Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.109718
Revised: July 5, 2025
Accepted: August 13, 2025
Published online: September 14, 2025
Processing time: 109 Days and 4.2 Hours
Elevated plasma homocysteine (Hcy) levels are associated with increased risk of colorectal cancer (CRC), particularly in patients with systemic inflammation or chronic conditions.
To evaluate serum Hcy levels as a predictive marker of lesion risk and CRC to prioritize patients undergoing diagnostic colonoscopy.
We conducted a prospective cohort study of 301 fecal occult blood test-positive patients at San Agustín University Hospital in Asturias, Spain. Plasma Hcy levels were measured prior to the colonoscopy and classified into three thresholds: ≤ 12, 12-15, and > 15 μmol/L. Colonoscopy and histopathology determined the pre
Median Hcy levels rose progressively with lesion severity, reaching 15.3 μmol/L in adenocarcinoma (P < 0.001). Higher levels were also observed in men and in
Serum Hcy is a clinically useful biomarker for identifying high-risk colorectal lesions and cancer, particularly when interpreted in combination with age and sex. This composite model improves predictive accuracy and enables a structured three-tiered triage system that supports faster colonoscopy scheduling for at-risk groups. The traffic light approach offers a low cost, scalable strategy to reduce delays and optimize resource use in CRC screening, especially in public health systems with limited endoscopic capacity.
Core Tip: In this study, we propose a traffic-light triage model based on serum homocy
- Citation: Cano FX, Duque JM, Seoane L, Puga-Tejada M, Espinoza de los Monteros A, Bermeo P, Junquera E, Pérez D, Martin-Delgado J, Santelli M, Pérez C, Pérez Rivera FJ. Serum homocysteine-based traffic light triage colonoscopy screening in colorectal cancer at-risk patients: A prospective cohort study. World J Gastroenterol 2025; 31(34): 109718
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/109718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.109718
Colorectal cancer (CRC) is a major cause of mortality worldwide, and hyperhomocysteinemia has been identified as a part of CRC oncogenic pathways[1-3]. Homocysteine (Hcy) is a sulfur-containing amino acid generated from methionine metabolism through the S-adenosylmethionine-dependent transmethylation pathway[4]. Preliminary research has established a correlation between elevated serum Hcy levels and an increased probability of developing high-risk polyps in the colon, which can be identified as precursors of CRC[2,3,5]. Furthermore, low blood levels of folate and vitamin B12 are associated with elevated Hcy levels, which are implicated in the occurrence of DNA hypomethylation and therefore induce oxidative stress and chromosomal instability, favoring carcinogenesis[3,6,7].
Early detection through screening programs, including fecal occult blood test (FOBT) followed by colonoscopy, has been shown to decrease both the incidence and mortality[1,8,9]. However, delays in performing diagnostic colonoscopies in FOBT-positive patients, often due to resource limitations and extensive waiting lists, may lead to the progression of neoplastic lesions to adenocarcinoma, compromising patient outcomes and decreasing the probability[1,10]. Therefore, given its wide availability and low cost, Hcy holds promise for addressing this gap[11,12], as Hcy strongly interacts with comorbid conditions such as renal dysfunction, cardiovascular disease, and metabolic syndrome, rendering it a relevant marker for identifying and stratifying risk in a diverse patient population and serving as a potential aid in triaging and prioritizing patients for timely diagnostic interventions[4,13,14]. The present study aimed to evaluate the role of serum Hcy levels for triaging of patients undergoing colonoscopy for the early detection of CRC.
This prospective, analytical, monocentric, endoscopist-blind cohort study sought to determine the utility of serum Hcy as a predictive marker of lesion risk for prioritizing patients waitlisted for diagnostic colonoscopies in the Spanish Colon and Rectal Cancer Screening Program (CCCR). All participants were informed in advance of the research objectives and signed informed consent forms prior to sample collection or colonoscopy. This study was approved by the Hospital Ethics Committee of Asturias (official letter No. 244-18) in addition to the Heads of the Digestive and Clinical Bioche
The study was conducted in the Endoscopy Unit of the San Agustín University Hospital, located in in the city of Avilés, autonomous community of Asturias, Spain. In this unit, endoscopic assessments of patients recruited within the CCCR program were conducted.
Inclusion criteria: First-time patients recruited to the CCCR were invited to participate. Additionally, we also invited patients from the general population to whom a colonoscopy was requested cause of presumptive CRC diagnosis based on computed tomography (CT).
Exclusion criteria: Subjects who, due to clinical or technical circumstances, could not meet the endoscopic quality criteria established by the Spanish guidelines for CRC screening[10,15], subjects with a previous diagnosis of inflammatory bowel disease (IBD), non-IBD colitis (e.g. microscopic or eosinophilic colitis), active colonic inflammatory pathology of the ischemic type or associated with diverticula, familial polyposis, active extracolonic neoplastic pathology or under treatment at the time of the test, or any patient with criteria for colonoscopy surveillance cause to preexistences were excluded. Systemic autoimmune diseases, chronic kidney disease ≥ stage III, chronic infections known to be treated or not (human immunodeficiency virus, tuberculosis, hepatitis C virus, hepatitis B virus), or any patient with criteria for colonoscopy surveillance due to preexistences were also excluded.
Stratification of CRC risk: The samples obtained were categorized according to colonoscopy findings as the diagnostic reference standard, defined by the quality and surveillance criteria of the Spanish Guide for Colon and Rectal Cancer Screening, which are detailed in Table 1.
| Group number | Criteria |
| Absence of lesion/no polyps | No pathological findings in the diagnostic colonoscopy |
| Low-risk lesion | Classified as low risk are those subjects who present the finding of polyps with low-risk endoscopic characteristics according to number (< 3), size (< 1 cm), endoscopic criteria of the Paris staging, and results of the pathological anatomy of the piece(s) obtained classified as adenoma |
| High-risk lesion | Polyps with high-risk endoscopic characteristics according to number (> 3), size (> 1 cm), endoscopic criteria of Paris staging, and pathological anatomy results of the piece(s) obtained compatible with a villous component, high-grade dysplasia, or intramucosal adenocarcinoma |
| Adenocarcinoma | Local and/or distant invasive adenocarcinoma |
Hcy cut-off values: The cohort classification in this study was defined by the three Hcy cut-off levels validated by receiver operating curves (ROC) analysis, and further detailed in the statistical analysis section. Hcy values ≥ 15 μmol/L are consistent with the validation study by Chen et al[2] as a reference standard.
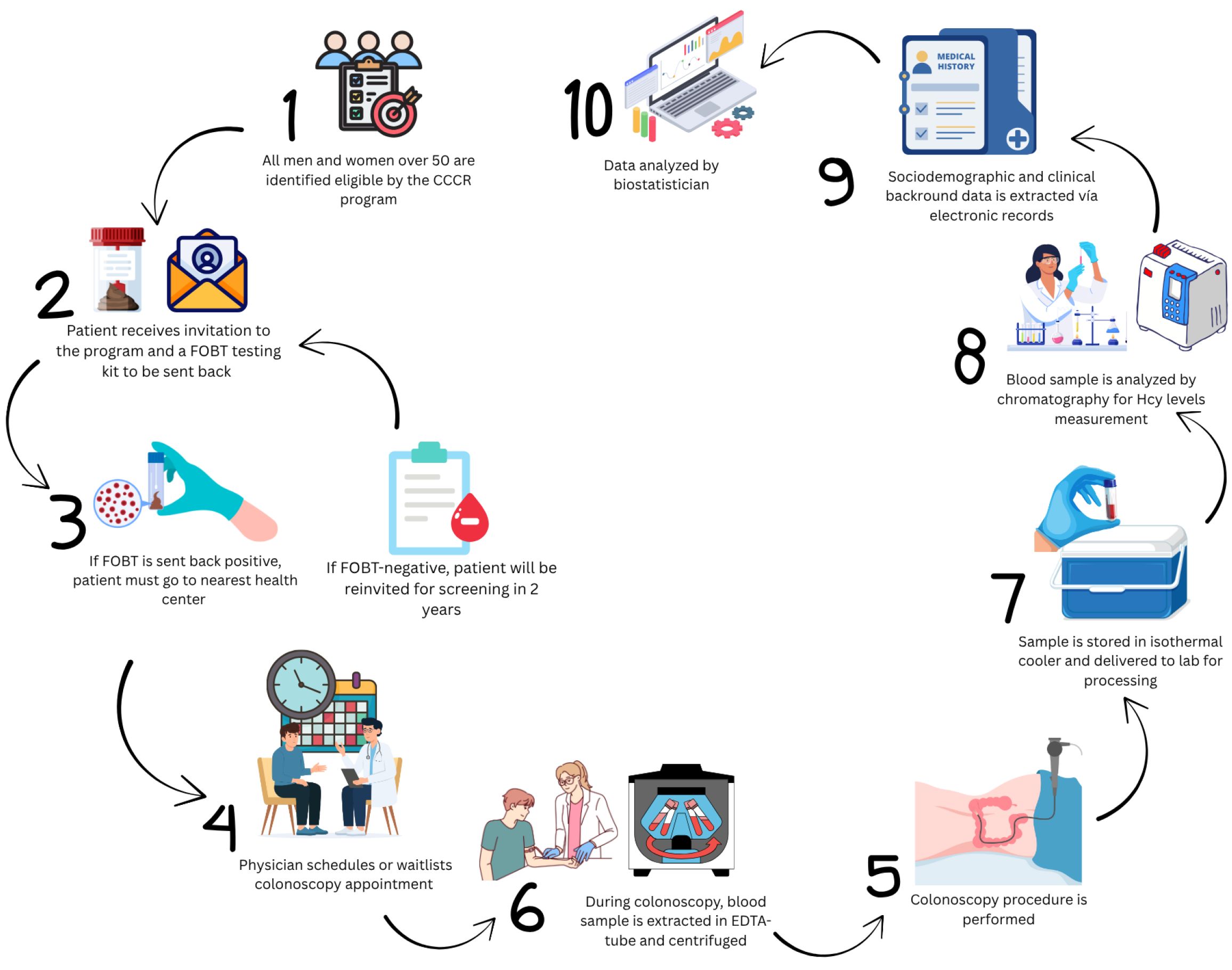
CCCR: The CCCR program is a nationwide preventive intervention that aims at performing periodical screenings for people at risk of CRC. The program identifies and invites all men and women between 50 and 69 years old for FOBT by mail. Individuals who accepted participation were mailed with a fecal occult immunochemical test kit that must be returned to the health system. If the result was negative, the patient was informed by confidential mail that they should test again in two years. If a patient’s result was positive, the patient was called for an appointment to be enrolled in the screening colonoscopy waiting list. Figure 1 summarizes the procedure used in this study.
Blood sample analysis: Before the colonoscopy procedure, a sample of 10 cc of peripheral venous blood was extracted. This extraction was performed at a puncture site different from the venous route used for sedation administration. Plasma samples were collected in tubes with ethylenediaminetetraacetic acid and immediately cold-centrifuged using the Allegra™ 6R centrifuge. The plasma was then stored in aliquots of 0.5 mL at -80 °C.
The blood samples were transferred and processed at the clinical analysis service of the Central University Hospital of Asturias in isothermal containers, strictly maintaining the cold chain at -80 °C. Plasma Hcy levels were determined by reversed-phase high-performance liquid chromatography using the breeze waters chromatograph equipment. The determination included derivatization with 7-fluorobenzene-2-oxa-1,3-diazol-4-ammonium sulfonate, and detection was performed by fluorescence, guaranteeing accuracy in the analysis.
Technical considerations: Statistically significant results were considered in those with a P value less than 0.05. The data analysis was carried out using R v4.0 (R Foundation for Statistical Computing; Vienna, Austria).
Sample size: Calculation was based on the need to compare two means, with a 95% confidence interval (95%CI) and a statistical power of 90%, resulting in a minimum sample of 258 cases. The population (n) was defined based on CRC incidence data from the Spanish Network of Cancer Registries (REDECAN), which reported 44231 cases in 2020.
Descriptive statistics: Continuous variables were described by means (SD) or median (IQR), in agreement to their statistical distribution according to the Kolmogorov-Smirnov normality test. Categorical variables were described by frequencies (%).
Inferential statistics: The Pearson’s χ2 or exact Fisher’s test was applied to evaluate associations for categorical variables. For quantitative variables, Mann-Whitney and Kruskal-Wallis U tests were applied for two or more group comparisons, respectively, including ad hoc Bonferroni correction to adjust for multiplicity and type I errors. Diagnostic accuracy of Hcy was calculated in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and observed agreement. Hcy Optimal cut-off points were obtained through ROC analysis and Youden’s index.
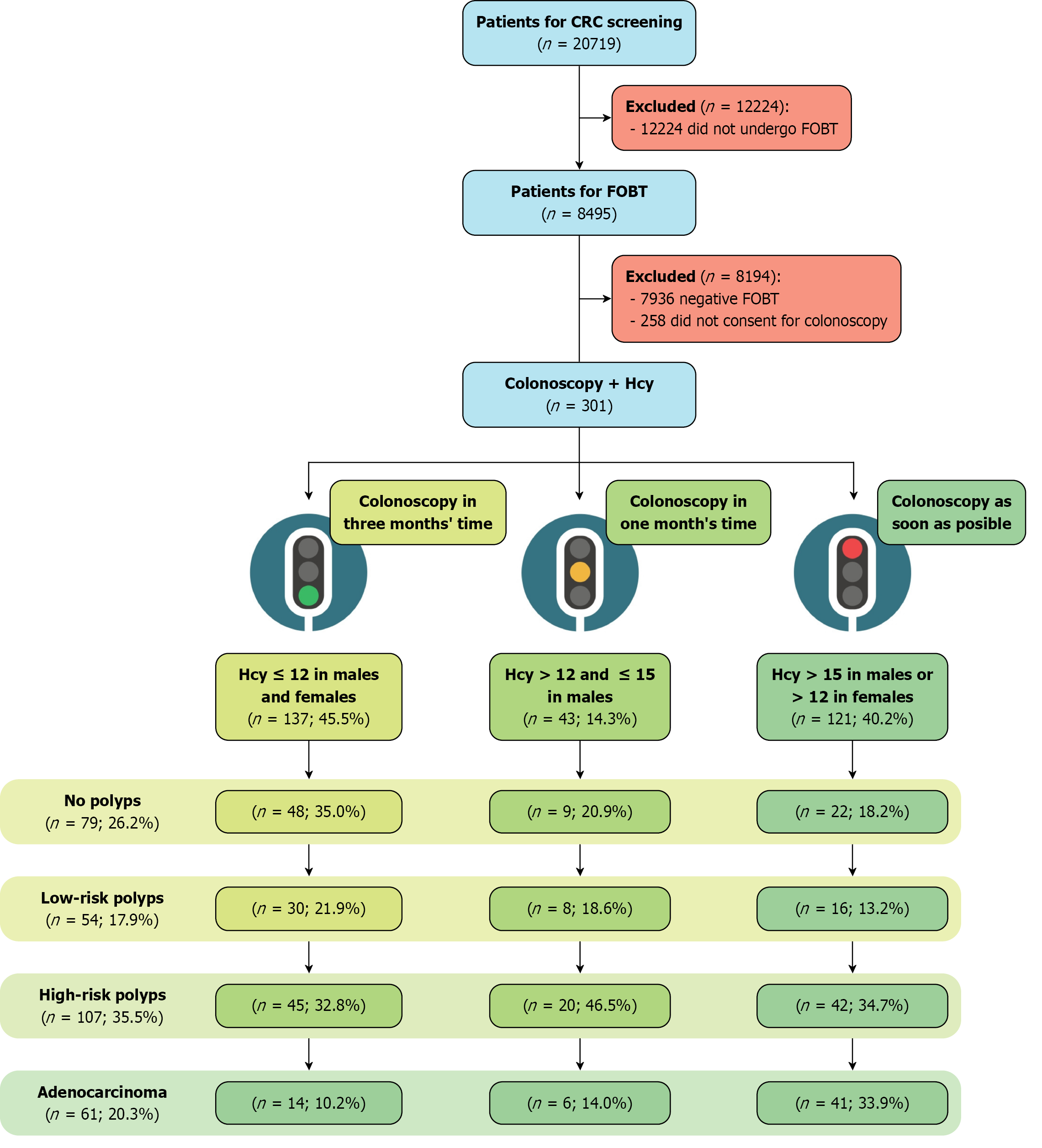
In 2018, the Asturias principality invited 20719 individuals by mail to participate in the program via mail. A total of 13515 participants did not respond, leaving 8495 participants who submitted fecal samples for FOBT. Among them, 629 were eligible for colonoscopy; however, 328 could not be enrolled because of non-attendance or inconclusive FOBT results. A total of 270 patients who underwent diagnostic colonoscopy as part of the screening program at San Agustín de Avilés University Hospital between November 2018 and June 2019 were included in this study. Furthermore, 31 patients diagnosed with CRC based on CT findings and requiring colonoscopy confirmation were enrolled. Figure 2 summarizes patient enrollment and cohort classification.
Table 2 shows the baseline and clinical characteristics of 301 patients. The median age of those who had no polyps or low-risk differed significantly from that of those with adenocarcinoma, and the highest proportion of cases was < 65 years old. Similarly, males dominated the sample (56.1%) and had higher proportions of high-risk polyps (70.1%) and adenocarcinoma (62.3%) than females. Analysis of the patients’ clinical histories revealed that smoking was significantly associated with low- and high-risk polyps.
| Total (n = 301) | Colonoscopy findings | P value | Homocysteine | P value | |||||
| No polyps (n = 79) | Low-risk polyps (n = 54) | High-risk polyps (n = 107) | Adenocarcinoma | ≤ 15 (n = 213) | > 15 (n = 88) | ||||
| Age (years), median (IQR) | 63.0 (57.0-67.0) | 61.0 (55.0-65.0) | 61.0 (57.0-65.0) | 62.0 (58.5-67.0) | 69.0 (63.0-79.0) | < 0.0001 | 62.0 (57.0-67.0) | 64.0 (60.0-70.0) | 0.0046 |
| < 65 | 177 (58.8) | 55 (69.6) | 36 (66.7) | 65 (60.7) | 21 (34.4) | 0.0001 | 131 (61.5) | 46 (52.3) | 0.1767 |
| ≥ 65 | 124 (41.2) | 24 (30.4) | 18 (33.3) | 42 (39.3) | 40 (65.6) | 82 (38.5) | 42 (47.7) | ||
| Sex | 0.0001 | 0.0003 | |||||||
| Female | 132 (43.9) | 48 (60.8) | 29 (53.7) | 32 (29.9) | 23 (37.7) | 108 (50.7) | 24 (27.3) | ||
| Male | 169 (56.1) | 31 (39.2) | 25 (46.3) | 75 (70.1) | 38 (62.3) | 105 (49.3) | 64 (72.7) | ||
| Alcohol intaking | 73 (24.3) | 14 (17.7) | 16 (29.6) | 24 (22.4) | 19 (31.1) | 0.2162 | 49 (23.0) | 24 (27.3) | 0.5235 |
| Smoking | 63 (20.9) | 11 (13.9) | 14 (25.9) | 31 (29.0) | 7 (11.5) | 0.0139 | 40 (18.8) | 23 (26.1) | 0.2036 |
| Diabetes | 45 (15.0) | 12 (15.2) | 4 (7.4) | 15 (14.0) | 14 (23.0) | 0.1349 | 29 (13.6) | 16 (18.2) | 0.4049 |
| Hypertension | 118 (39.2) | 28 (35.4) | 17 (31.5) | 47 (43.9) | 26 (42.6) | 0.3735 | 80 (37.6) | 38 (43.2) | 0.4359 |
| Obesity | 95 (31.6) | 26 (32.9) | 19 (35.2) | 33 (30.8) | 17 (27.9) | 0.8481 | 67 (31.5) | 28 (31.8) | 1.0000 |
| Dyslipidemia | 139 (46.2) | 40 (50.6) | 28 (51.9) | 47 (43.9) | 24 (39.3) | 0.4411 | 104 (48.8) | 35 (39.8) | 0.1916 |
| Coronaropathy | 23 (7.6) | 5 (6.3) | 3 (5.6) | 8 (7.5) | 7 (11.5) | 0.6598 | 14 (6.6) | 9 (10.2) | 0.3970 |
| Chronic kidney injury | 11 (3.7) | 2 (2.5) | 1 (1.9) | 4 (3.7) | 4 (6.6) | 0.5979 | 4 (1.9) | 7 (8.0) | 0.0168 |
| Family history of CRC | 13 (4.3) | 4 (5.1) | 3 (5.6) | 5 (4.7) | 1 (1.6) | 0.7309 | 9 (4.2) | 4 (4.5) | 1.0000 |
| Colonoscopy findings | No significant connection | < 0.0001 | |||||||
| No polyps | 79 (26.2) | 79 (100.0) | - | - | - | 66 (31.0) | 13 (14.8) | ||
| Low-risk polyps | 54 (17.9) | - | 54 (100.0) | - | - | 46 (21.6) | 8 (9.1) | ||
| High-risk polyps | 107 (35.5) | - | - | 107 (100.0) | - | 73 (34.3) | 34 (38.6) | ||
| Adenocarcinoma | 61 (20.3) | - | - | - | 61 (100.0) | 28 (13.1) | 33 (37.5) | ||
| Medication associated with increased Hcy | 118 (39.2) | 35 (44.3) | 19 (35.2) | 40 (37.4) | 24 (39.3) | 0.7109 | 81 (38.0) | 37 (42.0) | 0.6034 |
| Medication associated with decreased Hcy | 15 (5.0) | 4 (5.1) | - | 6 (5.6) | 5 (8.2) | 0.1920 | 5 (2.3) | 10 (11.4) | 0.0023 |
| Homocysteine (µmol/L), median (IQR) | 12.5 (10.8-15.5) | 11.5 (9.74-13.5) | 11.8 (10.8-13.3) | 12.5 (10.9-16.0) | 15.3 (12.4-19.3) | < 0.0001 | 11.3 (10.1-12.6) | 17.7 (16.1-21.8) | n/c |
| Homocysteine | < 0.0001 | n/c | |||||||
| ≤ 15 | 213 (70.8) | 66 (83.5) | 46 (85.2) | 73 (68.2) | 28 (45.9) | 213 (100.0) | - | ||
| > 15 | 88 (29.2) | 13 (16.5) | 8 (14.8) | 34 (31.8) | 33 (54.1) | - | 88 (100.0) | ||
As for Hcy levels, Table 2 shows how the median increases in accordance with the severity of lesions, with the group with adenocarcinoma showing the highest levels (P < 0.0001). In connection, patients who had no polyps or low-risk lesions were associated with Hcy < 15 μmol/L. Differences were also found according to sex, where females had Hcy levels < 15 μmol/L (50.7%) as compared to males who predominantly had Hcy > 15 μmol/L (72.7%; P < 0.0003). A small proportion (8%) of cases with CKD were found to have Hcy levels > 15 μmol/L (P < 0.0168), and most presented with risk lesions or adenocarcinoma. Similarly, the proportion of patients who took medication related to decreased Hcy levels was significantly associated with Hcy > 15 μmol/L.
Table 3 presents the characteristics of the 873 colorectal samples collected, of which 84 samples presented with no polyps. Most lesions were found in the left colon (69.3%), where high-risk polyps (88.6%) and adenocarcinomas (98.4%) tended to be larger (≥ 10 mm). Regarding morphology, 479 Low-risk polyps presented an Is pattern, whereas adenocarcinomas were predominant in the lateral spread tumor (LST) group (64.5%). As for histology, 74.9% of low-risk polyps were identified as tubular adenomas in similar fashion to high-risk polyps (51.9%).
| Total (n = 873) | No polyps (n = 84) | Low-risk polyps (n = 542) | High-risk polyps (n = 185) | Adenocarcinoma (n = 62) | |
| Location | |||||
| Left colon | 552 (69.3) | 5 (62.5) | 353 (65.1) | 146 (78.9) | 48 (77.4) |
| Right colon | 245 (30.7) | 3 (37.5) | 189 (34.9) | 39 (21.1) | 14 (22.6) |
| Size (mm) | |||||
| < 10 | 570 (71.5) | 6 (75.0) | 542 (100.0) | 21 (11.4) | 1 (1.6) |
| ≥ 10 | 227 (28.5) | 2 (25.0) | - | 164 (88.6) | 61 (98.4) |
| Paris classification | |||||
| Is | 554 (69.5) | 7 (87.5) | 479 (88.4) | 65 (35.1) | 3 (4.8) |
| Ip | 82 (10.3) | 1 (12.5) | 20 (3.7) | 59 (31.9) | 2 (3.2) |
| Isp | 48 (6.0) | - | 20 (3.7) | 26 (14.1) | 2 (3.2) |
| IIa | 36 (4.5) | - | 16 (3.0) | 20 (10.8) | - |
| IIb | 17 (2.1) | - | 7 (1.3) | 10 (5.4) | - |
| IIc | 8 (1.0) | - | - | 2 (1.1) | 6 (9.7) |
| IIa + IIc | 7 (0.9) | - | - | 3 (1.6) | 4 (6.5) |
| IIc + IIa | 5 (0.6) | - | - | - | 5 (8.1) |
| Lateral spread tumor | 40 (5.0) | - | - | - | 40 (64.5) |
| Histology | |||||
| Adenocarcinoma | 62 (7.8) | - | - | - | 62 (100.0) |
| Tubulovillous adenoma | 77 (9.7) | - | - | 77 (41.6) | - |
| Tubular adenoma | 502 (63.0) | - | 406 (74.9) | 96 (51.9) | - |
| Serrated adenoma | 3 (0.4) | - | 2 (0.4) | 1 (0.5) | - |
| Hyperplastic | 90 (11.3) | - | 86 (15.9) | 4 (2.2) | - |
| Non-retrieved specimen | 44 (5.5) | - | 41 (7.6) | 3 (1.6) | - |
| Non-resected specimen | 11 (1.4) | - | 7 (1.3) | 4 (2.2) | - |
| Inflammatory polyp | 5 (0.6) | 5 (62.5) | - | - | - |
| Lipoma | 1 (0.1) | 1 (12.5) | - | - | - |
| Normal colonic tissue | 2 (0.3) | 2 (25.0) | - | - | - |
| Colonoscopy without findings | 76 (8.7) | 76 (90.5) | - | - | - |
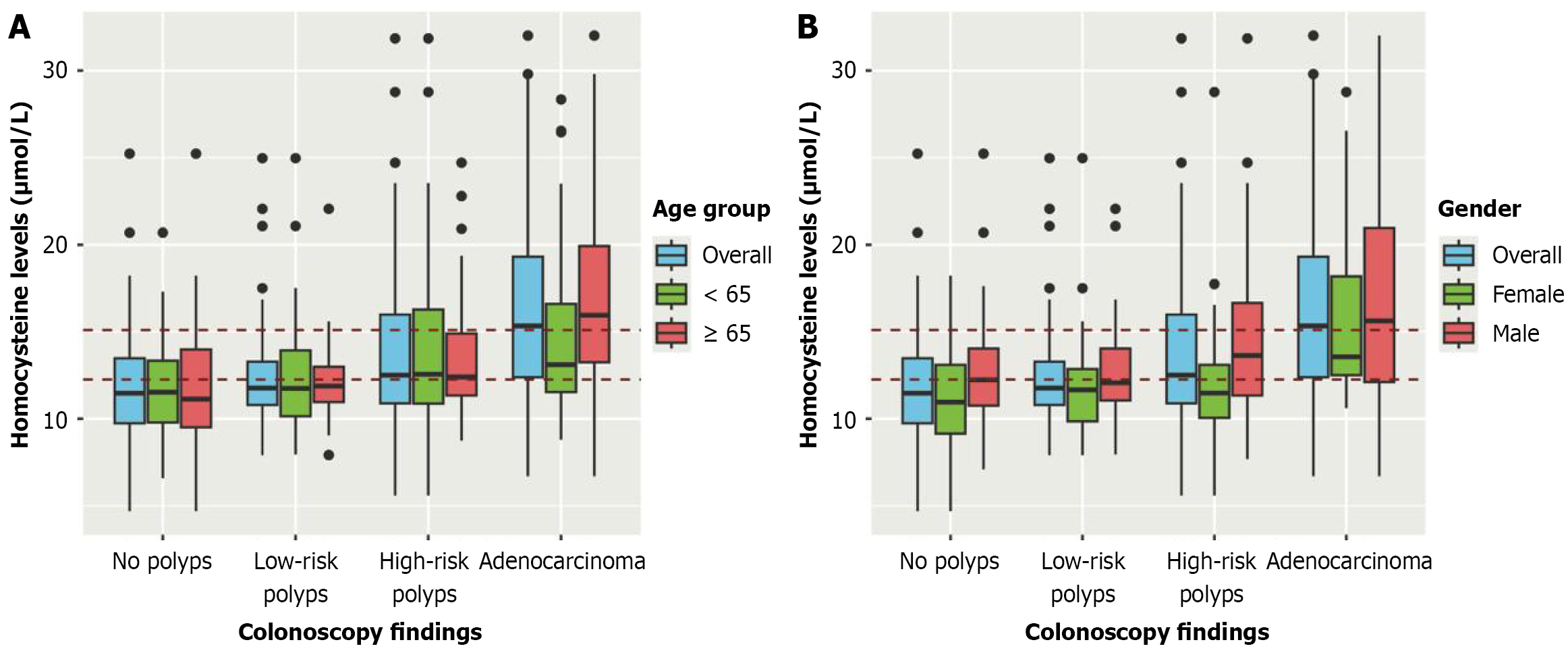
In Figure 3, red dashed lines represent Hcy cut-off values at 12 μmol/L and 15 μmol/L, respectively. Figure 3A shows the differences in Hcy levels. For low-risk and high-risk polyps, the median of Hcy was close to the established cut-off of 12 μmol/L; nonetheless, greater variability in IQR and outliers were more frequent in high-risk lesions, particularly for patients < 65. In stark contrast, for adenocarcinoma lesions, Hcy levels surpassed the lowest cut-off, and it was noted that, particularly, serum Hcy rose significantly for people > 65 years old.
Similar to age, Figure 3B reveals that Hcy levels by sex increase as lesions evolve, with males exhibiting higher serum Hcy levels than females across all groups. It was also noted that females have similar Hcy levels for low- and high-risk lesions, with little variability compared to that of the males; however, for adenocarcinomas, most male and female cases were well above the 12 μmol/L cut-off, but male Hcy levels had a higher median and variability in comparison.
In our multivariate model (Table 4), age [OR: 1.12 (95%CI: 1.06-1.17)], male sex [OR: 4.03 (95%CI: 1.48-11.1)], and higher serum Hcy [OR: 1.12 (95%CI: 1.06-1.19)], were independently associated with increased odds of presence of risk-lesions or adenocarcinoma. Similarly, an interaction was observed between male sex [OR: 1.12 (95%CI: 1.06-1.17)] and Hcy levels, indicating a strong relationship between these. No other clinical variables or medication interactions were significant.
| OR | 95%CI | P value | |
| Age (years) | 1.120 | 1.06-1.17 | < 0.001 |
| Sex (male) | 4.030 | 1.48-11.1 | 0.007 |
| Alcohol intaking | 1.060 | 0.74-1.52 | 0.7 |
| Smoking | 1.210 | 0.83-1.76 | 0.3 |
| Diabetes | 1.170 | 0.73-1.88 | 0.5 |
| Hypertension | 0.950 | 0.68-1.33 | 0.8 |
| Obesity | 0.960 | 0.69-1.34 | 0.8 |
| Dyslipidemia | 0.760 | 0.54-1.05 | 0.1 |
| Coronaropathy | 0.940 | 0.52-1.73 | 0.8 |
| Homocysteine (µmol/L) | 1.120 | 1.06-1.19 | < 0.001 |
| Age (≥ 65 years old) × Hcy | 0.980 | 0.94-1.03 | 0.5 |
| Sex (male) × Hcy | 0.920 | 0.86-0.99 | 0.035 |
| Medication associated with increased Hcy × Hcy | 1.000 | 0.97-1.02 | 0.7 |
| Medication associated with decreased Hcy × Hcy | 0.990 | 0.95-1.03 | 0.7 |
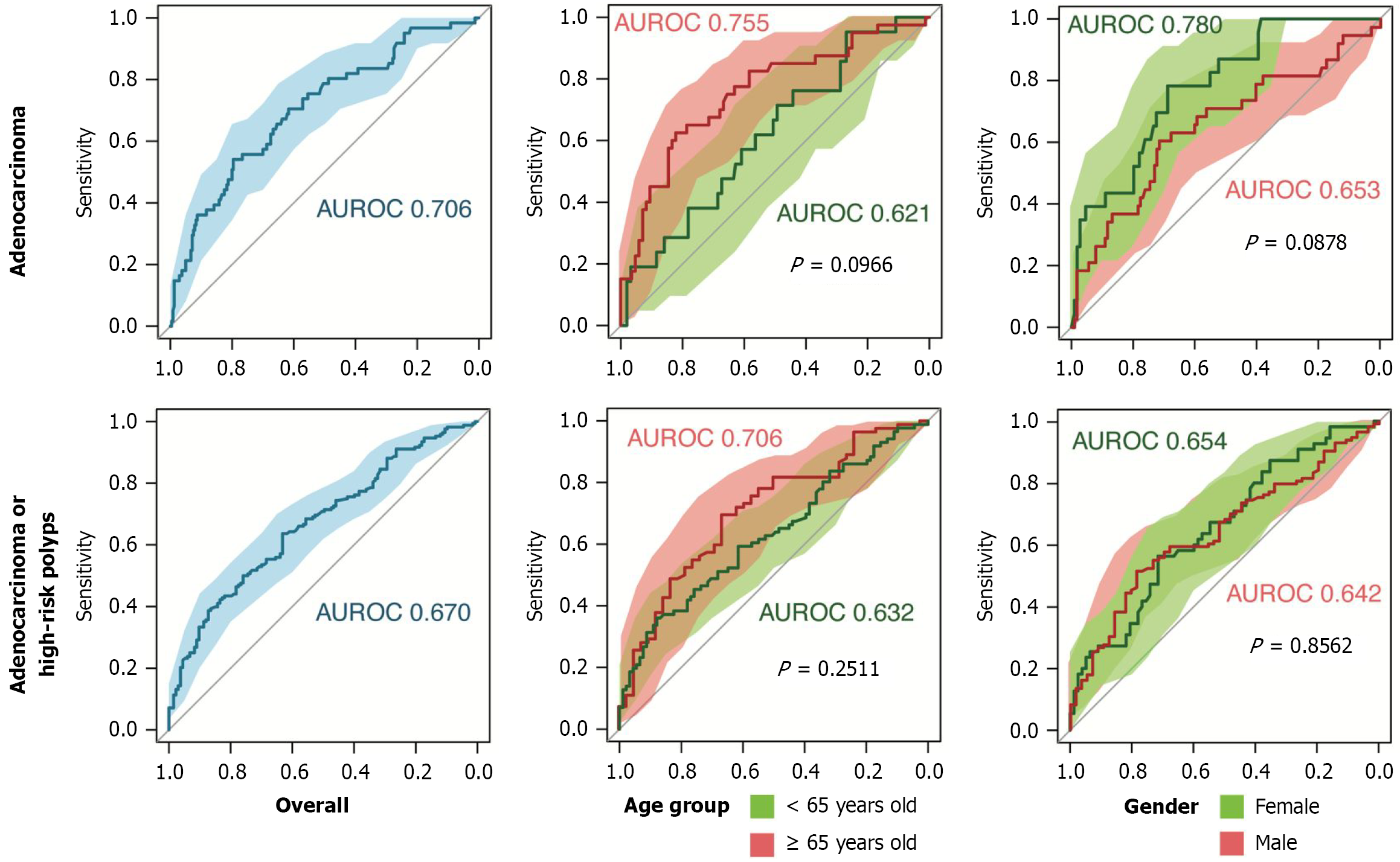
Table 5 illustrates the diagnostic performance of serum Hcy levels at two different cut-off points, adjusted for age and sex, to identify adenocarcinoma alone or in combination with high-risk lesions. Hcy was higher for males (15 μmol/L) than for females (12 μmol/L). Analysis of Hcy ≥ 15 μmol/L revealed overall good sensitivity (79.58%) in identifying adenocarcinoma, with a PPV of 87.21%. A higher sensitivity was observed in male patients (71.76%) than in female patients (68.81%). Figure 4 shows the area under the receiver operating characteristic curve (AUROC) for adenocarcinoma (0.706), indicating fair discrimination. Hcy improved the predictive power, reaching 82.14% sensitivity, for cases ≥ 65 years old.
| Hcy cut-off value | Sensitivity | Specificity | PPV | NPV | Observed agreement | AUROC | |
| Adenocarcinoma | |||||||
| Overall | ≥ 15.1 | 191/240; 79.58 (73.92-84.5) | 33/61; 54.1 (40.85-66.94) | 191/219; 87.21 (82.05-91.33) | 33/82; 40.24 (29.56-51.66) | 224/301; 74.42 (69.1-79.25) | 0.706 (0.631-0.781) |
| < 65 years | ≥ 15.1 | 122/156; 78.21 (70.9-84.41) | 8/21; 38.1 (18.11-61.56) | 122/135; 90.37 (84.1-94.77) | 8/42; 19.05 (8.6-34.12) | 130/177; 73.45 (66.3-79.79) | 0.621 (0.496-0.747) |
| ≥ 65 years | ≥ 15.18 | 69/84; 82.14 (72.26-89.65) | 25/40; 62.5 (45.8-77.27) | 69/84; 82.14 (72.26-89.65) | 25/40; 62.5 (45.8-77.27) | 94/124; 75.81 (67.3-83.04) | 0.755 (0.660-0.850) |
| Females | ≥ 12.5 | 75/109; 68.81 (59.22-77.34) | 18/23; 78.26 (56.3-92.54) | 75/80; 93.75 (86.01-97.94) | 18/52; 34.62 (21.97-49.09) | 93/132; 70.45 (61.89-78.07) | 0.780 (0.683-0.877) |
| Males | ≥ 15.1 | 94/131; 71.76 (63.23-79.27) | 23/38; 60.53 (43.39-75.96) | 94/109; 86.24 (78.32-92.09) | 23/60; 38.33 (26.07-51.79) | 117/169; 69.23 (61.68-76.09) | 0.653 (0.546-0.761) |
| Adenocarcinoma or high-risk polyps | |||||||
| Overall | ≥ 12.26 | 84/133; 63.16 (54.36-71.35) | 107/168; 63.69 (55.93-70.96) | 84/145; 57.93 (49.46-66.07) | 107/156; 68.59 (60.68-75.78) | 191/301; 63.46 (57.74-68.91) | 0.670 (0.609-0.730) |
| < 65 years | ≥ 15.1 | 80/91; 87.91 (79.4-93.81) | 31/86; 36.05 (25.97-47.12) | 80/135; 59.26 (50.47-67.63) | 31/42; 73.81 (57.96-86.14) | 111/177; 62.71 (55.14-69.85) | 0.632 (0.551-0.714) |
| ≥ 65 years | ≥ 12.16 | 28/42; 66.67 (50.45-80.43) | 57/82; 69.51 (58.36-79.2) | 28/53; 52.83 (38.64-66.7) | 57/71; 80.28 (69.14-88.78) | 85/124; 68.55 (59.6-76.59) | 0.706 (0.610-0.802) |
| Females | ≥ 12.46 | 55/77; 71.43 (60-81.15) | 31/55; 56.36 (42.32-69.7) | 55/79; 69.62 (58.25-79.47) | 31/53; 58.49 (44.13-71.86) | 86/132; 65.15 (56.37-73.23) | 0.654 (0.559-0.748) |
| Males | ≥ 14.48 | 44/56; 78.57 (65.56-88.41) | 58/113; 51.33 (41.74-60.84) | 44/99; 44.44 (34.45-54.78) | 58/70; 82.86 (71.97-90.82) | 102/169; 60.36 (52.56-67.78) | 0.642 (0.557-0.727) |
Serum Hcy at ≥ 12 μmol/L moderately detected both high-risk lesions and adenocarcinoma, with an overall sensitivity of 63.16% and the AUROC slightly lower (0.670), with a modest improvement in patients > 65 (0.706). Mild differences in performance capability were also observed between males (AUROC: 0.642; sensitivity: 78.57%) and females (AUROC: 0.654; sensitivity: 71.43%). In terms of predictive value, the overall PPV for these groups was 57.93%, reflecting a better reliability in females (69.62%) than in males (44.4%). The overall specificity for adenocarcinoma alone was moderate (54.1%), and the NPV was 40.24%, indicating a moderate likelihood of false negatives at this threshold.
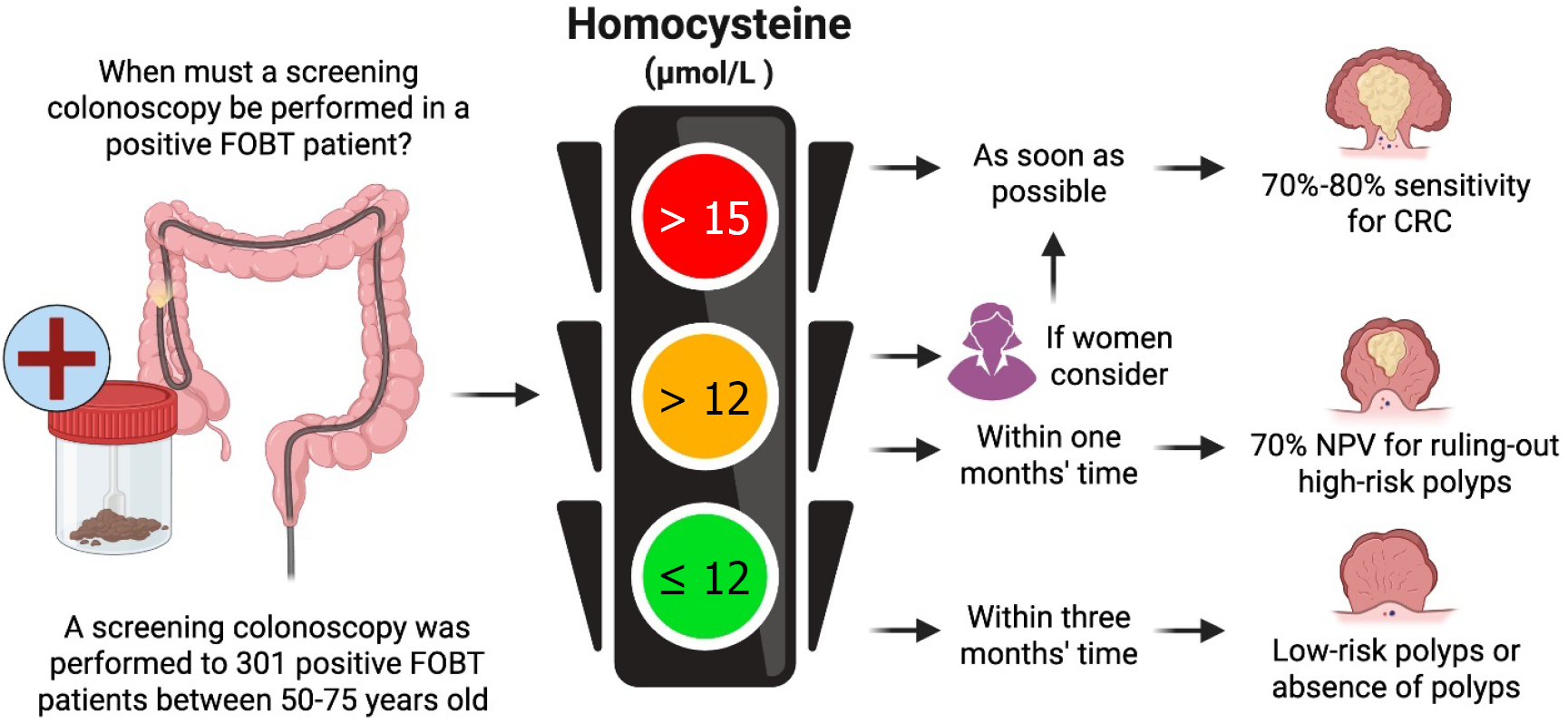
Figure 5 shows an effective visual representation that simplifies risk stratification and conveys the urgency levels for faster colonoscopy scheduling based on plasma Hcy levels, age, and sex. This approach facilitates clinical decision-making and helps clinicians prioritize patients who require immediate intervention and those who can wait without compromising their health. Traffic light levels are described as follows.
Red level (> 15.1 μmol/L in males and > 12 μmol/L in females): This level reflects a high urgency for immediate scheduling of a colonoscopy intervention in both sexed. But higher priority should be provided if the patient is female and > 65.
Yellow level (> 12 μmol/L and ≤ 15 μmol/L in males): Although the risk is lower than in the red level, there is still a considerable likelihood of risk-lesions or adenocarcinoma, which warrant a colonoscopy in the short term, in which males ≤ 65 with Hyc < 15 μmol/L have a better NPV (82.86%) than females, warranting the former to be scheduled within a month for intervention and the latter for urgent colonoscopy.
Green level (≤ 12 μmol/L in males and females): Over 83% of samples with low-risk polyps or no polyps at all were stratified at this Hcy level, reflecting a low probability of injury, and allowing the colonoscopy to be scheduled up to three months in both sexes without compromising the patient’s health.
The findings of this study introduce and validate the potential of Hcy as a triage instrument to be considered in the nationwide Spanish CCCR program for FOBT-positive patients, to prioritize faster scheduling of colonoscopy screening for those at the highest risk. Thus, the innovative traffic light triage system proposed in this study uses a composite triad of Hcy, age, and sex to provide a robust risk profile. This system could be useful in improving waiting times and response capacity by the health system, as Spanish guidelines reinforce that a 6-week delay of colonoscopy intervention in FOBT-positive patients is harmful[10,16], this recommendation is supported by Forbes et al’s systematic review[17] which analyzed a significant increase in CRC incidence in patients who underwent colonoscopy 6 months, 9 months, 10 months or 12 months after the FOBT-positive test. Furthermore, our findings are consistent with evidence linking elevated Hcy with the presence of risk lesions and adenocarcinoma, highlighting how pathological increase in free serum Hcy plays a mechanistic role in cellular dysregulation and carcinogenesis[2,3,5,18].
Previous studies have analyzed alterations in Hcy levels due to multimorbidity and medication, in which metabolic pathways can be disrupted due to chronic inflammatory states, vitamin deficiencies or decreased dietary intake of methionine that increase metabolic demands and biochemical processes[6,7,13,19]. In this study, most at-risk patients had over three chronic conditions, as well as polypharmacy, compounded by the current inflammatory state. Among these, CKD patients were more likely to have homocysteinemia, of which 7/11 stage II cases had serum Hcy > 15 μmol/L; in these cases, the reduced renal clearance compounded by deficiencies in vitamins B6, B9 and B2 could have led to Hcy accumulation, promoting endothelial dysfunction, oxidative stress and heightened cardiovascular risk[13,19]. These potential confounders were included in the logistic regression model, justifying age and sex as the only significant characteristics in the composite diagnostic accuracy AUROC model.
Variability was observed in homocysteinemia according to age, with higher levels detected in patients ≥ 65 years. This observation aligned with anticipated physiological changes in renal clearance associated with advancing age. These patients typically present with vitamin deficiencies and chronic conditions that can further increase serum Hcy levels[13,14]. Sex differences in Hcy levels were also observed, an observation consistent with existing literature[20,21]. Males exhibited higher levels of Hcy across all lesion types as compared to females. Logistic regression revealed that men had more than four times the odds for presence of risk-lesions or adenocarcinoma, independent of age and Hcy levels. However, while Hcy concentrations were higher in men, the clinical meaning of elevated Hcy is more pronounced in women. In particular, Hcy levels 12-15 were already associated with substantially increased likelihood of adenocarcinoma in females, while in males this range more often corresponded to high-risk polyps. This sex-dependent pattern reflects known differences in responses to oxidative stress and methylation efficiency, partially mediated by estrogen’s effect on Hcy remethylation and clearance[13,18,22,23].
A predominance of high-risk polyps and adenocarcinoma were found in the left colon, consistent with prior associations between left-sided lesions and adenocarcinoma[24]. However, over 40% of right-sided lesions in the sample were also classified as high-risk, as reported in a similar Spanish study by Álvarez-Delgado et al[8], highlighting the importance of evaluating the entire colon as also supported by colonoscopy quality guidelines[15]. Regarding lesion size, our findings align with previous evidence that smaller lesions (97.7%) are typically indicators of no or low risk, with only one adenocarcinoma and 21 at-risk polyps identified, whereas larger polyps were mostly high-risk, with 87.9% classified as precancerous and 98.4% as adenocarcinomas. In terms of morphology based on the Paris classification, Is-type lesions were most common in low-risk cases (33.9%), while Ip (32.8%) and LST (64.5%) patterns were associated with high-risk lesions and adenocarcinomas, consistent with similar studies and warrant prompt intervention according to Spanish guidelines[3,10]. Though less frequent overall, IIc and IIa/IIc morphologies were mostly observed in risk lesions and adenocarcinomas. Histologically, findings aligned with previous investigations[5,10,25]. In our sample, tubular adenomas represented the most frequent subtype (63.9%) and were mainly found in low-risk lesions (55.2%). None of the adenocarcinomas showed tubular adenoma histology, while tubulovillous adenomas were more common in high-risk lesions (44.3%).
While our model shows strong internal validity within the Spanish CRC screening context, its generalizability to other populations should be interpreted with caution. Future research is warranted in neighboring European countries that implement similar FOBT-to-colonoscopy screening programs to stablish contextual Hcy thresholds[1,26]. Examples include the United Kingdom, Ireland, and Italy as sites that could potentially benefit from this tool in prioritizing patients and improving resource allocation, as these national programs do not account for clinical or demographic risk stratification. Although earlier investigations combined Hcy with folate and vitamins B6/B12[27-30], we selected Hcy alone, because the marker already captures the combined metabolic, nutritional, and inflammatory variables that would otherwise require multiple testing. Adding micronutrient testing to a population-level programme would raise costs and logistical complexity, whereas Hcy provides an integrated signal of dysregulation and oxidative stress[6,31-33]. Its robustness was evident in our cohort, where elevated levels of Hcy persisted even among patients receiving medication that ordinarily would lower it. We therefore reinforce that Hcy combined with age and sex offers a biologically grounded, cost-conscious triage tool for public health systems in which simplicity and feasibility are paramount. Nonetheless, we recognize that future multicenter work should explore whether adding micronutrient markers yields increased benefit in diverse screening settings.
This study demonstrated that elevated serum Hcy levels are significantly associated with the presence of high-risk colorectal lesions and adenocarcinoma in FOBT-positive patients. When combined with age and sex, Hcy level improved the accuracy of risk stratification, supporting its use as a practical adjunct in CRC screening programs. Notably, moderate elevations 12-15 were already linked to malignancy in women, while men exhibited a more linear Hcy-risk gradient, and older adults showed higher predictive sensitivity. Based on these findings, we propose a clinically actionable three-tiered traffic light triage system that integrates age and sex to prioritize colonoscopy scheduling. This system offers a low cost biologically grounded tool for improving risk-stratification and resource allocation in CRC screening. Its simplicity and scalability make it particularly suited for public health programs facing endoscopic capacity constraint. Future validation across broader populations is warranted to support its integration into routine screening protocols.
The author expresses sincere gratitude to the “Consejería de Sanidad del Principado de Asturias” for collaborating with biochemical analyses, including granting access to laboratory facilities and reagents. Acknowledgments are also extended to the Department of Gastroenterology and Clinical Analysis of the Hospital Universitario San Agustín de Asturias for their valuable collaboration, which was essential for the successful completion of this study.
| 1. | Jacobsson M, Wagner V, Kanneganti S. Screening for Colorectal Cancer. Surg Clin North Am. 2024;104:595-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Chen FP, Lin CC, Chen TH, Tsai MC, Huang YC. Higher plasma homocysteine is associated with increased risk of developing colorectal polyps. Nutr Cancer. 2013;65:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Liu Z, Cui C, Wang X, Fernandez-Escobar A, Wu Q, Xu K, Mao J, Jin M, Wang K. Plasma Levels of Homocysteine and the Occurrence and Progression of Rectal Cancer. Med Sci Monit. 2018;24:1776-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Koklesova L, Mazurakova A, Samec M, Biringer K, Samuel SM, Büsselberg D, Kubatka P, Golubnitschaja O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021;12:477-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Sun M, Sun M, Zhang L, Shi S. Colorectal polyp risk is linked to an elevated level of homocysteine. Biosci Rep. 2018;38:BSR20171699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Kathpalia M, Kumar P, Mohapatra S. Homocysteine Metabolism as a Biomarker for Cancer. In: Dubey GP, Misra K, Kesharwani RK, Ojha RP, editors. Homocysteine Metabolism in Health and Disease. Singapore: Springer, 2022. [DOI] [Full Text] |
| 7. | Desouza C, Keebler M, McNamara DB, Fonseca V. Drugs affecting homocysteine metabolism: impact on cardiovascular risk. Drugs. 2002;62:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Álvarez-Delgado A, García MLP, García-González JM, de Sena HI, Chamorro AJ, Gómez MFL, Marcos M, Mirón-Canelo JA. Improvements in the Effectiveness of Early Detection in Colorectal Cancer with Open-Label Randomised Study. J Clin Med. 2021;10:5072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Ran T, Cheng CY, Misselwitz B, Brenner H, Ubels J, Schlander M. Cost-Effectiveness of Colorectal Cancer Screening Strategies-A Systematic Review. Clin Gastroenterol Hepatol. 2019;17:1969-1981.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Cubiella J, Marzo-Castillejo M, Mascort-Roca JJ, Amador-Romero FJ, Bellas-Beceiro B, Clofent-Vilaplana J, Carballal S, Ferrándiz-Santos J, Gimeno-García AZ, Jover R, Mangas-Sanjuán C, Moreira L, Pellisè M, Quintero E, Rodríguez-Camacho E, Vega-Villaamil P; Sociedad Española de Medicina de Familia y Comunitaria y Asociación Española de Gastroenterología. Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 Update. Gastroenterol Hepatol. 2018;41:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C, Lindor NM, Minsky BD, Monzon FA, Sargent DJ, Singh VM, Willis J, Clark J, Colasacco C, Rumble RB, Temple-Smolkin R, Ventura CB, Nowak JA. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Smith AD, Refsum H. Homocysteine - from disease biomarker to disease prevention. J Intern Med. 2021;290:826-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 13. | Ferroni P, Palmirotta R, Martini F, Riondino S, Savonarola A, Spila A, Ciatti F, Sini V, Mariotti S, Del Monte G, Roselli M, Guadagni F. Determinants of homocysteine levels in colorectal and breast cancer patients. Anticancer Res. 2009;29:4131-4138. [PubMed] |
| 14. | Suresh S, Waly MI. Metabolic Role of Hyperhomocysteinemia in the Etiology of Chronic Diseases. In: Waly MI, editor. Nutritional Management and Metabolic Aspects of Hyperhomocysteinemia. Cham: Springer, 2021. [DOI] [Full Text] |
| 15. | Jover R, Herráiz M, Alarcón O, Brullet E, Bujanda L, Bustamante M, Campo R, Carreño R, Castells A, Cubiella J, García-Iglesias P, Hervás AJ, Menchén P, Ono A, Panadés A, Parra-Blanco A, Pellisé M, Ponce M, Quintero E, Reñé JM, Sánchez del Río A, Seoane A, Serradesanferm A, Soriano Izquierdo A, Vázquez Sequeiros E; Spanish Society of Gastroenterology; Spanish Society of Gastrointestinal Endoscopy Working Group. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (5)] |
| 16. | Safont MJ, García-Figueiras R, Hernando-Requejo O, Jimenez-Rodriguez R, Lopez-Vicente J, Machado I, Ayuso JR, Bustamante-Balén M, De Torres-Olombrada MV, Domínguez Tristancho JL, Fernández-Aceñero MJ, Suarez J, Vera R. Interdisciplinary Spanish consensus on a watch-and-wait approach for rectal cancer. Clin Transl Oncol. 2024;26:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Forbes N, Hilsden RJ, Martel M, Ruan Y, Dube C, Rostom A, Shorr R, Menard C, Brenner DR, Barkun AN, Heitman SJ. Association Between Time to Colonoscopy After Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review. Clin Gastroenterol Hepatol. 2021;19:1344-1354.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Dahlin AM, Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Palmqvist R. Plasma vitamin B12 concentrations and the risk of colorectal cancer: a nested case-referent study. Int J Cancer. 2008;122:2057-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Chen W, Feng J, Ji P, Liu Y, Wan H, Zhang J. Association of hyperhomocysteinemia and chronic kidney disease in the general population: a systematic review and meta-analysis. BMC Nephrol. 2023;24:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 20. | Ali I, Högberg J, Hsieh JH, Auerbach S, Korhonen A, Stenius U, Silins I. Gender differences in cancer susceptibility: role of oxidative stress. Carcinogenesis. 2016;37:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Allegra A, Caserta S, Genovese S, Pioggia G, Gangemi S. Gender Differences in Oxidative Stress in Relation to Cancer Susceptibility and Survival. Antioxidants (Basel). 2023;12:1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 22. | Scheppach W, Bingham S, Boutron-Ruault MC, Gerhardsson de Verdier M, Moreno V, Nagengast FM, Reifen R, Riboli E, Seitz HK, Wahrendorf J. WHO consensus statement on the role of nutrition in colorectal cancer. Eur J Cancer Prev. 1999;8:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Wei EK, Giovannucci E, Selhub J, Fuchs CS, Hankinson SE, Ma J. Plasma vitamin B6 and the risk of colorectal cancer and adenoma in women. J Natl Cancer Inst. 2005;97:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Baile-Maxía S, Mangas-Sanjuan C, Sala-Miquel N, Barquero C, Belda G, García-Del-Castillo G, García-Herola A, Penalva JC, Picó MD, Poveda MJ, de-Vera F, Zapater P, Jover R. Incidence, characteristics, and predictive factors of post-colonoscopy colorectal cancer. United European Gastroenterol J. 2024;12:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 25. | Sullivan BA, Noujaim M, Roper J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest Endosc Clin N Am. 2022;32:177-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 26. | Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 956] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 27. | Fu H, He J, Li C, Deng Z, Chang H. Folate intake and risk of colorectal cancer: a systematic review and up-to-date meta-analysis of prospective studies. Eur J Cancer Prev. 2023;32:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Gylling B, Van Guelpen B, Schneede J, Hultdin J, Ueland PM, Hallmans G, Johansson I, Palmqvist R. Low folate levels are associated with reduced risk of colorectal cancer in a population with low folate status. Cancer Epidemiol Biomarkers Prev. 2014;23:2136-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Haas CB, Su YR, Petersen P, Wang X, Bien SA, Lin Y, Albanes D, Weinstein SJ, Jenkins MA, Figueiredo JC, Newcomb PA, Casey G, Le Marchand L, Campbell PT, Moreno V, Potter JD, Sakoda LC, Slattery ML, Chan AT, Li L, Giles GG, Milne RL, Gruber SB, Rennert G, Woods MO, Gallinger SJ, Berndt S, Hayes RB, Huang WY, Wolk A, White E, Nan H, Nassir R, Lindor NM, Lewinger JP, Kim AE, Conti D, Gauderman WJ, Buchanan DD, Peters U, Hsu L. Interactions between folate intake and genetic predictors of gene expression levels associated with colorectal cancer risk. Sci Rep. 2022;12:18852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Martínez ME, Henning SM, Alberts DS. Folate and colorectal neoplasia: relation between plasma and dietary markers of folate and adenoma recurrence. Am J Clin Nutr. 2004;79:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Jakubowski H. Protein N-Homocysteinylation and Colorectal Cancer. Trends Cancer. 2019;5:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Ribeiro ML, Priolli DG, Miranda DD, Arçari DP, Pedrazzoli J Jr, Martinez CA. Analysis of oxidative DNA damage in patients with colorectal cancer. Clin Colorectal Cancer. 2008;7:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, Halašová E, Lehotský J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int J Mol Sci. 2016;17:1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/