Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.109825
Revised: June 29, 2025
Accepted: August 5, 2025
Published online: September 14, 2025
Processing time: 105 Days and 16.7 Hours
Colorectal cancer (CRC) is a major global health burden. B cell CLL/lymphoma 10 (BCL10), a key component of the caspase recruitment domain protein-BCL10-mucosa-associated lymphoid tissue lymphoma paracaspase complexes, is upre
To explore the role of BCL10 in regulating the sensitivity of CRC cells to cup
A series of in vitro and in vivo experiments were conducted using CRC cell lines and CRC mouse models to evaluate the effects of BCL10 on CRC cell proliferation, migration, invasion, and sensitivity to copper-induced cell death. Mechanistic studies were performed to elucidate the underlying molecular pathways.
BCL10 promoted CRC cell proliferation, migration, and invasion, while its knockdown had the opposite effects. BCL10 also influenced the sensitivity of CRC cells to cuproptosis, with BCL10 overexpression enhancing resistance and its knockdown increasing sensitivity. The mechanism involved BCL10 modulating the expression of DLAT, a key protein in the copper-induced cell death pathway, through activation of the nuclear factor kappa-B (NF-κB) signaling pathway.
BCL10 promotes CRC growth and regulates the sensitivity of CRC cells to cuproptosis by activating the NF-κB signaling pathway and modulating DLAT expression. These findings provide a molecular basis for developing BCL10-targeted therapies for CRC.
Core Tip: B cell CLL/lymphoma 10 (BCL10) drives colorectal cancer (CRC) progression by promoting tumor cell proliferation, migration, and invasion, correlating with poor prognosis. It modulates CRC sensitivity to copper-induced cell death (cuproptosis), with overexpression increasing resistance and knockdown enhancing susceptibility. BCL10 activates nuclear factor kappa-B (NF-κB), suppressing DLAT-a key cuproptosis mediator. BCL10 knockdown increases DLAT oligomerization, boosting cuproptosis, while overexpression protects cells. Targeting BCL10 or the NF-κB-DLAT axis may improve CRC treatment by enhancing cuproptosis sensitivity. In vivo studies show BCL10 knockdown improves tumor response to copper therapy, supporting its therapeutic potential. These findings reveal BCL10 as a key CRC regulator, offering new treatment strategies.
- Citation: Xiao PT, Li CF, Liu YD, Zhong J, Cui XL, Liu C, Yang W. B cell CLL/lymphoma 10 promotes colorectal cancer cell proliferation and regulates cuproptosis sensitivity through the NF-κB signaling pathway. World J Gastroenterol 2025; 31(34): 109825
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/109825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.109825
Colorectal cancer (CRC) is a serious health burden worldwide, and has become the third most common cancer and the second leading cause of cancer-related death in the world[1]. The disease originates from the abnormal proliferation of epithelial cells in the colon or rectum, leading to the formation of malignant tumors[2]. The occurrence of CRC is a com
B-cell CLL/Lymphoma 10 (BCL10) is a protein-coding gene that plays a critical role in immune response and inflammation[16]. As a key component of the caspase recruitment domain protein-BCL10-mucosa-associated lymphoid tissue lymphoma paracaspase (CBM) complex, BCL10 plays a central role in activation of the NF-κB signaling pathway[17-19]. The NF-κB signaling pathway is a key factor regulating inflammatory response, immune response and cell survival[20-22]. Its abnormal activation is closely related to the occurrence of a variety of tumors, including CRC[21]. A study found that the expression of BCL10 was significantly up-regulated in CRC tissues and cell lines, suggesting that it may play a role in the occurrence and development of CRC. The therapeutic potential of targeting BCL10 Lies in its ability to modulate the NF-κB pathway. Inhibitors that disrupt BCL10 or its downstream signaling could restore the balance of pro-apoptotic and anti-apoptotic signals, thereby inducing apoptosis in CRC cells[22]. Moreover, recent findings indicate that targeting BCL10 may enhance the efficacy of existing chemotherapeutics by overcoming resistance mechanisms mediated by the NF-κB pathway[22]. These insights underscore the importance of further investigating BCL10 as a therapeutic target in CRC.
Cuproptosis, also called copper-induced cell death, is a newly identified form of programmed cell death which is diffe
Recent studies have demonstrated that cuproptosis can be exploited as a novel therapeutic strategy for cancer treatment[30-32]. Copper-based compounds have been shown to induce cuproptosis in various cancer cell lines, including CRC cells, suggesting their potential as anticancer agents[33]. However, the molecular mechanisms underlying cuproptosis and its regulation in cancer cells are not completely clear.
Given the roles of BCL10 in activation of the NF-κB signaling pathway and the potential of cuproptosis as a therapeutic strategy for CRC, it is of great significance to explore the potential connection between these factors. The NF-κB signaling pathway plays a role in regulating various aspects of cell survival and inflammation, and its activation has been shown to modulate the sensitivity of cancer cells to different forms of cell death, including cuproptosis[34]. Our research hypothesizes that BCL10 may affect the sensitivity of CRC cells to cuproptosis through activation of the NF-κB signaling pathway. Specifically, BCL10 has been shown to activate the NF-κB signaling pathway, leading to the upregulation of anti-apoptotic proteins and the suppression of cell death. Furthermore, the NF-κB signaling pathway has been implicated in the regulation of copper homeostasis and the response to oxidative stress, which are the key mechanisms of cuproptosis.
Emerging evidence suggests crosstalk between NF-κB signaling and copper homeostasis. The NF-κB pathway has been shown to regulate metallothioneins (MTs), a family of cysteine-rich metal-binding proteins that modulate copper avai
Therefore, it is hypothesized that BCL10 may promote the growth of CRC by activating the NF-κB signaling pathway and affect the sensitivity of CRC cells to cuproptosis. Elucidating the molecular mechanisms underlying this potential connection could provide valuable insights into the pathogenesis of CRC and identify novel therapeutic targets for the treatment of this disease.
NCM460, HCT116, SW480 and SW620 cell lines were obtained from the American Type Culture Collection. HCT116, SW480 and SW620 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (Gibco) at 37 °C in 5% CO2. SW480 and SW620 were paired CRC cell lines derived from the same patient’s primary tumor and metastatic lymph node, respectively. NCM460 cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% FBS. Elesclomol (HY-12040), BAY 11-7082 (HY-13453), cell counting kit-8 (HY-K0301) and Ammonium Tetrathiomolybdate (TTM, HY-128530) were purchased from MedChemExpress (MCE). CuCl2 was purchased from Aladdin (Shanghai, China).
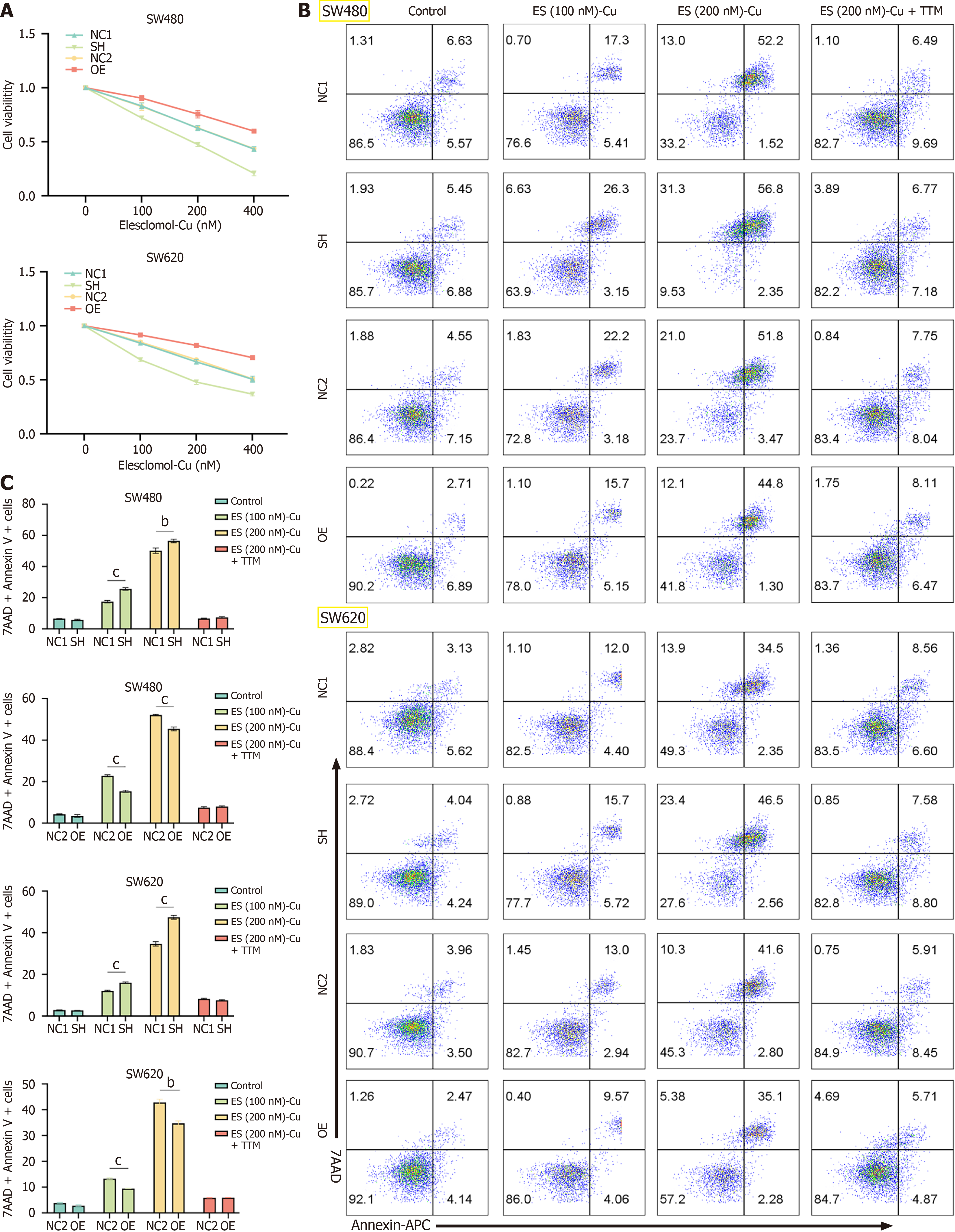
Cells in the logarithmic growth phase were seeded in a 96-well plate at a density of 3000 cells per well and treated with elesclomol-Cu/elesclomol-Cu-TTM/blank for 24 hours, the Cell Counting Kit-8 reagent (10 µL) was added and the plates were incubated at 37 °C for 1 hour. The absorbance was measured at 450 nm using a microplate reader.
Total RNA was extracted using Trizol (Lablead, China) and cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Lablead, China). Quantitative PCR (qPCR) reactions were performed using Power SYBR Green PCR Master Mix (Lablead, China). Relative expression levels of target genes were calculated using the 2-ΔΔCT method. Primer information is provided in Supplementary Table 1.
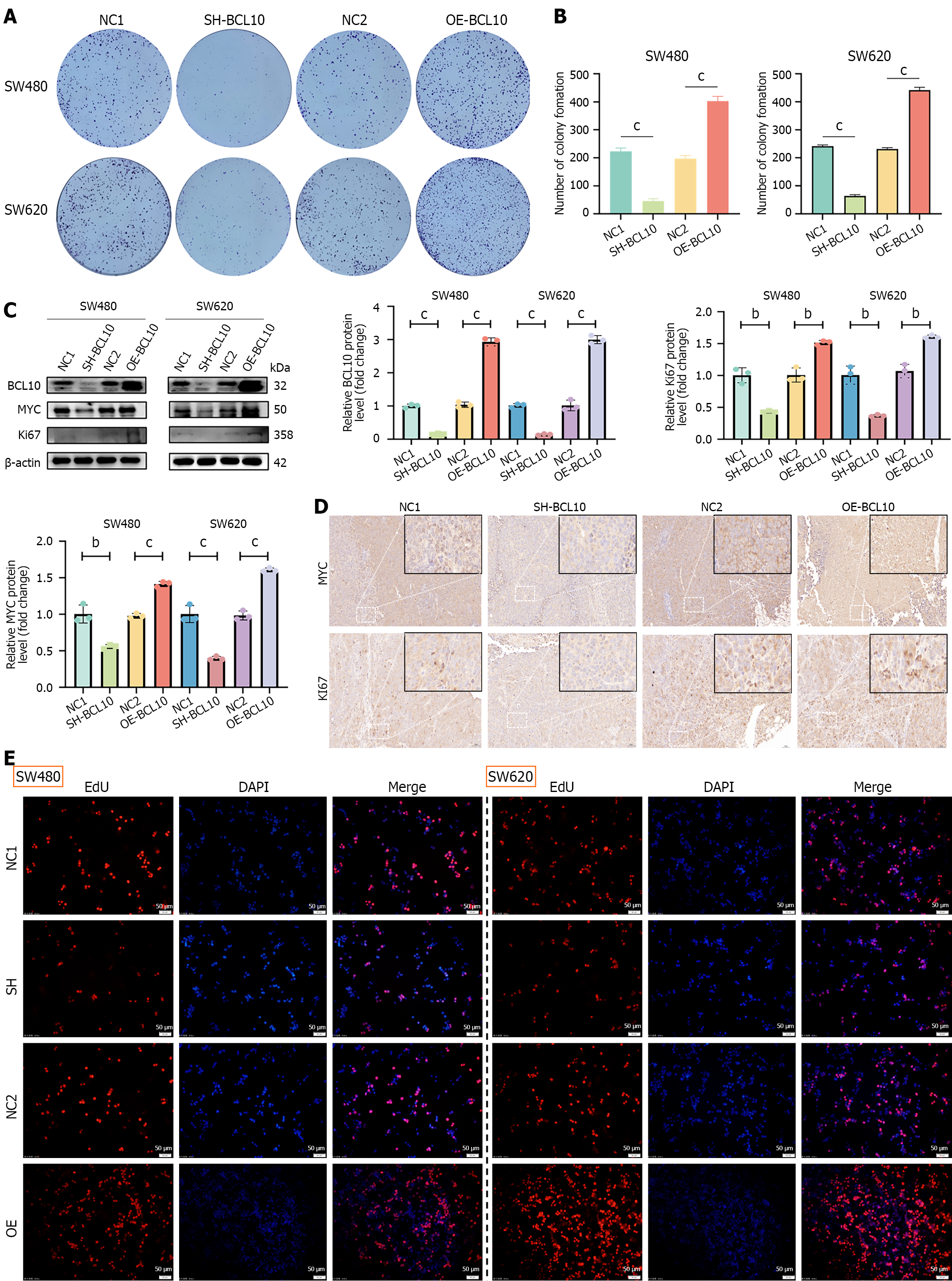
The cells were seeded in 6-well plates and cultured for 14 days. Subsequently, the cells were fixed with 4% paraformaldehyde for 20 minutes. Finally, the cells were stained using 0.5% crystal violet for 10 minutes. The colonies were photographed. Image J software was then used to count the number of colonies. The images were imported into the software, and the color and parameters were set. Finally, the software calculated the specific number of colonies.
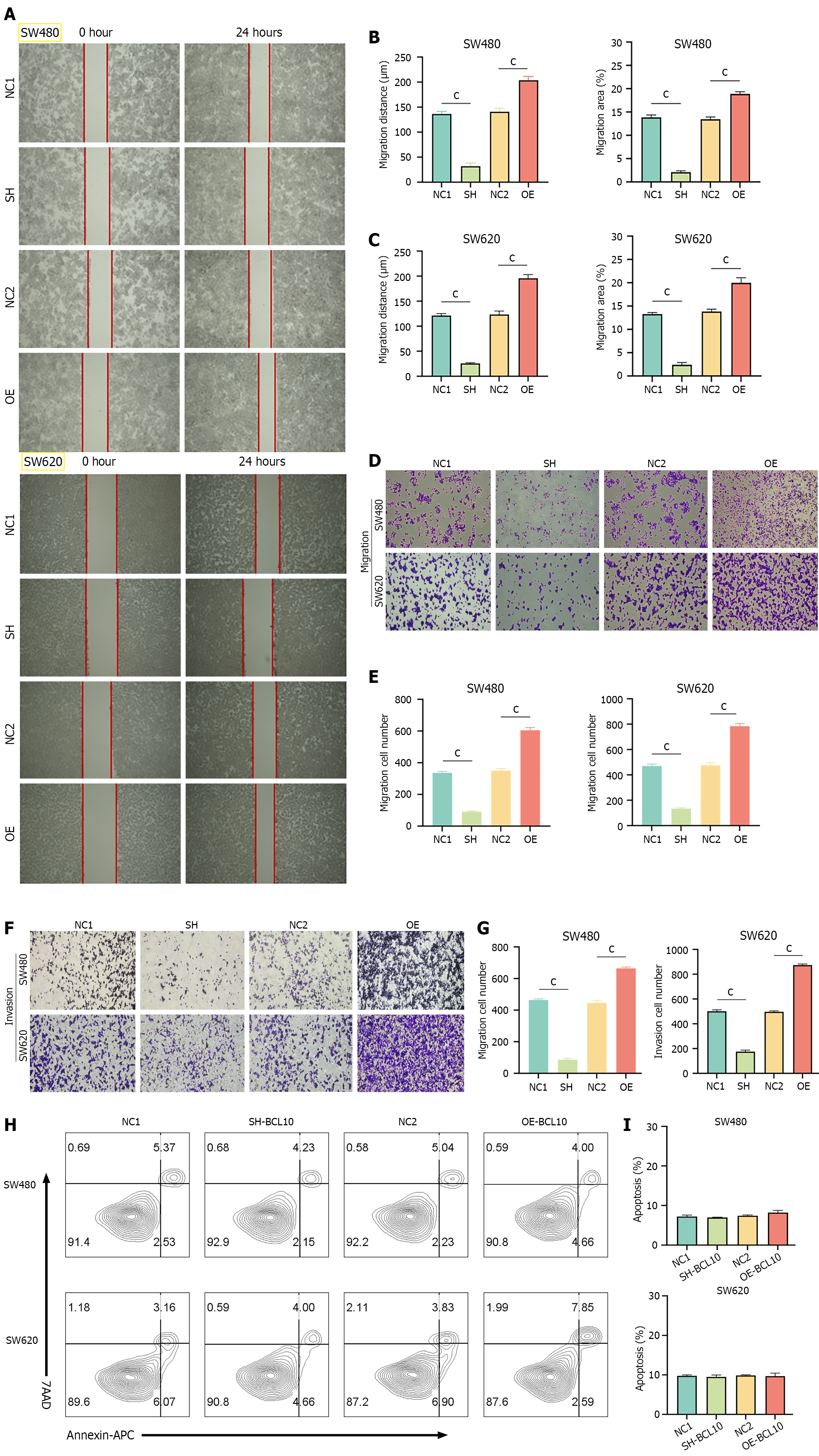
For the wound healing assay, horizontal lines were drawn using a marker on the back of the 6-well plates, which were then used to seed the cells. After 12 hours, the cells were scratched using a 1250 µL sterile tip. The cells were washed three times using PBS and photographed at 0 hour and 24 hours with an Olympus BX51 microscope (Olympus, Tokyo, Japan). Image J software was used to analyze the width of wound healing, and the differences between the widths at 0 hour and 24 hours were calculated. The percentage wound score is the ratio of the differences to the width at 0 hour.
The process of cell invasion was simulated by adding matrix gel (Lablead, China) to transwell chambers (Labelect, China), and cell metastasis was simulated without adding matrix gel. Matrix gel was diluted with DMEM medium at a ratio of 1:7, and 50 μL of diluted matrix gel was added to the upper layer of the chambers. Then, 6 × 104 cells were added to the upper layer of the chambers in 200 μL DMEM medium. Subsequently, 600 μL of 1640 DMEM supplemented with FBS was added to the lower chambers. After 24 hours, the chambers were removed, and the upper cells and matrix gel were wiped off. The migrated cells were fixed with 4% paraformaldehyde for 20 minutes, stained with crystal violet for 15 minutes, rinsed with PBS, air-dried, and photographed. Image J software was used to count the number of migrated/invaded cells.
Cells were seeded in 6-well plates and cultured for 24 horus. After treatment, the cells were incubated with 10 μM 5-ethynyl-2'-deoxyuridine (EdU) (FineTest, China) for 6 hours at 37 °C. The cells were then fixed with 4% paraformaldehyde for 30 minutes and permeabilized with 0.1% Triton X-100 (Lablead, China) for 1 hour. Subsequently, the cells were stained with Alexa Fluor 594 AffiniPure Goat Anti-Rabbit IgG (FineTest, FNSA-0064, 1:100), then in order to see the nuclei, the coverslips were counter-stained with DAPI (Lablead, 28718-90-3). The cells were then examined using an upright fluorescence microscope (Zeiss, Axio Imager M2, Germany) at a magnification of × 20.
To modulate BCL10 expression in the CRC cell lines, both knockdown and overexpression strategies were employed. BCL10 knockdown: Lentivirus-mediated short hairpin RNA (shRNA) targeting BCL10 was utilized. The specific sequences for the shRNA were purchased from Heyuan (Shanghai, China) and are as follows: RNAi# 5′-GTTG
Apoptosis was evaluated using the Annexin V/7-AAD apoptosis kit (Beyotime, China). The cells were suspended in 100 µL of Annexin V binding buffer. APC-Annexin V (5 µL) and 7-AAD (10 µL) were added and the mixture was vortexed. The cells were incubated for 15 minutes at room temperature. Finally, an additional 100 µL of Annexin V binding buffer was added before testing, and apoptosis was analyzed using flow cytometry (Luminex, United States).
Cells were incubated at 37 °C on coverslips in 24-well plates. After rinsing the cells with PBS, they were fixed for 1 hour with 4% paraformaldehyde. The cells were permeabilized by incubating them in PBS with 0.1% Triton X-100 (Lablead, China) for a duration of 15 minutes. The cells were then blocked for 1 hour at room temperature using 3% BSA in PBS. Subsequently, the cells were stained with Alexa Fluor 594 AffiniPure Goat Anti-Rabbit IgG (FineTest, FNSA-0064, 1:100) for 1 hour at room temperature after being incubated with the primary antibodies DLAT (FineTest, FNab02404, 1:50) and P65 (FineTest, FNab09875, 1:50) for the entire night at 4 °C. The coverslips were then counter-stained with DAPI (Lablead, 28718-90-3). The subcellular localization of DLAT proteins (red) and P65 proteins (red), was then examined using an upright fluorescence microscope (Zeiss, Axio Imager M2, Germany) (× 40).
The cells were digested and collected in a centrifuge tube, then centrifuged for 5 minutes at 900 rpm, and the supernatant was discarded. A 2.5% glutaraldehyde fixing solution was slowly applied along the tube wall, and left overnight at 4 °C. The previously fixed cell sample was then post-fixed with osmic acid after three rinses. Following post-fixation, the sample was dehydrated for 20 minutes using ethanol solutions of varying concentrations. After dehydration, the sample was treated for 20 minutes with pure acetone, followed by a combination of acetone and embedding agent (V/V = 1/1), transferred to a mixture of acetone and embedding agent (V/V = 3/1), and an overnight treatment with pure embedding agent. The sample was then heated to 70 °C for a specific duration and embedded. The embedded samples were sectioned using an ultrathin sectioning machine into 70-90 nm slices. The slices were stained with lead citrate and uranium acetate solutions, dried, and viewed under a transmission electron microscope.
Paraffin-embedded tissues or tumors were serially sectioned at a thickness of 4 µm. The tissue pieces were dewaxed and then heated for 20 minutes at 98 °C in citrate buffer (pH 6.0) (Sigma, C9999). Of 3% hydrogen peroxide was added for 15 minutes to inhibit the endogenous peroxidase activity. The presence of these proteins was subsequently ascertained by following a standard protocol and employing anti-DLAT (FineTest, FNab02404, 1:50), anti-BCL10 (FineTest, FNab00835, 1:50), anti-MKI67 (FineTest, FNab09788, 1:50), anti-MYC (FineTest, FNab01791, 1:50), anti-P65 (FineTest, FNab09875, 1:50) or anti-Phospho-NF-KB p65 (Ser536) (MCE, HY-P80839, 1:50) antibodies. Independently, two pathologists assessed the different antibody immunostaining levels. Immunostaining results were assessed based on the percentage of positively stained tumor cells and staining intensity. Tissue sections were stained with hematoxylin and eosin (HE), and six random fields per slide were evaluated for necrosis.
Cells 1 × 106 were plated in each well of a 6-well plate and treated with a gradient chemical for 24 hours. After digestion and collection, the cells were washed twice with PBS. Disuccinimidyl suberate (DSS; Solarbio, D9480) was applied to achieve a final concentration of 0.1 mmol/L. Tris buffer (pH 7.5) was added to the tubes, and the mixture was gently rotated for 30 minutes at room temperature. The reaction was quenched for 15 minutes by adding Tris buffer to a final concentration of 50 mmol/L. The cells were washed twice, and proteins were cleaved according to the previously mentioned method. Proteins were separated using 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and crosslinked products were detected using antibodies on Western blots.
Proteins were extracted from the cells, and their concentrations were determined using a BCA protein assay (Beyotime, Shanghai, China). The proteins were separated via SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was incubated with primary antibodies (diluted at 1:1000 in TBST) for 18 hours and secondary antibodies (diluted at 1:5000 in TBST) for 1 hour. After incubation, the membranes were washed three times with Tris-buffered saline plus Tween®20. The membrane was then imaged using ECL reagents (MCE, China) with the imaging system (Thermo Fisher Scientific). In this study, we used β-actin as an internal control. The optical density of the target protein bands was divided by the optical density of the corresponding β-actin bands in the same sample. This normalization process allowed us to obtain relative expression levels that are comparable across different samples and experimental replicates.
The research protocol was approved by an Institutional Reviewer Board: All procedures followed were in accordance with the ethics committee of the School of Basic Medicine, Jilin University (2024425). Six-week-old male Balb/c nude mice (5 per group) were obtained from Charles River (Beijing, China). The animals were maintained in collective cages at 22 ± 1 °C under a 12 hours light-dark cycle with free access to food and water. A total of 6 × 106 SW620 cells in 150 µL PBS was subcutaneously injected into the right flank of nude mice. After 10 days, tumor-bearing mice were randomly assigned to control or elesclomol groups (5 per group) using a stratified randomization method based on initial tumor volume (GraphPad Random Number Generator). Investigators performing tumor measurements and endpoint analyses were blinded to group allocations throughout the experiment. Mice in the elesclomol group were administered elesclomol (10 mg/kg) via subcutaneous injection every two days, with at least 3 mice per group. The tumor volume was calculated using the following formula: [(length × width × width)/2]. Mice were euthanized (CO2 inhalation) after 11-day treatment. The transplanted tumors, liver, and kidney were excised and fixed with 4% paraformaldehyde and embedded in paraffin for immunohistochemistry and HE staining. Histopathological evaluations (IHC/HE staining) were conducted by two independent pathologists blinded to treatment groups.
The mRNA expression levels in patients with CRC in TCGA and GTEx were acquired from the UCSC Xena project (http://xena.ucsc.edu). The quantity of CRC BCL10 protein expression was obtained from the NIH (PDC000116). The prognosis analysis of patients with CRC was downloaded from GSE106584 (www.ncbi.nlm.nih.gov).
The data were expressed as mean ± SD. Data normality was assessed using the Shapiro-Wilk test. If normality was confirmed, parametric statistical tests were used. Differences between two groups were assessed using the Student’s t-test, while one-way analysis of variance was used for multiple groups. Statistical analyses were conducted using SPSS 22.0 and GraphPad Prism 9.0 software. In addition, P < 0.05 was considered statistically significant.
According to HPA data, the expression level of consensus transcripts of BCL10 gene in 50 normal tissue samples showed that the expression level of BCL10 in colon tissue was the highest (Supplementary Figure 1A). In addition, the expression level of BCL10 in CRC was also relatively high in different tumor tissues (Supplementary Figure 1B). By pairing the TPM expression of normal samples from GTEX with that of tumor samples from TCGA, it was found that the expression of BCL10 in CRC tissues was significantly higher than that in adjacent normal tissues (Supplementary Figure 1C). At the protein level, the data of coad_pnnl cohort showed that the expression level of BCL10 protein in CRC was also significantly increased (Supplementary Figure 1D).
In the cell line study, we observed that the expression level of BCL10 mRNA in SW480, SW620 and HCT116 cell lines was higher than that in NCM480 human colon mucosal epithelial cells (Supplementary Figure 1E). In addition, the expression level of BCL10 was significantly correlated with poor prognosis, tumor stage and grade in patients (Supplementary Figure 1F and G). Receiver operating characteristic curve analysis showed that the expression of BCL10 had high accuracy in the identification of CRC, and its area under the curve value was 0.808 (Supplementary Figure 1H). These results suggest that the differential expression of BCL10 in CRC may not only participate in the occurrence and development of tumors, but may also be closely related to the prognosis of CRC patients.
Stable knockdown and overexpression of BCL10 were constructed in vitro in SW480 and SW620 cell lines, including SW480-NC1/SW620-NC1: Negative control for knockdown (scrambled shRNA); SW480-SH/SW620-SH: BCL10 knockdown (shRNA #1); SW480-NC2/SW620-NC2: Negative control for overexpression (empty vector); and SW480-OE/SW620-OE: BCL10 overexpression. Among the shRNAs tested, shRNA #1 exhibited the highest knockdown efficiency and was therefore selected for subsequent functional studies (Supplementary Figure 2A). A clone formation assay showed that overexpression of BCL10 significantly promoted the proliferation of SW480 and SW620 CRC cells, while knockdown of BCL10 significantly inhibited the proliferation of these cells (Figure 1A and B). Western blot analysis showed that overexpression or knockdown of BCL10 resulted in the corresponding up-regulation or down-regulation of the expression levels of MYC and Ki67, downstream molecules related to proliferation (Figure 1C). These in vitro findings were further corroborated by in vivo evidence showing consistent alterations in MYC and Ki67 expression patterns. Specifically, Western blot (Figure 1C) and IHC (Figure 1D) demonstrated parallel changes in MYC/Ki67 Levels upon BCL10 modulation. In addition, the CCK-8 proliferation assay also confirmed that overexpression or knockdown of BCL10 significantly affected the proliferation activity of CRC cells (Supplementary Figure 3). The results of the EdU proliferation test were consistent with the clone formation assay and CCK-8 assay, which showed that BCL10 could pro
In the scratch test, BCL10 overexpressed cell lines showed significantly enhanced migration ability compared with BCL10 knockdown cell lines (Figure 2A-C). Transwell assay results showed that the number of cells traversed by BCL10 overexpressed cell lines increased significantly regardless of whether matrix glue was used, indicating that BCL10 endowed CRC cells with stronger invasion and migration ability (Figure 2D-G). Although BCL10 affects a variety of phenotypes of CRC cells, it has no significant effect on the baseline apoptosis of cells (Figure 2H and I). These results show that BCL10 plays an important role in the migration and invasion of CRC cells, but it has no significant effect on the baseline level of apoptosis.
Recent studies have highlighted cuproptosis, a novel form of cell death induced by copper accumulation, as a critical player in cancer cell survival and resistance to therapy. Given that BCL10 is known to be involved in various cellular processes, including apoptosis and inflammation, we hypothesized that BCL10 may also play a role in regulating cuproptosis sensitivity in CRC. In this study, different concentrations of elesclomol-Cu (100 nm, 200 nm) were added to SW480 and SW620 cell lines, respectively. Elesclomol is a copper ionophore that selectively transports copper (Cu) into cells, inducing cuproptosis, a novel form of copper-dependent cell death. The results showed that BCL10 overexpressed cell lines showed stronger activity, while BCL10 knockdown cell lines showed significantly lower activity after copper induction (Figure 3A). In addition, we tested the effects at different time points (0 hour, 24 hours, 48 hours, 72 hours) on cell viability and cuproptosis induction, and the optimal experimental time was 24 hours (Supplementary Figure 4). The results of flow cytometry showed that the proportion of 7-AAD+ and Annexin V+ cells in all CRC cell lines increased with the increase in copper concentration. However, after adding TTM, which is a sulfur-containing ligand that forms stable complexes when reacting with Cu2+, cell death almost did not occur. In addition, compared with BCL10 knockdown cell lines, the proportion of 7-AAD+ and Annexin V+ cells in BCL10 overexpression cell lines was signi
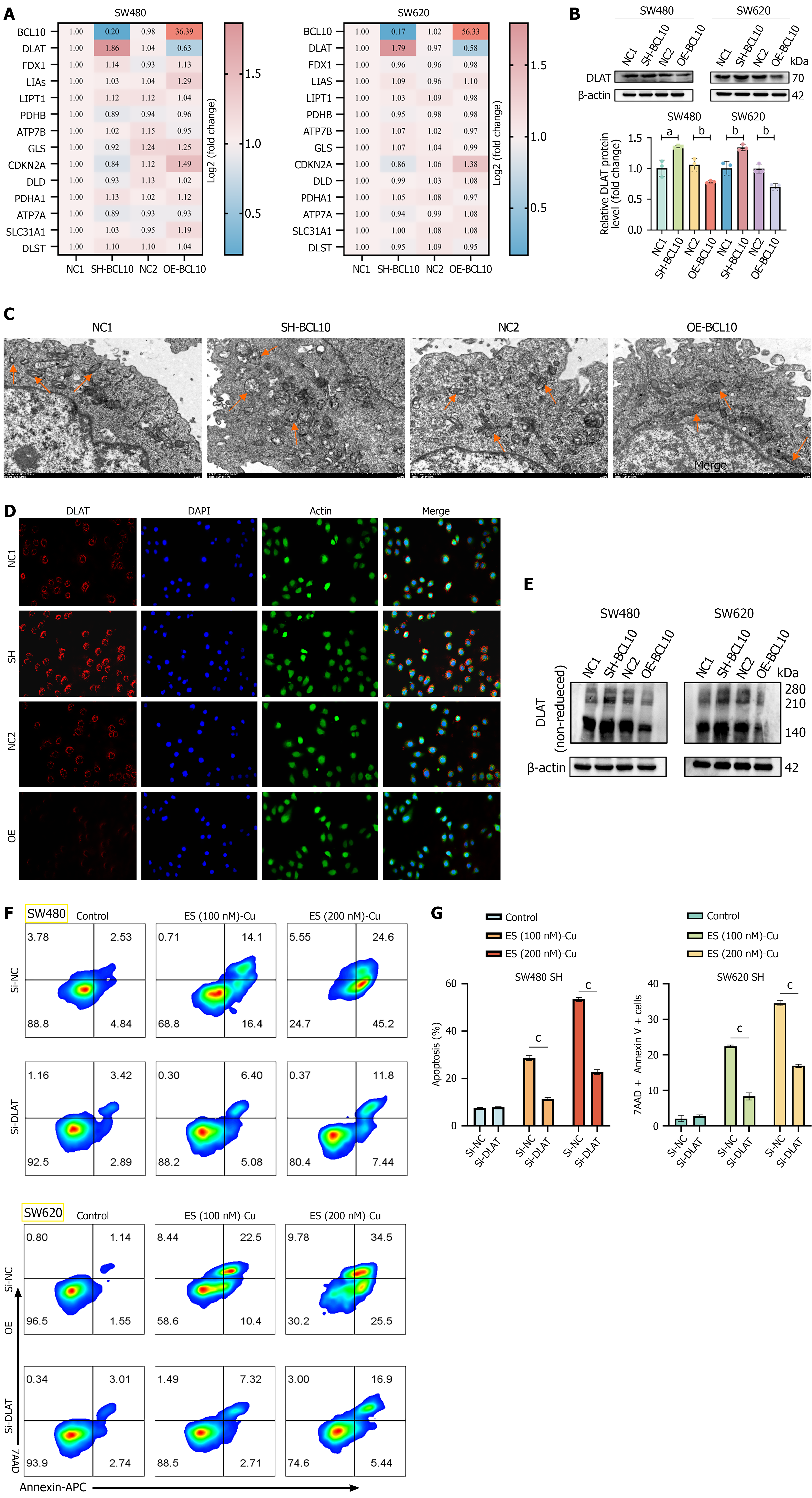
Following overexpression or knockdown of BCL10, the mRNA levels of 13 genes related to cuproptosis were detected by qPCR[39]. The results showed that DLAT was significantly negatively correlated with BCL10 (Figure 4A). At the protein level, there was also a negative correlation between BCL10 and DLAT (Figure 4B). Copper at the concentration of 200 nm was used to induce the occurrence of cuproptosis in the SW620 cell line. Under an electron microscope, it was observed that mitochondrial damage was more significant in BCL10 knockdown cell lines, while mitochondrial damage was less severe in BCL10 overexpression cell lines (Figure 4C). A landmark event of cuproptosis is the oligomerization of DLAT[23]. The expression level of DLAT in clinical colon adenocarcinoma (COAD) tissue samples was significantly lower than that in normal tissues (Supplementary Figure 5A). In addition, the expression level of DLAT in COAD adjacent tissues was significantly lower than that in normal tissues (Supplementary Figure 5B). The low expression level of DLAT was significantly correlated with a poor prognosis in patients (Supplementary Figure 5C). Under a fluorescence microscope, the aggregation of DLAT protein in the BCL10 knockout group with more cuproptosis was more significant (Figure 4D). Further experimental results showed that the expression of oligomeric DLAT protein in BCL10 knockdown cell lines was significantly increased in the total protein of cells after the occurrence of cuproptosis (Figure 4E). To test this hypothesis, we used siRNA to knock down DLAT in BCL10 knockdown cell lines with high DLAT expression, and then used different concentrations of copper (50 nm, 100 nm, 200 nm, 400 nm, 800 nm) to induce cuproptosis in cells. CCK8 test results showed that cell death in the Si-DLAT group was significantly inhibited (Supplementary Figure 5D and E). Flow cytometry results showed that the proportion of 7-AAD+ and Annexin V+ cells in the Si-DLAT group was significantly reduced (Figure 4F and G). In conclusion, we speculated that BCL10 regulated the sensitivity of CRC cells to cuproptosis by affecting the expression of DLAT.
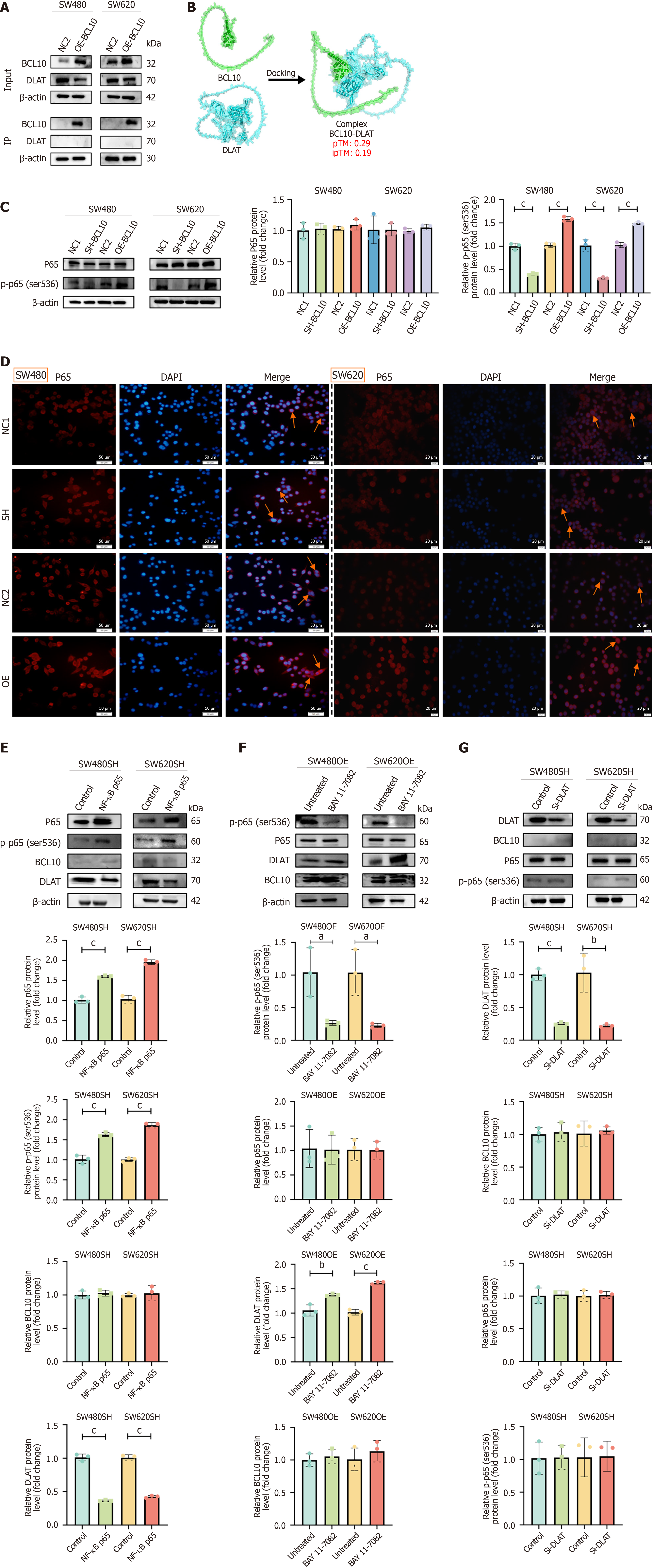
To further explore the relationship between BCL10 and DLAT, we first performed co-immunoprecipitation experiments. The results showed that there was no interaction between BCL10 and DLAT (Figure 5A). The analysis using alphafold3 prediction software also confirmed this result (Figure 5B). As there is no interaction between BCL10 and DLAT, how does BCL10 regulate DLAT expression? Our study found that BCL10 promotes the phosphorylation of p65 (Figure 5C). In addition, overexpression of BCL10 could also enhance the nuclear translocation of p65 (Figure 5D). We transfected rela (NF-κB p65) plasmid into BCL10 knockdown cell lines and found that the activity of the NF-κB signaling pathway increased, while the expression of DLAT was down-regulated, but the expression of BCL10 did not change significantly (Figure 5E). In contrast, in BCL10 overexpressing cell lines, the expression of DLAT was upregulated after treatment with BAY 11-7082, an inhibitor of NF-κB, while the level of BCL10 was unchanged (Figure 5F). To further validate the role of DLAT, we constructed a siRNA-DLAT cell line. Among the siRNAs tested, siRNA #1 exhibited the highest knockdown efficiency and was therefore selected for subsequent functional studies (Supplementary Figure 2B). Further experiments proved that in BCL10 knockdown cell lines, knockdown of DLAT did not affect the NF-κB signaling pathway and the expression of BCL10 (Figure 5G). In conclusion, we speculate that BCL10 may negatively regulate the expression of DLAT by activating the NF-κB signaling pathway and promoting the phosphorylation and nuclear translocation of p65, thereby affecting the sensitivity of CRC cells to copper-induced cuproptosis. However, there was no interaction between BCL10 and DLAT.
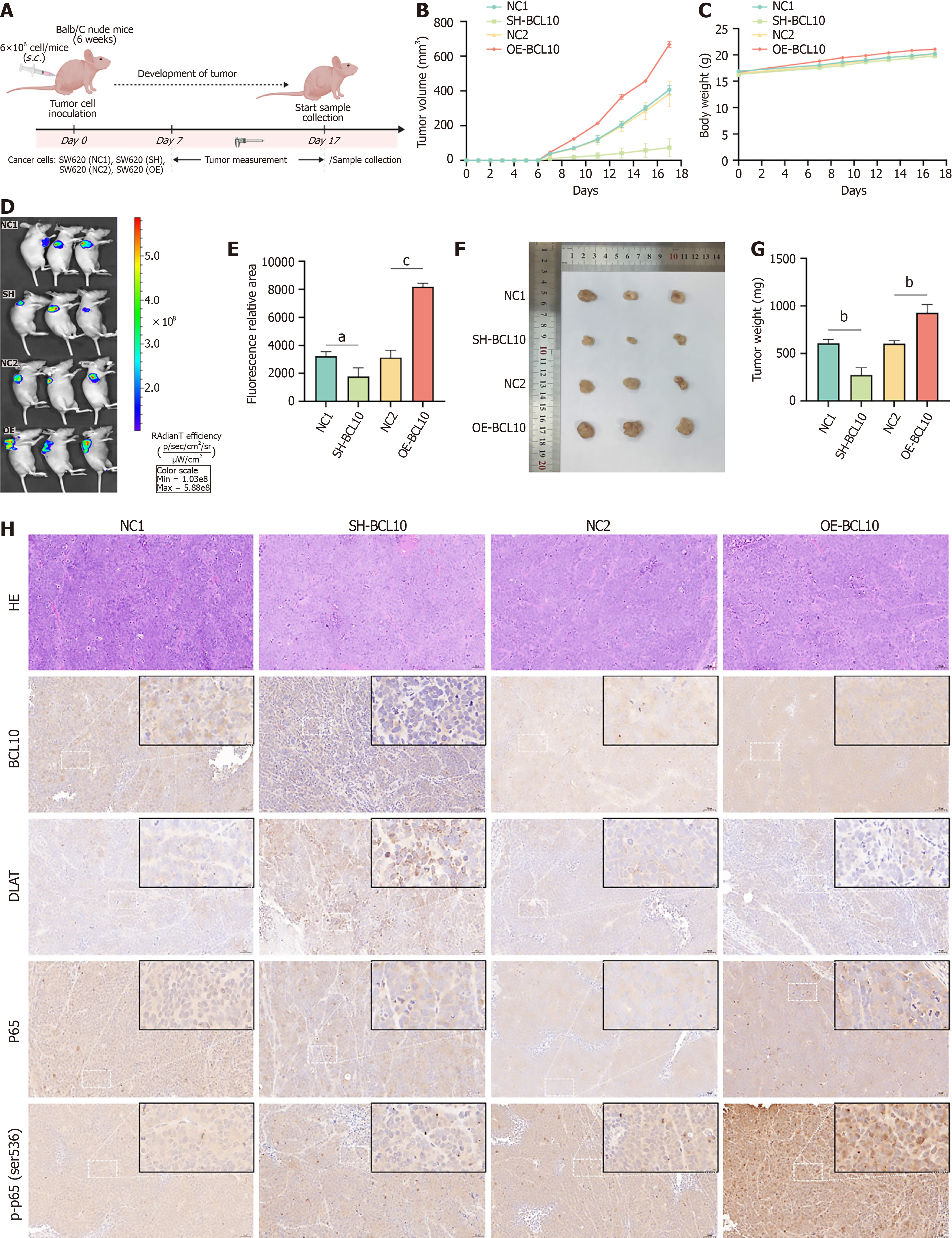
To elucidate the potential therapeutic implications of our findings, we conducted in vivo experiments to verify the effects of BCL10 and DLAT on cuproptosis sensitivity in a mouse model. In the in vivo experiments, the flow chart of CRC mouse models was as follows (Figure 6A): At the 6th week, the tumor volume in the OE group was significantly larger than that of the SH group (Figure 6B). There was no significant difference in the weight of mice (Figure 6C). On the 17th day of tumor bearing, the mice underwent in vivo imaging after anesthesia. The tumor volume in the OE group was significantly larger than that in the SH group (Figure 6D and E). The tumor tissues were then obtained and weighed. The tumor weight in the OE group was also significantly larger than that in the SH group (Figure 6F and G). The immunohistochemical results of tumor tissues were consistent with our previous in vitro Western blot findings (Figures 5C, E-G and Figure 6H; Supplementary Figure 6). BCL10 may regulate DLAT by promoting translation protein modification (phosphorylation) and nuclear translocation of p65, and by negative feedback.
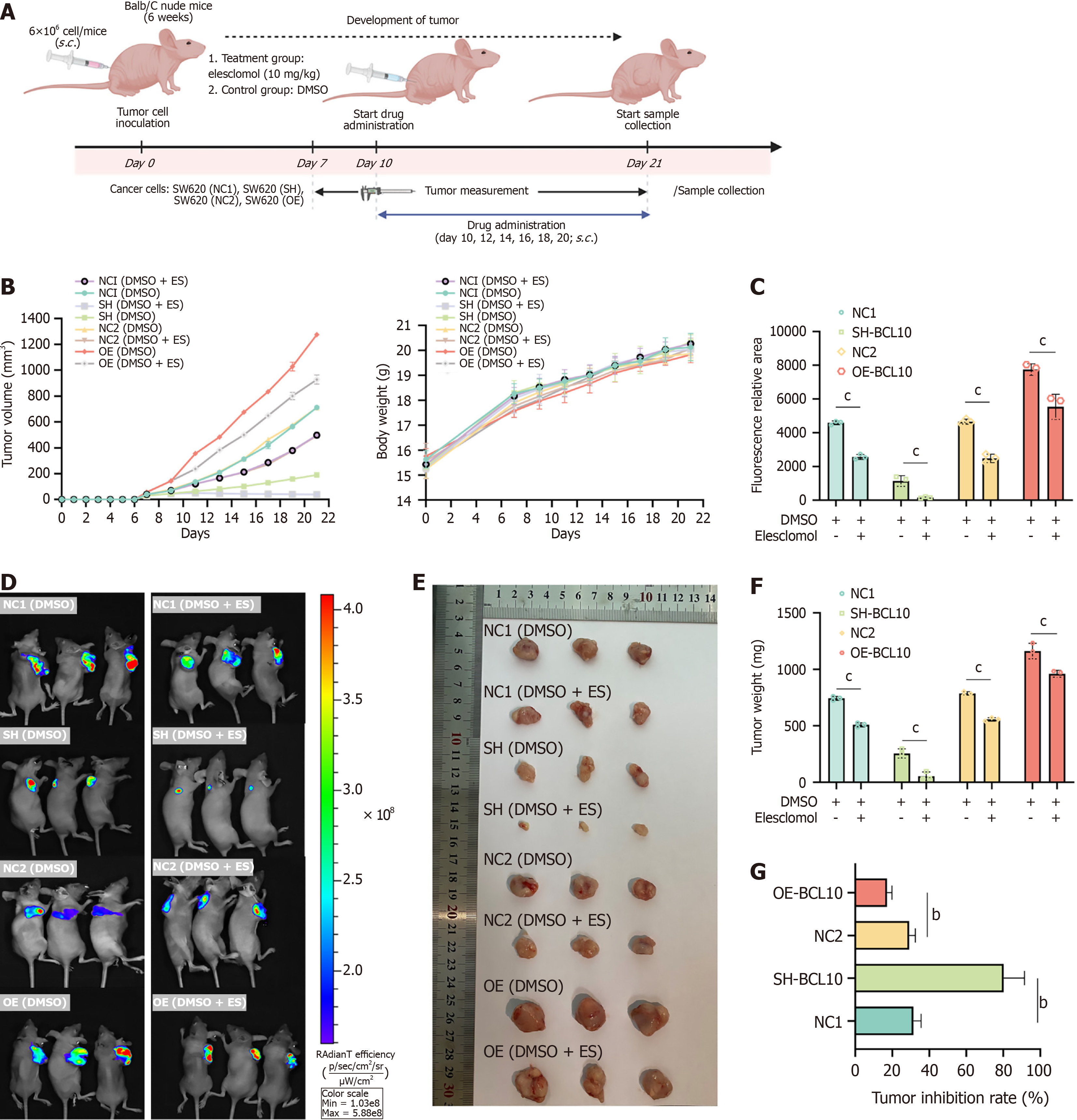
In the in vivo experiments, to verify the effect of BCL10 on copper treatment sensitivity in CRC mice, the flow chart of drug administration in CRC mice was as follows (Figure 7A): On the 10th day of tumor bearing, the treatment group was subcutaneously injected with elesclomol at the dose of 10 mg/kg based on previous studies demonstrating its efficacy in xenograft models[40], while the control group was subcutaneously injected with DMSO at the dose of 10 mg/kg. The results showed that after elesclomol treatment, the tumor volume in each group decreased, and the weight of mice in each group showed no significant difference (Figure 7B). On the 21st day of tumor bearing, in vivo imaging of mice after anesthesia demonstrated that tumor volume in the OE group was significantly larger than that in the SH group. In addition, compared with the control group, the tumor volume in each group was reduced, and the relative reduction in tumor volume in the SH group was much larger than that in the OE group (Figure 7C and D). Tumor tissue was then obtained and weighed, and the tumor mass in the OE group was significantly larger than that in the SH group. In addition, the tumor mass in the treatment group was reduced compared with that in control group, and the relative reduction in tumor mass in the SH group was much larger than that in the OE group (Figure 7E and F). Tumor growth inhibition rate was calculated as follows: Tumor growth inhibition rate = (1 - average tumor weight in the treatment group/average tumor weight in the control group), and the results showed that the reduction in tumor load in the SH group was significantly greater than that in the OE group (Figure 7G). The liver ALT and AST indices in mice were measured by the ELISA method and no significant change was observed between the treatment group and the control group (Supplementary Figure 7A). In addition, HE staining of liver and kidney in mice in the treatment group and the control group showed no evident destruction (Supplementary Figure 7B). Immunohistochemical results showed that after treatment with elesclomol, the SH group with the highest tumor growth inhibition rate also showed significantly high expression of DLAT (Supplementary Figure 7B). Therefore, in vivo, BCL10 also affected the sensitivity of CRC therapy and the sensitivity to copper treatment in the SH group increased. While our in vivo data demonstrated enhanced therapeutic efficacy of elesclomol in BCL10-knockdown tumors (Figure 7), we acknowledge that direct evidence of copper accumulation and DLAT oligomerization in tumor tissues would strengthen the cuproptosis connection. Several lines of indirect evidence support this mechanism. The SH group showing highest tumor inhibition (Figure 7G) concurrently exhibited elevated DLAT expression by IHC (Supplementary Figure 6 and 7B), consistent with DLAT's role as a cuproptosis mediator[23]. Our in vitro experiments confirmed that BCL10 knockdown increased DLAT oligomerization (Figure 4E) and mitochondrial damage (Figure 4C) upon copper treatment. Prior studies have established that elesclomol's antitumor effects require copper-dependent DLAT aggregation[41]. Technical limitations in measuring intra-tumoral copper levels (e.g., requiring mass spectrometry) precluded direct quantification. Future work could employ copper-sensitive fluorescent probes to visualize spatial copper distribution.
CRC remains a significant global public health challenge, underscoring the urgent need for novel therapeutic targets to enhance patient outcomes[42]. While existing studies primarily focused on BCL10's role in immune signaling[17], our research provides the first evidence that BCL10 is a critical player in CRC progression and its sensitivity to cuproptosis.
BCL10 is a key component of the CBM complex. It has been confirmed that the expression of BCL10 is up-regulated in CRC tissues and is associated with poor prognosis[43], which indicates that BCL10 may play an important role in the occurrence and development of CRC. Our study found that overexpression of BCL10 promoted the proliferation, migration and invasion of CRC cells, while knockdown of BCL10 inhibited these malignant biological behaviors. These observations are consistent with previous studies[44], suggesting that BCL10 plays a key role in cell proliferation, invasion, migration and anti-apoptosis. Specifically, BCL10 modulates the expression of key downstream molecules, MYC and Ki67, which are closely involved in cell proliferation. This interaction may elucidate the mechanistic basis by which BCL10 promotes CRC development and progression.
Cuproptosis, a newly identified form of programmed cell death, has emerged as a promising strategy for cancer therapy[30,39]. Our study further clarified the mechanism of BCL10 regulating the sensitivity of CRC cells to cuproptosis. In contrast to the prevailing view of NF-κB as a metallothionein inducer[35], our data reveal a paradoxical suppression of DLAT by BCL10-activated NF-κB (Figure 4B). The results showed that overexpression of BCL10 enhanced the resistance of CRC cells to cuproptosis, while knockdown of BCL10 increased the sensitivity of CRC cells to cuproptosis. This explains why BCL10-OE tumors showed only 17% volume reduction after elesclomol vs 79% in SH group (Figure 7G), a divergence from the uniform copper sensitivity reported in unstratified models. This phenomenon may be achieved by regulating DLAT, which is a key protein in the cuproptosis pathway. Our data suggest that BCL10 negatively regulates DLAT expression, which is crucial for the sensitivity of CRC cells to cuproptosis. In order to clarify the molecular mechanism of BCL10 regulating cuproptosis, we investigated the interaction between BCL10 and DLAT. The results showed that BCL10 did not directly interact with DLAT. On the contrary, BCL10 activated the NF-κB signaling pathway by promoting p65 phosphorylation and nuclear translocation. Activation of the NF-κB signaling pathway in turn negatively regulated the expression of DLAT, which was a key factor in the sensitivity of CRC cells to cuproptosis. This regulatory mechanism has been verified in both in vitro and in vivo experiments, in which BCL10 overexpression leads to an increase in p65 phosphorylation and nuclear translocation, thereby down-regulating DLAT and enhancing resistance to cuproptosis.
An examination of the novel mechanistic insights of our study reveals the need to consider alternative pathways that may interact with or mediate the effects of BCL10 on NF-κB activation and cuproptosis sensitivity. For instance, the PI3K/Akt pathway, known for its role in promoting cell survival and proliferation, may also influence the effects of BCL10 on CRC. Investigating potential cross-talk between BCL10 and the PI3K/Akt pathway could reveal additional layers of regulation that contribute to CRC cell behavior. Another relevant pathway is the MAPK/ERK pathway, which is associated with cell growth and differentiation. Given that both NF-κB and MAPK pathways can be activated by similar stimuli, it would be valuable to explore whether BCL10 indirectly influences MAPK signaling. Such investigations could help elucidate the broader context of BCL10’s role in CRC and its potential as a therapeutic target. In addition, the interaction between BCL10 and other proteins involved in apoptosis and cellular stress responses should be examined. For example, the protein p53, which plays a critical role in cell cycle regulation and apoptosis, may interact with BCL10 in the context of CRC. Although our NF-κB inhibition experiments (Figure 5F) support its mediatory role, this contrasts with reported NF-κB-independent BCL10 functions in lymphomas[16], warranting tissue-specific investigations. Examining these relationships could provide insights into off-target mechanisms and further clarify the specific role of BCL10 in modulating cancer cell fate.
Our findings hold significant clinical implications. Firstly, BCL10 could serve as a prognostic biomarker for CRC due to its association with poor prognosis. Secondly, targeting BCL10 may be a feasible strategy for the treatment of CRC, as it can promote tumor growth and enhance the resistance to cuproptosis. Thirdly, the BCL10-NF-κB-DLAT axis was identified as a new therapeutic target to improve the sensitivity of CRC cells to cuproptosis.
However, there are still some limitations. Firstly, the specific mechanisms by which BCL10 activates the NF-κB signaling pathway and regulates DLAT expression warrant further investigation. While our data suggest that NF-κB plays a role in the BCL10-mediated regulation of DLAT, it is important to note that this does not determine the direct regulatory role of NF-κB in the BCL10-regulated sensitivity to cuproptosis. Further studies are needed to determine whether NF-κB signaling is essential for the observed effects of BCL10 on cuproptosis sensitivity or whether additional mechanisms are involved. Future studies should investigate whether NF-κB signaling is directly required for BCL10-regulated sensitivity to cuproptosis. For example, experiments involving NF-κB inhibition or genetic knockout in the context of BCL10 modulation could help clarify whether NF-κB is an essential mediator of this process. Additionally, it would be valuable to explore whether NF-κB influences other cellular pathways or targets that contribute to cuproptosis sensitivity. Secondly, it is essential to assess whether targeting BCL10 can enhance the efficacy of copper-based therapies in CRC patients. Lastly, potential side effects and long-term efficacy of targeting BCL10 need thorough evaluation to ensure the safety and effectiveness of such therapeutic interventions.
Our study showed that BCL10 promoted the growth of CRC by activating the NF-κB signaling pathway and affected the sensitivity of CRC cells to cuproptosis by regulating DLAT. These findings provide a new molecular basis for developing CRC therapeutic strategies targeting BCL10.
I would like to sincerely thank teachers for their invaluable contributions to the development of this manuscript.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12553] [Article Influence: 6276.5] [Reference Citation Analysis (6)] |
| 2. | Sun L, Xing J, Zhou X, Song X, Gao S. Wnt/β-catenin signalling, epithelial-mesenchymal transition and crosslink signalling in colorectal cancer cells. Biomed Pharmacother. 2024;175:116685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 3. | Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 4. | Li B, Ming H, Qin S, Zhou L, Huang Z, Jin P, Peng L, Luo M, Zhang T, Wang K, Liu R, Liou YC, Nice EC, Jiang J, Huang C. HSPA8 Activates Wnt/β-Catenin Signaling to Facilitate BRAF V600E Colorectal Cancer Progression by CMA-Mediated CAV1 Degradation. Adv Sci (Weinh). 2024;11:e2306535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Li J, Li S, Xing X, Liu N, Lai S, Liao D, Li J. FTO-mediated ZNF687 accelerates tumor growth, metastasis, and angiogenesis in colorectal cancer through the Wnt/β-catenin pathway. Biotechnol Appl Biochem. 2024;71:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Zhou X, Zhang K, Wang C, Teng Y, Yu P, Cai W, Gao W, Li M, Ding Y, Sun P, Chen F, Wang Y, Ma J, Maeshige N, Ma X, Li Q, Liang X, Zhang Y, Su D. Isthmin-1 promotes growth and progression of colorectal cancer through the interaction with EGFR and YBX-1. Cancer Lett. 2024;590:216868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Wei PL, Lin JC, Hung CS, Makondi PT, Batzorig U, Chang TC, Huang CY, Chang YJ. Human α-defensin 6 (HD6) suppresses CRC proliferation and metastasis through abolished EGF/EGFR signaling pathway. Int J Med Sci. 2022;19:34-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | He Y, Shi Q, Ling Y, Guo H, Fei Y, Wu R, Tang C, Zhang X, Yao L. ABLIM1, a novel ubiquitin E3 ligase, promotes growth and metastasis of colorectal cancer through targeting IĸBα ubiquitination and activating NF-ĸB signaling. Cell Death Differ. 2024;31:203-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Laskar RS, Qu C, Huyghe JR, Harrison T, Hayes RB, Cao Y, Campbell PT, Steinfelder R, Talukdar FR, Brenner H, Ogino S, Brendt S, Bishop DT, Buchanan DD, Chan AT, Cotterchio M, Gruber SB, Gsur A, van Guelpen B, Jenkins MA, Keku TO, Lynch BM, Le Marchand L, Martin RM, McCarthy K, Moreno V, Pearlman R, Song M, Tsilidis KK, Vodička P, Woods MO, Wu K, Hsu L, Gunter MJ, Peters U, Murphy N; Colorectal Transdisciplinary (CORECT) Study, the Colon Cancer Family Registry (CCFR), Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO). Genome-wide association studies and Mendelian randomization analyses provide insights into the causes of early-onset colorectal cancer. Ann Oncol. 2024;35:523-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 10. | Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY, Coker OO, Chan AWH, Chan FKL, Sung JJY, Yu J. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. 2019;4:2319-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 11. | Wan ML, Wang Y, Zeng Z, Deng B, Zhu BS, Cao T, Li YK, Xiao J, Han Q, Wu Q. Colorectal cancer (CRC) as a multifactorial disease and its causal correlations with multiple signaling pathways. Biosci Rep. 2020;40:BSR20200265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2020;22:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 13. | Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB; US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 1356] [Article Influence: 271.2] [Reference Citation Analysis (0)] |
| 14. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1732] [Article Influence: 346.4] [Reference Citation Analysis (1)] |
| 15. | Ohishi T, Kaneko MK, Yoshida Y, Takashima A, Kato Y, Kawada M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int J Mol Sci. 2023;24:1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 16. | Moud BN, Ober F, O'Neill TJ, Krappmann D. MALT1 substrate cleavage: what is it good for? Front Immunol. 2024;15:1412347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | O'Neill TJ, Tofaute MJ, Krappmann D. Function and targeting of MALT1 paracaspase in cancer. Cancer Treat Rev. 2023;117:102568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 18. | Phelan JD, Young RM, Webster DE, Roulland S, Wright GW, Kasbekar M, Shaffer AL 3rd, Ceribelli M, Wang JQ, Schmitz R, Nakagawa M, Bachy E, Huang DW, Ji Y, Chen L, Yang Y, Zhao H, Yu X, Xu W, Palisoc MM, Valadez RR, Davies-Hill T, Wilson WH, Chan WC, Jaffe ES, Gascoyne RD, Campo E, Rosenwald A, Ott G, Delabie J, Rimsza LM, Rodriguez FJ, Estephan F, Holdhoff M, Kruhlak MJ, Hewitt SM, Thomas CJ, Pittaluga S, Oellerich T, Staudt LM. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560:387-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 19. | Schlauderer F, Seeholzer T, Desfosses A, Gehring T, Strauss M, Hopfner KP, Gutsche I, Krappmann D, Lammens K. Molecular architecture and regulation of BCL10-MALT1 filaments. Nat Commun. 2018;9:4041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 1473] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 21. | Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M, Zeng C, Zhou T, Zhang J. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 1043] [Article Influence: 521.5] [Reference Citation Analysis (0)] |
| 22. | Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 1542] [Article Influence: 257.0] [Reference Citation Analysis (0)] |
| 23. | Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, Eaton JK, Frenkel E, Kocak M, Corsello SM, Lutsenko S, Kanarek N, Santagata S, Golub TR. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 3044] [Article Influence: 761.0] [Reference Citation Analysis (1)] |
| 24. | Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 610] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 25. | Xiong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30:876-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 172] [Reference Citation Analysis (0)] |
| 26. | Zhang Z, Xue H, Xiong Y, Geng Y, Panayi AC, Knoedler S, Dai G, Shahbazi MA, Mi B, Liu G. Copper incorporated biomaterial-based technologies for multifunctional wound repair. Theranostics. 2024;14:547-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 27. | Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 926] [Article Influence: 231.5] [Reference Citation Analysis (0)] |
| 28. | Doguer C, Ha JH, Collins JF. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr Physiol. 2018;8:1433-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 622] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 30. | Liu WQ, Lin WR, Yan L, Xu WH, Yang J. Copper homeostasis and cuproptosis in cancer immunity and therapy. Immunol Rev. 2024;321:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 31. | Guo B, Yang F, Zhang L, Zhao Q, Wang W, Yin L, Chen D, Wang M, Han S, Xiao H, Xing N. Cuproptosis Induced by ROS Responsive Nanoparticles with Elesclomol and Copper Combined with αPD-L1 for Enhanced Cancer Immunotherapy. Adv Mater. 2023;35:e2212267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 261] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 32. | Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z, Feng T, Cui Z, Zhu T, Li Y, Qiu Z, Fan G, Huang C. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14:6523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 298] [Reference Citation Analysis (0)] |
| 33. | Yang W, Wang Y, Huang Y, Yu J, Wang T, Li C, Yang L, Zhang P, Shi L, Yin Y, Tao K, Li R. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed Pharmacother. 2023;159:114301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 122] [Reference Citation Analysis (0)] |
| 34. | Li L, Zhou H, Zhang C. Cuproptosis in cancer: biological implications and therapeutic opportunities. Cell Mol Biol Lett. 2024;29:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 35. | Xue Q, Kang R, Klionsky DJ, Tang D, Liu J, Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;19:2175-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 432] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 36. | Park C, Jeong J. Synergistic cellular responses to heavy metal exposure: A minireview. Biochim Biophys Acta Gen Subj. 2018;1862:1584-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Masnikosa R, Cvetković Z, Pirić D. Tumor Biology Hides Novel Therapeutic Approaches to Diffuse Large B-Cell Lymphoma: A Narrative Review. Int J Mol Sci. 2024;25:11384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 38. | Zhao Q, Qi T. The implications and prospect of cuproptosis-related genes and copper transporters in cancer progression. Front Oncol. 2023;13:1117164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 39. | Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 510] [Reference Citation Analysis (1)] |
| 40. | Lei G, Sun M, Cheng J, Ye R, Lu Z, Horbath A, Huo D, Wu S, Alapati A, Aggarwal S, Xu Z, Mao C, Yan Y, Yao J, Li Q, Chen X, Lee H, Zhuang L, Jiang D, Pataer A, Roth JA, Navin N, Koong AC, You MJ, Lin SH, Gan B. Radiotherapy promotes cuproptosis and synergizes with cuproptosis inducers to overcome tumor radioresistance. Cancer Cell. 2025;43:1076-1092.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 41. | Zheng P, Zhou C, Lu L, Liu B, Ding Y. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J Exp Clin Cancer Res. 2022;41:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 237] [Reference Citation Analysis (0)] |
| 42. | Housini M, Dariya B, Ahmed N, Stevens A, Fiadjoe H, Nagaraju GP, Basha R. Colorectal cancer: Genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene. 2024;892:147857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 43. | Liu X, Zhang G, Li S, Liu Y, Ma K, Wang L. Identification of gut microbes-related molecular subtypes and their biomarkers in colorectal cancer. Aging (Albany NY). 2024;16:2249-2272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Chen H, Li Z, Wu F, Ji W, Lu L, Wu Z, Huang Y, Wang W, Li S. BCL10 correlates with bad prognosis and immune infiltration of tumor microenvironment in hepatocellular carcinoma. IUBMB Life. 2023;75:207-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/