endoscopic resection techniques, such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), surgical procedures ranging from local excision to more extensive resections, and systemic therapies in cases of advanced disease.
The management of rectal NETs is primarily guided by the tumor size, depth of invasion, and histological grade. Well-differentiated rectal NETs < 10 mm are thought to have a very low risk of distant metastasis, generally less than 3%[1]. They have a low risk of lymphovascular and muscularis propria invasion, rarely exhibit malignant potential, and can be completely removed endoscopically. Furthermore, rectal NETs > 20 mm are likely to have a higher risk of involvement of the muscularis propria and a high metastatic risk (60%-80%), and are candidates for surgical resection[5]. There is controversy over the treatment method for intermediate-sized rectal NETs (10-19 mm), in which the metastatic risk is 10%-15%[6-10]. To determine the utility of endoscopic resection as a treatment for NETs measuring 1-1.5 cm, we compared the outcomes after endoscopic resection of 0 cm ≤ size < 1 cm and 1 cm ≤ size < 1.5 cm-sized rectal NETs.
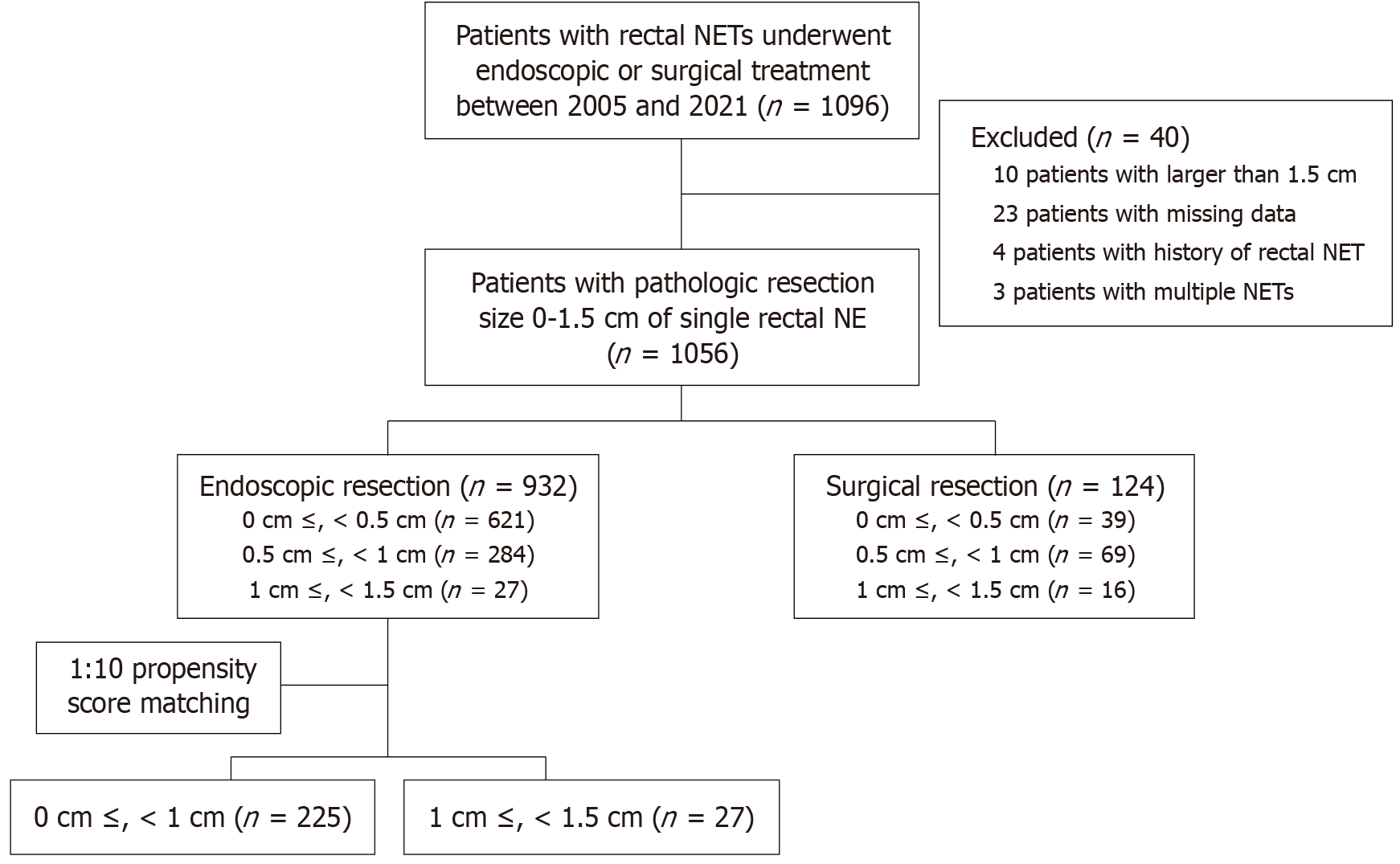
This retrospective study was conducted from January 2005 to June 2021 at the Samsung Medical Center, a tertiary institution in Seoul, South Korea. We reviewed the electronic medical records to obtain the variables for each patient. We enrolled 1096 patients with rectal NETs who underwent endoscopic or surgical treatment between 2005 and 2021 (Figure 1). After excluding patients with a pathological size > 1.5 cm of NET, missing data, or a history of rectal NET or multiple NETs (defined as synchronous or multifocal lesions identified at diagnosis), 1056 patients were included in the study. A total of 932 patients underwent endoscopic resection, and 124 patients underwent surgical resection. In the endoscopic resection group, there were 621 patients with NETs having the pathologic size 0 cm ≤ size < 0.5 cm, 284 patients with 0.5 cm ≤ size < 1 cm, and 27 patients with 1 cm ≤ size < 1.5 cm. In this study, propensity score matching was conducted at 1:10, with 27 patients in 1 cm ≤ size < 1.5 cm group, and 225 patients in the 0 cm ≤ size < 1 cm group.
Clinicopathological factors including age, sex, date of cancer diagnosis, date of surgical or endoscopic resection, tumor location, pathological data of the specimen [longest size, depth, World Health Organization (WHO) grade, resection margin (RM), mitosis count, lymphovascular invasion (LVI), and perineural invasion], death, latest outpatient clinic date, and computed tomography (CT) findings after resection were assessed. The variables were based on the pathology of surgical resection in the primary surgery, post-endoscopic, and surgery combination groups, and the pathology of endoscopic resection in the post-endoscopic specimens. Information, including surgery date, endoscopic resection date, recurrence date, recurrence type, death date, survival status, and cause of death, was obtained from hospital records and test results. The follow-up duration was defined as the duration between the dates of the first and last outpatient visits. These data were extracted from the clinical data warehouse Darwin-C of Samsung Medical Center. The study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center (Approval No. SMC 2024-10-073-001). The study was conducted in accordance with the principles of the Declaration of Helsinki. Information was used only if the patient consented to the electronic medical record access. The board waived the requirement for informed consent because all data were analyzed anonymously.
All patients underwent an endoscopic evaluation, including colonoscopy, before the intervention. Additionally, endoscopic ultrasound was performed in patients if the tumor was quite large endoscopically or if it was not proven in the biopsy to assess the depth of the tumor before resection. The CT scan was performed to identify distant organ or lymph node metastases. Removal methods for rectal NETs are determined by the tumor size, depth, and patient factors. Endoscopic excision was performed for NETs < 10 mm without muscularis propria involvement. With NETs ranging from 10 mm to 20 mm, endoscopic resection is preferred when the tumor is considered localized and the patient is reluctant to undergo surgery. The endoscopic procedure was performed by a skilled endoscopist with extensive experience in colonoscopy. Before excision, a mixture of saline, indigo carmine, and diluted epinephrine (1:10000) was injected to elevate the tumor from the rectal muscle layer. The endoscopist selected the most suitable excision technique based on variations in standard EMR, such as EMR with a precut, EMR with a ligation, or ESD. This strategy aimed to provide a tailored, minimally invasive treatment for rectal NETs, considering the tumor characteristics and patient preferences. Transanal endoscopic microsurgery (TEM) transanal rectal resection, and lower anterior resection were performed. The RM was classified as follows: Negative, defined as a margin that clearly contained no tumor cells; positive, defined as a margin with definite tumor cell involvement; and indeterminate, defined as a margin that could not be assessed because of fragmented specimens or electrocautery artifacts. The invasion depths were classified as the muscularis mucosa, submucosa, and propria muscularis.
Upon initial diagnosis, a CT scan was performed to assess lymph node and distant metastases. Following endoscopic resection, patients underwent follow-up examinations, including abdominopelvic and chest CT, along with sigmoidoscopy or colonoscopy. These were initially performed at 6-12 months intervals and then annually according to the investigators’ protocol. In cases where pathological examination revealed positive RMs, salvage treatments such as additional endoscopic resection or surgery were recommended and performed for some patients. Patients who declined salvage treatment underwent an additional sigmoidoscopic examination one-month post-initial endoscopic resection. If no recurrence or residual disease was detected during the follow-up endoscopy, subsequent endoscopy and CT were scheduled six months later. The term synchronous rectal NETs denote rectal NETs identified in a single patient within six months. Metachronous tumors were defined as NETs diagnosed at different sites at least six months after the initial resection.
Baseline characteristics were analyzed using descriptive and frequency analyses. Categorical variables were analyzed using the χ2 test or Fisher’s exact test. Continuous variables were compared using the Kruskal-Wallis rank-sum test or Wilcoxon signed-rank test. Statistical significance was set at P < 0.05. All statistical analyses were performed using R version 4.4.0. A 1:10 propensity score matching was performed using the nearest neighbor method without replacement, with a caliper width of 0.2 times the standard deviation of the logit-transformed propensity score. Kaplan-Meier curves were used to compare the recurrence curves between the two size groups.
The baseline characteristics, including the clinical and pathological characteristics of the patients, are shown in Table 1. A total of 1056 patients were included in the study, categorized into three groups based on tumor size: 0 cm ≤ size < 0.5 cm (n = 659, 62.4%), 0.5 cm ≤ size < 1 cm (n = 354, 33.5%), and 1 cm ≤ size < 1.5 cm (n = 43, 4.1%). The median age of the total population was 46 years [interquartile range (IQR): 38-54 years], with no significant difference between the groups (P = 0.44). The proportion of male patients was 60.1% (n = 635). The median tumor size was 0.4 cm (IQR: 0.3-0.6 cm), with a median distance from the anal verge of 7.0 cm (IQR: 5-10 cm). Tumors in the 1 cm ≤ size < 1.5 cm group were located slightly closer to the anal verge compared to smaller tumors (P = 0.13). Among all patients, 88.3% (n = 932) underwent endoscopic resection and 11.7% (n = 124) underwent surgical resection. The proportion of patients who underwent surgical resection was significantly higher in the 1-1.5 cm group (37.2%) than in the 0-1 cm group (10.7%) (P < 0.01). ESD was performed in 180 (19.3%) and 752 (80.7%) patients, respectively. The proportion of ESD was significantly higher in the 1 cm ≤ size < 1.5 cm group (48.1%) compared to the 0 cm ≤ size < 1 cm group (18.5%) (P < 0.01). Among the surgical procedures, TEM was the most frequently performed technique (79.2%), followed by transanal rectal resection (14.4%), and lower anterior resection (6.4%), with no significant differences between the groups (P = 0.69).
| Study population (n = 1056) | ≥ 0 cm and < 0.5 cm (n = 659) | ≥ 0.5 cm and < 1 cm (n = 354) | ≥ 1 cm and < 1.5 cm (n = 43) | P value | |
| Age (years) | 46 (38-54) | 46 (38-54) | 45 (37-55) | 48 (38-61.5) | 0.44 |
| Sex | 0.87 | ||||
| Male | 636 (60.2) | 401 (71) | 209 (59) | 26 (60.5) | |
| Female | 421 (39.8) | 259 (29) | 145 (41) | 17 (39.5) | |
| Rectal NET size (cm) | 0.4 (0.3-0.6) | 0.3 (0.2-0.4) | 0.6 (0.5-0.7) | 1.0 (1.0-1.1) | < 0.01 |
| Distance from anal verge (cm) | 7 (5-10) | 7 (5-10) | 8 (5-10) | 6.5 (5-8) | 0.13 |
| Therapy methods | < 0.01 | ||||
| Endoscopic resection | 932 (88.2) | 621 (94.1) | 284 (80.2) | 27 (62.8) | |
| Surgical resection | 125 (11.8) | 39 (5.9) | 70 (19.8) | 16 (37.2) | |
| Endoscopic methods | < 0.01 | ||||
| ESD | 180 (19.3) | 67 (10.8) | 100 (35.2) | 13 (48.1) | |
| EMR | 752 (80.7) | 554 (89.2) | 184 (64.8) | 14 (51.9) | |
| Surgical methods | 0.69 | ||||
| TEM | 99 (79.2) | 32 (82.1) | 54 (77.1) | 13 (81.3) | |
| Transanalrectal resection | 18 (14.4) | 5 (12.8) | 12 (17.1) | 1 (6.3) | |
| Lower anterior resection | 8 (6.4) | 2 (5.1) | 4 (5.7) | 2 (12.4) | |
| WHO grade | 0.02 | ||||
| Grade 1 | 627 (59.3) | 401 (60.8) | 194 (54.8) | 32 (74.4) | |
| Grade 2 | 19 (1.8) | 7 (1.1) | 12 (3.4) | 0 | |
| Grade 3 | 0 | 0 | 0 | 0 | |
| Missing | 411 (38.9) | 252 (38.2) | 148 (41.8) | 11 (25.6) | |
| Resection margin | 0.57 | ||||
| Negative | 1025 (97.0) | 641 (97.1) | 343 (96.9) | 41 (95.4) | |
| Positive | 26 (2.5) | 14 (2.1) | 10 (2.8) | 2 (4.6) | |
| Uncheckable | 6 (0.5) | 5 (0.8) | 1 (0.3) | 0 (0) | |
| Depth | < 0.01 | ||||
| 1a | 1014 (95.9) | 660 (100.0) | 353 (99.7) | 1 (2.3) | |
| 1b | 41 (3.9) | 0 (0) | 0 (0) | 41 (95.4) | |
| 2 | 2 (0.2%) | 0 (0) | 1 (0.3) | 1 (2.3) | |
| Lymphovascular invasion | 0.47 | ||||
| Absent/not identified | 1040 (98.4) | 648 (98.2) | 350 (98.9) | 42 (97.7) | |
| Present | 5 (0.5) | 2 (0.3) | 3 (0.9) | 0 (0.0) | |
| Missing | 12 (1.1) | 10 (1.5) | 1 (0.2) | 1 (2.3) |
Histopathological examination revealed that WHO grade 1 was the predominant classification (59.3% of cases), with grade 2 tumors identified in 1.8% of cases. Notably, all tumors in the 1 cm ≤ size < 1.5 cm group were WHO grade 1, with no cases of grade 2 or 3. The proportion of negative RMs was 97.0% (n = 1025) across all patients, with 95.4% (n = 41) in the 1 cm ≤ size < 1.5 cm group (P = 0.45). Regarding depth of invasion, 95.9% of tumors were confined to the muscularis mucosa, whereas 95.4% of tumors in the 1 cm ≤ size < 1.5 cm group invaded the submucosa (P < 0.01). The presence of LVI was low across all groups (0.4% overall, none in the 1 cm ≤ size < 1.5 cm group) (P = 0.47).
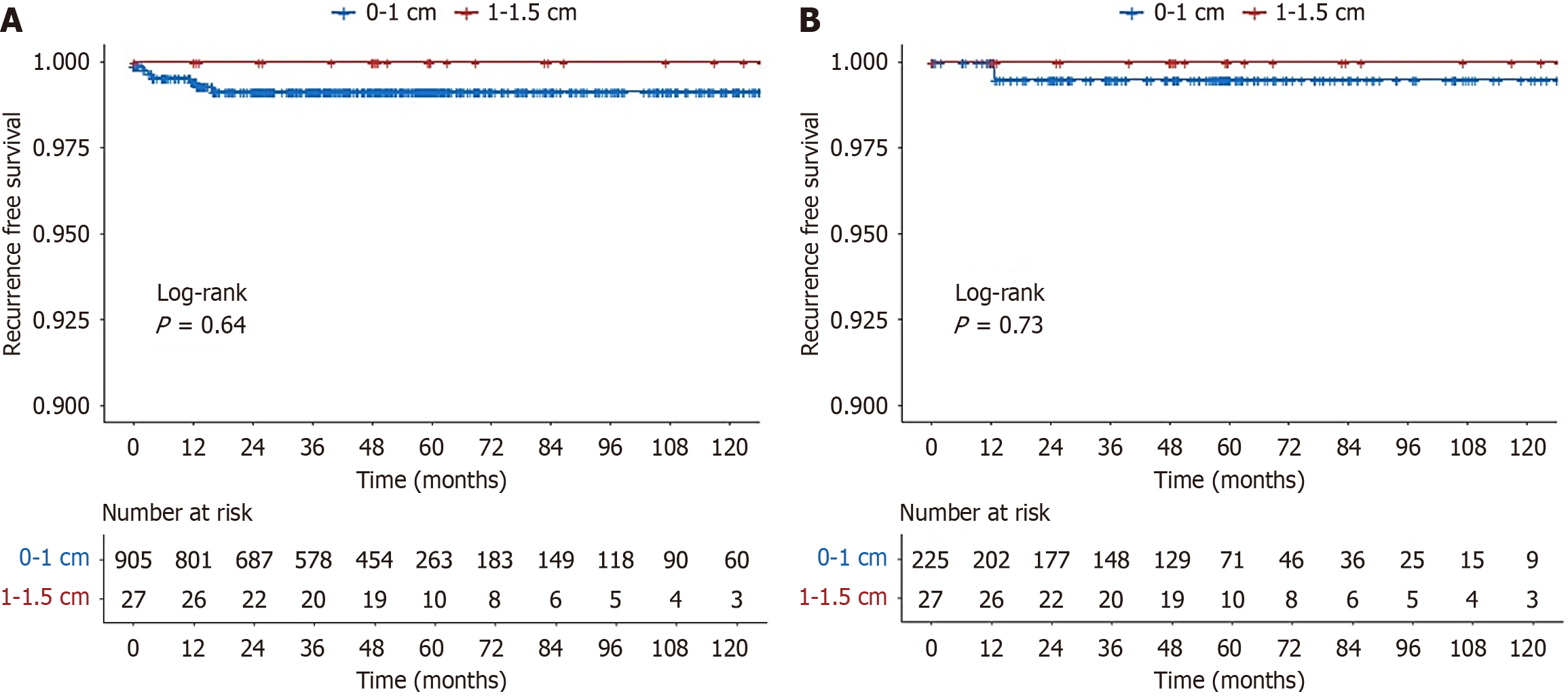
To adjust for potential confounders such as age, sex, and endoscopic resection method, propensity score matching was performed, yielding 225 patients in the < 1 cm group and 27 patients in the 1 cm ≤ size < 1.5 cm group (Table 2). After matching, treatment outcomes were compared between the two groups (Table 3). There were no significant differences between the two groups in terms of the negative RM (R0) rate, LVI, or recurrence rate. The follow-up period for recurrence in the 0 cm ≤ size < 1 cm, and 1 cm ≤ size < 1.5 cm groups was 54.0 months, 51.2 months, and 65.9 months, respectively. During a median follow-up period of 54 months, no recurrence was observed in the 1 cm ≤ size < 1.5 cm group, whereas one case of metachronous recurrence was noted in the < 1 cm group (P = 1.00). Kaplan-Meier analysis showed no statistically significant difference in recurrence-free survival between the two groups before (P = 0.55) or after propensity matching (P = 0.48) (Figure 2). Kaplan-Meier analysis also showed no statistically significant difference in recurrence-free survival between the ESD and EMR groups, both before and after propensity score matching (Supplementary Figures 1 and 2).
| Group | Before PS matching | After PS matching | ||||||
| Total (n = 932) | ≥ 0 cm and < 1 cm (n = 905) | ≥ 1 cm and < 1.5 cm (n = 27) | P value | Total (n = 252) | ≥ 0 cm and < 1 cm (n = 225) | ≥ 1 cm and < 1.5 cm (n = 27) | SMD | |
| Age, median (IQR) | 46.0 (38.0, 54.0) | 46.0 (38.0, 54.0) | 49.0 (38.0, 61.5) | 0.07 | 46.0 (38.0, 58.0) | 46.0 (38.0, 58.0) | 49.0 (38.0, 61.5) | 0.17 |
| Sex | 0.27 | 0.02 | ||||||
| Male | 560 (60.0) | 541 (59.7) | 19 (70.4) | 174 (69.1) | 155 (68.9) | 19 (70.4) | ||
| Female | 373 (40.0) | 365 (40.3) | 8 (29.6) | 78 (31.0) | 70 (31.1) | 8 (29.6) | ||
| Endoscopic resection | < 0.01 | 0.12 | ||||||
| ESD | 180 (19.3) | 167 (18.43) | 13 (48.15) | 114 (45.2) | 101 (44.9) | 13 (48.2) | ||
| EMR | 753 (80.7) | 739 (81.57) | 14 (51.85) | 138 (54.8) | 124 (55.1) | 14 (51.8) | ||
| Total (n = 252) | 0-1 cm (n = 225) | 1-1.5 cm (n = 27) | P value | |
| Resection margin (R0) | 0.2210 | |||
| Negative RM | 245 (97.2) | 220 (97.8) | 25 (92.6) | |
| Positive RM | 6 (2.4) | 4 (1.8) | 2 (7.4) | |
| Uncheckable | 1 (0.4) | 1 (0.4) | 0 (0) | |
| Recurrence | 1.0000 | |||
| No recur | 251 (99.6) | 224 (99.6) | 27 (100) | |
| Intramural recurrence | 0 (0) | 0 (0) | 0 (0) | |
| Extracolonic recurrence | 1 (0.4) | 1 (0.4) | 0 (0) | |
| Synchronous recurrence | 0 (0) | 0 (0) | 0 (0) | |
| Lymphovascular invasion | ||||
| No | 248 (48.4) | 221 (98.2) | 27 (100) | |
| Missing | 4 (1.6) | 4 (1.8) | 0 (0) |
There has been controversy over the treatment method for 1 cm ≤ size < 1.5 cm rectal NETs. To the best of our knowledge, this is the first study to compare the long-term clinical outcomes of endoscopic resection of rectal NETs measuring 1, < 1.5, 0, and < 1 cm using a matched study. A propensity score analysis was used to generate precisely matched patient. No statistically significant intergroup differences were observed in recurrence-free survival. Increasing size, the presence of LVI, WHO grade, depth, and Ki-67 expression are known risk factors for lymph node metastasis in NET[11-14]. Conventionally, LVI is thought to increase the risk of micrometastasis in locoregional cancers[15]. Moreover, because rectal NETs are highly vascular, LVI is a potential high-risk factor for metastasis[16]. There was no patient with LVI among patients with size 1 cm ≤ size < 1.5 cm. In our data, there was no recurrence in the 1 cm ≤ size < 1.5 cm group after propensity score matching. Considering the results, it may be worthwhile to try endoscopic resection in the 1 cm ≤ size < 1.5 cm group of rectal NET.
In this study, positive RMs after endoscopic resection was observed in 2.4% of cases. Among them, there were only two positive RM patients in the 1 cm ≤ size < 1.5 cm group. The likelihood of a positive RM was also not significantly different between 0 cm (size < 1 cm) and 1 cm (size < 1.5 cm). These patients did not undergo salvage treatments. Patients with positive RM of 1 cm ≤ size < 1.5 cm were also followed up at 5.3 years and 4.9 years, respectively, with no recurrence. In patients with 1 cm or less, one patient with positive RM was treated with EMR with a precut, one with cold polypectomy with a snare, and the other two patients with no further management. All patients with 1 cm or less with positive RM also had no recurrence. Patients with positive RMs on actual histology can be divided into two groups: Those with truly partially resected and those with endoscopically complete resection but positive RMs with cautery artifacts[17-19]. Therefore, if endoscopic RM is clear, physicians should consider careful and regular colonoscopy surveillance without additional intervention. However, if the submucosal layer is not fully resected or if deep RM is positive, physicians may consider additional EMR. Our study showed no local recurrence in patients with positive endoscopic RMs or residual lesions on endoscopy. The low rate of positive RM in the 1 cm ≤ size < 1.5 cm group and the lack of recurrence in this group suggest that endoscopic resection may be considered as the primary treatment option.
Three patients experienced recurrence in the entire group before propensity score matching, and all three patients had a median follow-up of 4.5 months for metachronous recurrence. All the patients with metachronous recurrence underwent endoscopic resection. After propensity score matching, only one patient experienced recurrence. This patient experienced metachronous recurrence that occurred one year after ESD. The patient had no recurrence even though five years had passed since the second treatment. Chung et al[20] metachronous rectal NETs were diagnosed in 2.7% (n = 9) of the 333 patients, and metachronous lesions were associated with the number of synchronous lesions at initial diagnosis. In our study, the overall rate of metachronous rectal NETs was 0.28% (n = 3) in 1057 patients, which may be due to the exclusion of patients with pre-existing synchronous lesions from the analysis. Previous studies have reported that synchronous adenomas may contribute to metachronous neoplasia[21,22]. Therefore, the surrounding rectal mucosa must be carefully examined in patients who have undergone endoscopic resection of rectal NETs. The National Comprehensive Cancer Network guidelines for rectal NETs do not recommend routine follow-up for small rectal NETs measuring less than 10 mm. Similarly, the European Neuroendocrine Tumor Society guidelines do not recommend regular follow-up for completely treated tumors. However, the current study showed different results, which questions whether there is a need for a follow-up. At the study institution, patients with rectal NETs routinely undergo annual follow-ups with either sigmoidoscopy or colonoscopy, along with abdominal CT imaging. Although the current data are insufficient to establish formal guidelines, continued surveillance and future data collection will hopefully contribute to the development of evidence-based surveillance protocols. We plan to report outcomes once we accumulate more patients and longer follow-up durations, which we believe will provide valuable insights into long-term prognosis and guide future surveillance strategies.
Surgical resection (e.g., transanal excision, TEM, or low anterior resection) provides the advantage of complete local excision with regional lymph node dissection, which may be appropriate for cases with high-risk features, such as LVI, poor differentiation, or deep submucosal invasion. In our study, patients who underwent surgery did not experience any recurrence during the follow-up period. However, surgical resection is associated with higher morbidity, longer hospitalization, and a higher procedural burden, which may not be justified in cases with low metastatic risk. In a large French study, the overall in-hospital mortality rate of colorectal resections, including rectal surgeries, was 3.4%, with a morbidity rate of 35%[23]. Although the proportion of complications in rectal surgery may be lower than that in general colorectal resection, wound complications, clinical anastomotic dehiscence, postoperative hemorrhage, and urinary tract complications can occur. For endoscopic resection of rectal NETs, complication rates are generally low, with bleeding occurring in 0%-8.8% of cases, and perforation is rare across multiple studies[24,25]. Although these rates can vary based on factors such as tumor size, stage, grade, and specific surgical approach, endoscopic resection is associated with lower complication rates than more extensive surgical procedures. In our study, there were no life-threatening complications such as death, need for intensive care, excessive bleeding, or urgent surgery due to perforation in the endoscopic resection group. In the endoscopic group, the procedure was often outpatient-based, and the procedure time was significantly shorter than that in the surgical group, making endoscopic procedures more cost-efficient and time-efficient. From a cost-effectiveness perspective, endoscopic resection is typically performed in an outpatient setting, involves minimal anesthesia, and allows faster return to daily activities. These factors translate into lower direct medical costs (procedural fees and hospital stay) and indirect costs (loss of productivity) compared to surgery. Although endoscopic resection may require close surveillance and occasional retreatment in cases with positive margins, it remains a more cost-efficient option for carefully selected patients without high-risk features. Treatment decisions should be based on a comprehensive evaluation of tumor size, histologic features, patient comorbidities, and patient preferences. As the prognosis in our study shows that endoscopic resection of 1 cm ≤ size < 1.5 cm is favorable, patients would likely prefer to undergo endoscopic treatment rather than surgery.
This study has some limitations. First, being conducted at a single center may limit the generalizability of the findings to all rectal NET patient populations. Our institution is one of the largest referral centers for rectal NETs in Korea, receiving patients from a wide range of geographic regions across the country. Due to Korea’s well-developed national transportation infrastructure and centralized healthcare system, our patient population is not limited to a specific region but represents a diverse and heterogeneous group. Moreover, clinical practices and management protocols for rectal NETs at our institution closely follow national guidelines, which are consistently implemented throughout Korea. Demographic characteristics, including age, sex, and the proportion of patients undergoing ESD or EMR, were comparable to those reported in previous single-center and multicenter studies conducted in Korea. Although outcomes at high-volume institutions may be somewhat more favorable than those at smaller centers, this is likely attributable to the greater procedural experience of endoscopists at larger hospitals. Therefore, while this is a single-center study, we consider our findings to be broadly representative of the overall Korean rectal NET patient population. Nonetheless, we acknowledge that further validation through multicenter studies would enhance the generalizability of our results. Second, the sample size of the 1-1.5 cm group was small, which may not have provided sufficient statistical power to detect small but clinically meaningful differences. NETs have an incidence of approximately 0.86 per 100000 individuals, with a higher prevalence among Black males and Asian populations[26,27]. Rectal and gastrointestinal NETs account for 18% of total NETs and 27% of gastrointestinal NETs[27]. Given the notable prevalence of rectal NETs, this study had the largest sample size among single study groups. Even in previous studies and those cited by the European Neuroendocrine Tumor Society 2023 guidelines, the prevalence of rectal NETs measuring 1-1.9 cm remains low[27,28]. In most studies, this size category accounts for approximately 8% of all rectal NET cases[29]. Naturally, the number of tumors measuring 1-1.5 cm was even smaller. Therefore, although the number of 1-1.5 cm NETs in our study may be limited, this reflects the inherently low frequency of such tumors in clinical practice. It would be appropriate to mention this context to clarify that the small sample size was not a result of inadequate recruitment but rather the rarity of this tumor subset. Recent multicenter studies have reported similarly low frequencies of intermediate-sized tumors[27]. Owing to the rarity of rectal NETs, this study is considered as a preliminary investigation that may serve as a valuable foundation for future large-scale prospective research. Third, there were missing or incomplete data in pathological reports. The WHO grading system for NETs was first established in 2010 and subsequently revised in 2017 using more detailed criteria. Since our study enrolled patients beginning in 2005, some pathological data were inevitably missing or inconsistently reported owing to the absence of standardized grading criteria during earlier years. Because this was a retrospective study conducted over a median period of 54 months, variability in the completeness and details of pathological reporting was inevitable. This exclusion may have introduced a selection bias because the excluded patients could have differed systematically from those with complete data. To maintain analytical rigor, we established predefined criteria requiring the presence of essential pathological data for inclusion in the final analysis. Patients with missing information for any of the key prognostic indicators were excluded from outcome-related analyses. This exclusion is explicitly stated in the methods section and allowed us to ensure that comparisons between the groups were based on consistent and reliable pathological profiles. In addition, endoscopically resected specimens were fixed in 10% formalin and cut into 2-3 mm thick sections, whereas surgically resected specimens were cut into 5-6 mm thick sections. Therefore, surgically resected specimens may undergo different pathological evaluations than endoscopically resected specimens. Fourth, the retrospective nature of the study introduced a potential selection bias and limited the ability to control for confounding factors that could influence the outcomes. Fifth, although the current study demonstrated no recurrence in the 1-1.5 cm group during a median follow-up period of 54 months, the risk of late recurrence, although low, cannot be completely excluded. Considering the slow-growing nature of NETs, long-term follow-up is essential to evaluate the oncological safety of endoscopic resection, especially for intermediate-sized rectal NETs. Continued surveillance and long-term observations are warranted to better evaluate the long-term prognosis of patients with intermediate-sized rectal NETs. Sixth, although propensity score matching is a robust method for minimizing confounding factors in observational studies, it has inherent limitations. Specifically, propensity score matching only accounts for the measured confounders included in the matching algorithm. In the study, we matched for age, sex, and endoscopic resection method; however, unmeasured or unavailable variables, such as tumor histological features, operator experience, and patient comorbidities, may still influence treatment outcomes and introduce residual bias. The study used multivariate regression analysis to reduce the confounding factors. However, given the small sample size of the 1-1.5 cm group and the absence of recurrence events in this subgroup, it was not feasible to adjust for a larger number of covariates or perform multivariable analyses. It is also noted that the 1-1.5 cm group had a relatively small sample size owing to the inherent rarity of intermediate-sized tumors. Therefore, a 1:10 matching ratio was employed to maximize statistical power while retaining all eligible cases in this group. Even after applying 1:10 propensity score matching, the covariate balance remained acceptable, with absolute standardized mean differences of less than 0.2 for all variables. This indicated that the groups were well-matched and comparable after adjustment. Future studies should employ complementary statistical techniques to strengthen causal inferences.
Our findings suggest that endoscopic resection for 1-1.5 cm grade 1 rectal NETs results in comparable recurrence-free survival to < 1 cm tumors, with no observed recurrence or LVI in the matched study. Given the low morbidity, favorable oncological outcomes, and minimally invasive nature of endoscopic treatment, endoscopic resection should be considered a viable treatment option for selected patients with 1 cm to 1.5 cm rectal NETs. However, there are limitations, such as the relatively small sample size and short median follow-up period of approximately 54 months, which may still be insufficient to detect late recurrences, particularly given the slow-growing nature of rectal NETs. Future prospective multicenter studies are warranted to confirm these findings and refine treatment guidelines.
Endoscopic resection of 1-1.5 cm grade 1 rectal NETs yielded comparable outcomes to those < 1 cm in size, suggesting its feasibility as a treatment.
| 1. | Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, Cassese G, Pagano G, Tropeano FP, Luglio G, De Palma GD. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel). 2021;11:771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Ploeckinger U, Kloeppel G, Wiedenmann B, Lohmann R; representatives of 21 German NET Centers. The German NET-registry: an audit on the diagnosis and therapy of neuroendocrine tumors. Neuroendocrinology. 2009;90:349-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2663] [Article Influence: 295.9] [Reference Citation Analysis (5)] |
| 4. | Chang JS, Chen LT, Shan YS, Chu PY, Tsai CR, Tsai HJ. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci Rep. 2021;11:7881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Matsuhashi N, Takahashi T, Tomita H, Araki H, Ibuka T, Tanaka K, Tanahashi T, Matsui S, Sasaki Y, Tanaka Y, Okumura N, Yamaguchi K, Osada S, Yoshida K. Evaluation of treatment for rectal neuroendocrine tumors sized under 20 mm in comparison with the WHO 2010 guidelines. Mol Clin Oncol. 2017;7:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Nagai T, Torishima R, Nakashima H, Ookawara H, Uchida A, Kai S, Sato R, Murakami K, Fujioka T. Saline-assisted endoscopic resection of rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy. 2004;36:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 9. | Sakata H, Iwakiri R, Ootani A, Tsunada S, Ogata S, Ootani H, Shimoda R, Yamaguchi K, Sakata Y, Amemori S, Mannen K, Mizuguchi M, Fujimoto K. A pilot randomized control study to evaluate endoscopic resection using a ligation device for rectal carcinoid tumors. World J Gastroenterol. 2006;12:4026-4028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 11. | Choi JS, Kim MJ, Shin R, Park JW, Heo SC, Jeong SY, Park KJ, Ryoo SB. Risk Factor Analysis of Lymph Node Metastasis for Rectal Neuroendocrine Tumors: Who Needs a Radical Resection in Rectal Neuroendocrine Tumors Sized 1-2 cm? Ann Surg Oncol. 2024;31:2414-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Li R, Li X, Wang Y, Chang C, Lv W, Li X, Cao D. Risk factors for regional lymph node metastasis in rectal neuroendocrine tumors: a population-based study. Front Med (Lausanne). 2024;11:1383047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 13. | Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994;179:231-248. [PubMed] |
| 14. | Pang S, Zong Y, Zhang K, Zhao H, Wang Y, Wang J, Liu C, Wu Y, Li P. Multiple rectal neuroendocrine tumors: An analysis of 15 cases and literature review. Front Oncol. 2022;12:996306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Ishii M, Ota M, Saito S, Kinugasa Y, Akamoto S, Ito I. Lymphatic vessel invasion detected by monoclonal antibody D2-40 as a predictor of lymph node metastasis in T1 colorectal cancer. Int J Colorectal Dis. 2009;24:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yazdani S, Kasajima A, Tamaki K, Nakamura Y, Fujishima F, Ohtsuka H, Motoi F, Unno M, Watanabe M, Sato Y, Sasano H. Angiogenesis and vascular maturation in neuroendocrine tumors. Hum Pathol. 2014;45:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008;143:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Lee SH, Park SJ, Kim HH, Ok KS, Kim JH, Jee SR, Seol SY, Kim BM. Retraction notice to "endoscopic resection for rectal carcinoid tumors: comparision of polypectomy and endoscopic submucosal resection with band ligation". Clin Endosc. 2015;48:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS, Jung SA, Park DI, Baek IH, Kim SH, Chang DK. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Chung HG, Goh MJ, Kim ER, Hong SN, Kim TJ, Chang DK, Kim YH. Recurrence pattern and surveillance strategy for rectal neuroendocrine tumors after endoscopic resection. J Gastroenterol Hepatol. 2021;36:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ballesté B, Bessa X, Piñol V, Castellví-Bel S, Castells A, Alenda C, Paya A, Jover R, Xicola RM, Pons E, Llor X, Cordero C, Fernandez-Bañares F, de Castro L, Reñé JM, Andreu M; Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Detection of metachronous neoplasms in colorectal cancer patients: identification of risk factors. Dis Colon Rectum. 2007;50:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Moon CM, Cheon JH, Choi EH, Kim ES, Park JJ, Han SY, Kim DH, Kim TI, Kim WH. Advanced synchronous adenoma but not simple adenoma predicts the future development of metachronous neoplasia in patients with resected colorectal cancer. J Clin Gastroenterol. 2010;44:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K; Association Française de Chirurgie. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278-283, discussion 284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 384] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 24. | Hong SM, Baek DH. Endoscopic treatment for rectal neuroendocrine tumor: which method is better? Clin Endosc. 2022;55:496-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 25. | Zheng Y, Guo K, Zeng R, Chen Z, Liu W, Zhang X, Liang W, Liu J, Chen H, Sha W. Prognosis of rectal neuroendocrine tumors after endoscopic resection: a single-center retrospective study. J Gastrointest Oncol. 2021;12:2763-2774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Park SJ. Proper Treatment Option for Small Rectal Neuroendocrine Tumors Using Precut Endoscopic Mucosal Resection. Clin Endosc. 2017;50:516-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Moon CM, Huh KC, Jung SA, Park DI, Kim WH, Jung HM, Koh SJ, Kim JO, Jung Y, Kim KO, Kim JW, Yang DH, Shin JE, Shin SJ, Kim ES, Joo YE. Long-Term Clinical Outcomes of Rectal Neuroendocrine Tumors According to the Pathologic Status After Initial Endoscopic Resection: A KASID Multicenter Study. Am J Gastroenterol. 2016;111:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Kim J, Kim JH, Lee JY, Chun J, Im JP, Kim JS. Clinical outcomes of endoscopic mucosal resection for rectal neuroendocrine tumor. BMC Gastroenterol. 2018;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Shin JW, Lee EJ, Park SS, Han KS, Kim CG, Chang HC, Kim WY, Jeong EC, Choi DH. Endoscopic treatment of rectal neuroendocrine tumors: a consecutive analysis of multi-institutional data. Ann Coloproctol. 2025;41:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/