Published online Jun 14, 2025. doi: 10.3748/wjg.v31.i22.105157
Revised: April 20, 2025
Accepted: May 26, 2025
Published online: June 14, 2025
Processing time: 150 Days and 0.2 Hours
Hepatic ischemia-reperfusion (I/R) injury related to liver transplantation and hepatic resection remains a challenge in clinical practice. Accumulating evidence indicates that mitochondrial dysfunction is a critical cause of I/R injury. The protein 4-nitrophenylphosphatase domain and non-neuronal SNAP25-like protein homolog 1 (NIPSNAP1) is involved in the regulation of mitophagy and the recruitment of autophagy receptor proteins independent of PTEN induced putative kinase 1.
To clarify the protective mechanism of NIPSNAP1 against hepatic I/R, with a focus on mitophagy and mitochondrial dynamics, as well as the potential mechanism by which n6-methyladenosine (m6A) modification regulates NIPSNAP1.
Mice were administered an adeno-associated virus in vivo and a hepatic I/R model was established via portal vein interruption followed by reperfusion to explore the effect of NIPSNAP1 on hepatic I/R. HepG2 cells were subjected to hypoxia/reoxygenation treatment in vitro.
We observed a significant downregulation of both NIPSNAP1 and insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) expression in vivo and in vitro. NIPSNAP1 knockdown impaired mitophagy and disrupted mitochondrial dynamics; in contrast, NIPSNAP1 overexpression resulted in the opposite effects. Further studies revealed that IGF2BP2 functions as an m6A reader that targets and binds NIPSNAP1, thereby regulating its mRNA stability.
NIPSNAP1 prevents hepatic I/R injury by promoting mitophagy and maintaining mitochondrial homeostasis, serving as a novel target of the m6A reader IGF2BP2. Therefore, targeting the IGF2BP2/NIPSNAP1 axis may facilitate the development of better therapeutics for hepatic I/R.
Core Tip: We confirmed the effect of 4-nitrophenylphosphatase domain and non-neuronal SNAP25-like protein homolog 1 (NIPSNAP1) as a new target that controls mitophagy and mitochondrial dynamics in hepatic ischemia-reperfusion. Knockdown of NIPSNAP1 impaired mitophagy and disrupted mitochondrial dynamics, as evidenced by increased mitochondrial fission and reduced fusion. Further studies revealed that interacted with insulin-like growing factor 2 acts as an n6-methyladenosine reader to bind NIPSNAP1 mRNA and enhance its stability, thereby regulating NIPSNAP1 expression. These results suggest that the interacted with insulin-like growing factor 2/NIPSNAP1 axis may be a promising target for hepatic ischemia-reperfusion.
- Citation: Guo SS, Zhao Y, Hu Y, Wang XY, Zhao XZ, Zhong PY, Luan QR, Wang ZC, Yao JH. N6-methyladenosine reader IGF2BP2 regulates NIPSNAP1-mediated mitophagy and mitochondrial dynamics to alleviate hepatic ischemia-reperfusion injury. World J Gastroenterol 2025; 31(22): 105157
- URL: https://www.wjgnet.com/1007-9327/full/v31/i22/105157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i22.105157
Hepatic ischemia-reperfusion (I/R) injury is an unavoidable complication during liver resection and liver transplantation, which can directly lead to early graft failure, organ damage, acute and chronic tissue rejection and even liver failure, with high morbidity and mortality[1]. Currently, we lack efficient measures to address I/R injury in clinical practice. Efforts are therefore being made to elucidate the pathogenic mechanisms of I/R injury to prevent and minimize its consequences.
Although hepatic I/R is caused by a variety of intricate mechanisms, including inflammatory responses, apoptosis, autophagy, abnormal energy metabolism, and oxidative stress, mitochondrial dysfunction is still a cardinal event that leads to hepatic I/R damage[2,3]. Mitochondrial dysfunction results in bioenergy depletion, reactive oxygen species production, and cell death[4]. It is therefore highly important to maintain a healthy and functional mitochondrial network. Mitochondrial fusion and fission can separate impaired mitochondria and maintain the relative balance of mitochondrial components[5]. Mitophagy is responsible for the degradation and circulation of damaged mitochondria[6].
4-nitrophenylphosphatase domain and non-neuronal SNAP25-like protein homolog 1 (NIPSNAP1), initially identified by sequencing the human genome, is most highly expressed in the liver and belongs to a gene family that has been conserved throughout evolution[7]. Previous research on NIPSNAP1 has focused mainly on modulation at the neural level[8], but in 2019, Princely Abudu et al[9] first reported that NIPSNAP1/2 can act as an “eat me” signal to regulate mitophagy. NIPSNAP1/2 are expressed predominantly in the mitochondrial matrix under normal conditions; however, during membrane depolarization, NIPSNAP1/2 can be transported to the mitochondrial surface where these proteins recruit other proteins involved in selective autophagy, such as autophagy-related protein 8 proteins and autophagy receptors. Recently, we reported that PTEN induced putative kinase 1 and NIPSNAP1 synergistically regulate mitophagy and participate in liver fibrosis[10]. Appropriate NIPSNAP1/2 signaling is therefore necessary for the maintenance of mitochondrial homeostasis. For this investigation, we focused on the role of NIPSNAP1, which is more highly expressed in the liver than NIPSNAP2.
The n6-methyladenosine (m6A) modification, which is installed by m6A methyltransferases (“writers”), removed by m6A demethylases (“erasers”) and recognized by reader proteins (“readers”), plays a part in mRNA transcription, splicing, processing, translation and degradation[11]. In 2018, the insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) family was identified as novel m6A readers with two RNA recognition motif domains and four unique K-homology domains that are distinct from classical YT521-B domains. The third and fourth K-homology domains are responsible for recognizing the m6A sites of target mRNAs, increasing the affinity of IGF2BPs for RNA and their ability to regulate multiple target transcripts[12]. The IGF2BP2, a member of the m6A reader IGF2BP family, regulates a variety of biological processes as a posttranscriptional regulator of mRNA localization, stability, and translational control[13]. In pancreatic cancer patients, IGF2BP2, which acts as an m6A reader to modify the long non-coding RNA DANCR and stabilize its mRNA, is associated with a poor prognosis[14]. Greater IGF2BP2 expression is also linked to a poorer prognosis in individuals with hepatocellular carcinoma, as this protein directly recognizes and binds to the m6A site on flap structure-specific endonuclease 1 mRNA to increase its stability[15]. Additionally, IGF2BP2 has been reported to participate in the progression of certain liver diseases[16,17]. However, the function of IGF2BP2 in hepatic I/R is still unclear.
In the present study, we confirmed that NIPSNAP1 is a new target for the control of mitophagy and mitochondrial dynamics in hepatic I/R. Further characterization revealed that IGF2BP2 increases NIPSNAP1 mRNA stability by acting as a reader for m6A-modified NIPSNAP1, thereby alleviating hepatic I/R injury. Overall, this research demonstrates for the first time that the modulation of NIPSNAP1 is dependent on IGF2BP2-mediated m6A modification. A focus on the IGF2BP2/NIPSNAP1 axis could therefore be a unique therapeutic strategy for the treatment of hepatic I/R injury.
Male wild-type C57BL/6 mice weighing 18-22 g at 8 weeks of age were acquired from the Animal Center of Dalian Medical University (Dalian, Liaoning Province, China) and maintained in a typical laboratory setting. A nonlethal model of partial (70%) hepatic warm I/R was established, and the mice were grouped randomly (10-12 mice per group). Briefly, the mice were injected with 50 mg/kg pentobarbital before midline laparotomy. The artery and portal vein of each mouse were then occluded for 1 hour with a clip, after which blood flow was allowed to resume at the prescribed intervals. Following a 4-hour reperfusion period, liver tissue and plasma were collected for analyses. Adeno-associated virus (AAV) was obtained from Hanbio Biotechnology (Shanghai, China). Three weeks prior to hepatic I/R, the mice were administered AAV- thyroxine-binding globulin (TBG)-NIPSNAP1 shRNA or AAV-TBG-IGF2BP2. The sequences of the NIPSNAP1 shRNAs (Hanbio Biotechnology) are shown in Supplementary Table 1. The Dalian Medical University Ethics Committee approved all the animal procedures, No. AEE20004, which were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals (Dalian, China).
Liver tissue sections were fixed overnight in 4% paraformaldehyde after which they were stained with hematoxylin eosin (HE). Using the appropriate assays (Jiancheng Corp., Nanjing, Jiangsu Province, China), the blood levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured to assess hepatocellular damage following hepatic I/R.
Liver sections were fixed in 4% paraformaldehyde and embedded in paraffin. After deparaffinization and antigen retrieval in citrate buffer (pH = 6.0), the sections were blocked with 5% bovine serum albumin in phosphate buffered saline and coincubated overnight at 4 °C with anti-NIPSNAP1 (Santa Cruz) and anti-albumin (Boster) primary antibodies. Next, sections were incubated with secondary antibodies against the corresponding species for 1 hour. 4’,6-diamidino-2-phenylindole was used to counterstain the nuclei. Images were acquired using a confocal laser scanning microscope, and colocalization analysis was performed using ImageJ software.
Previous studies have shown that the hypoxia/reoxygenation (H/R) model has characteristics analogous to those of in vivo I/R conditions[18]. The HepG2 cell line was obtained from the National Collection of Authenticated Cell Cultures (Shanghai, China). The cells were cultured in a mixed minimum essential medium solution and maintained in 5% CO2. To induce H/R in vitro, the cells were incubated in serum-free minimum essential medium with 1% O2 for 12 hours; then, the cells were incubated in 21% O2 for the indicated durations of reoxygenation. Small interfering RNAs (siRNAs) targeting NIPSNAP1 and IGF2BP2 or pcDNA-NIPSNAP1 plasmids, pcDNA-IGF2BP2 plasmids, or negative controls (pcDNA 3.1 or si-control) were transfected into HepG2 cells 48 hours prior to H/R. The specific siRNA sequences (GenePharma, Suzhou, Jiangsu Province, China) used are listed in Supplementary Table 1.
Total extracted proteins were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels (10%-12%). The antibodies used are shown in Supplementary Table 2. Immunoreactive bands were analyzed using a Gel-Pro Analyzer.
Protein A/G magnetic beads (Bimake) were treated with specific antibodies for 4 hours. The extracted proteins were incubated with the antibody/bead mixtures for 1 hour. Following isolation and purification, the bead-bound proteins were subjected to western blotting.
The mitochondrial membrane potential was assessed using a Jung’s cyanine dye 1 staining (JC-1) assay kit (Beyotime). Impaired mitophagy results in depolarization of the mitochondrial membrane potential. The JC-1 fluorescence was used to analyze the change in the mitochondrial membrane potential (Δψm).
Mitochondrial morphology was investigated using transmission electron microscopy (TEM). The liver tissue was fixed in 2.5% glutaraldehyde and then incubated with osmium tetroxide. The sections were promptly stained with lead citrate after washes in ethanol.
Total RNA was extracted using RNAiso Plus (TaKaRa). An Evo M-MLV RT kit (Accurate Biotechnology, Changsha, Hunan Province, China) was then used to generate cDNA. The SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology) was then used to conduct real-time polymerase chain reaction (RT-PCR). The primer sequences are displayed in Supplementary Table 3.
A Magna MeRIPTM m6A kit (Millipore, Bedford, MA, United States; Cat# 17-10499) was used to assess the m6A modification levels of the target mRNAs. Briefly, total RNA from HepG2 cells was isolated and then cleaved into approximately 100-nt pieces. To enrich the m6A-containing mRNAs, the fragmented RNA was treated with an m6A-specific antibody (Millipore, Bedford, MA, United states; Cat# 17-10499). Finally, the levels were measured by quantitative RT-PCR (qRT-PCR).
An RNA-Binding Protein Immunoprecipitation Kit (Millipore, Cat# 17-700) was used for the RNA immunoprecipitation (RIP) experiments. Briefly, the cells were lysed in RIP lysis buffer. Ten percent of the lysate was used as input, and the remainder was incubated at 4 °C overnight with protein A/G magnetic beads coupled with an anti-IGF2BP2 antibody. Anti-rabbit IgG was used as the negative control. RNA was extracted with RNAiso Plus after the immunoprecipitates were eluted and treated with proteinase K. The RNA level was assessed using qRT-PCR and normalized to the input.
The binding between NIPSNAP1 and IGF2BP2 was assessed via RNA pull-down experiments. Briefly, a biotin-labeled NIPSNAP1-mRNA probe was synthesized and subsequently incubated with streptavidin-coated magnetic beads (Invitrogen, Cat# 20164) for 30 minutes. After a magnetic stand was used to retain the bead-RNA-protein precipitates, washing buffer was used to purify the mixture. The beads were added to the HepG2 cell lysate supernatant for 60 minutes at 4 °C with rotation. Finally, following a wash with elution solution, protein levels were examined by western blotting.
After HepG2 cells were transfected with si-IGF2BP2, they were exposed to actinomycin D (MCE, Cat# HY-17559, 5 μg/mL) for 0 hour, 2 hours, 4 hours, and 8 hours. RNAiso Plus was used to extract total RNA. qRT-qPCR was performed as described above to analyze the NIPSNAP1 mRNA levels.
All experimental data are presented as the means and standard deviations. Tukey’s post-hoc test (GraphPad Software) was used after Student’s unpaired t test (comparisons between two groups) or one-way analysis of variance (comparisons among multiple groups) to analyze the data. P values < 0.05 indicated statistical significance.
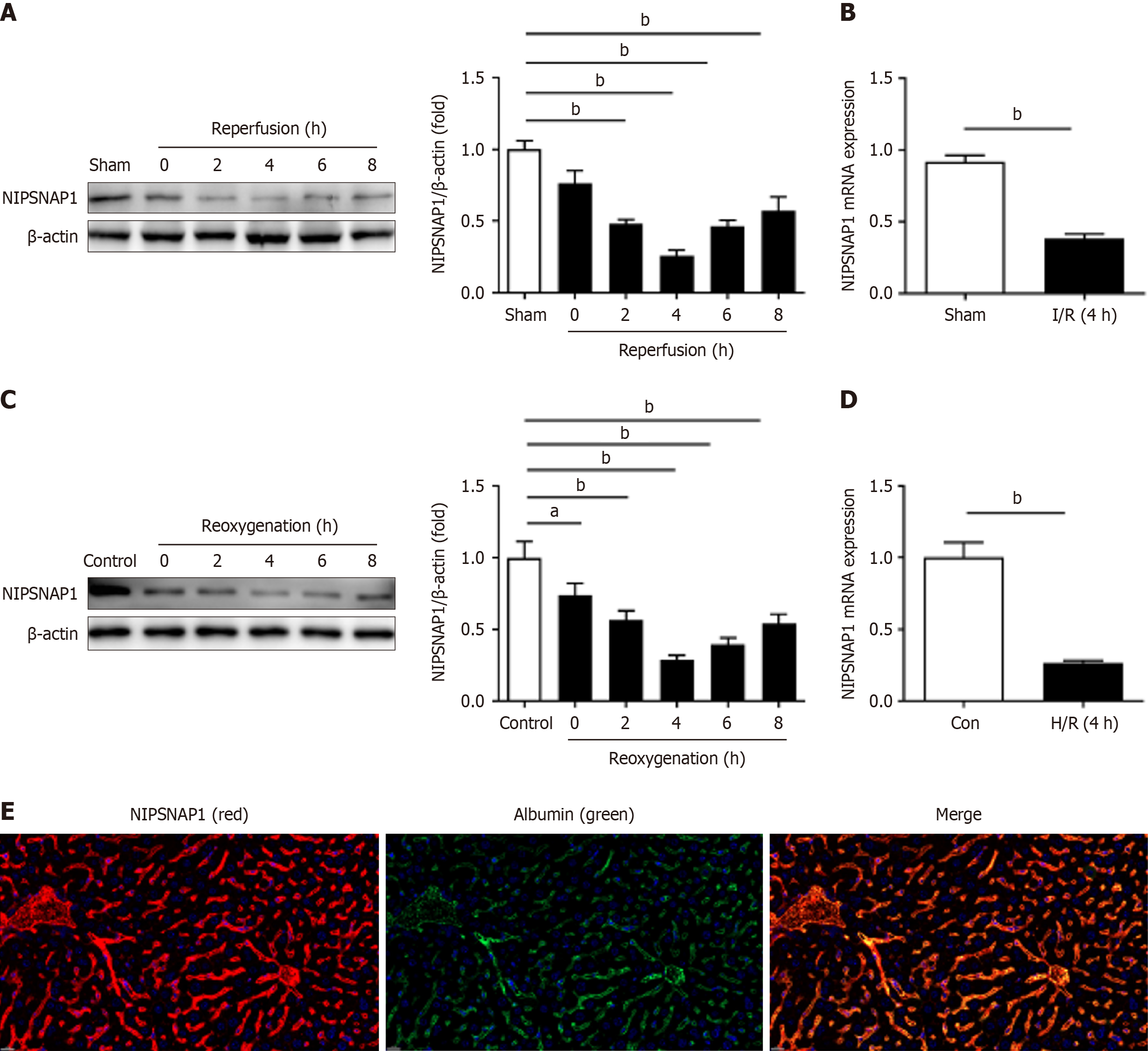
We first assessed NIPSNAP1 expression in vivo at different intervals following reperfusion in hepatic I/R. According to the data, NIPSNAP1 expression clearly decreased after 1 h of ischemia followed by 0-8 hours of reperfusion, particularly at 4 hours, and was then moderately restored throughout the next 6-8 hours of reperfusion (Figure 1A). Thus, 1 hour of ischemia and 4 hours of reperfusion were selected as the time points to explore the role of NIPSNAP1 in hepatic I/R. The in vivo mRNA levels of NIPSNAP1 also significantly decreased in our model (Figure 1B). In vitro H/R damage to HepG2 cells produced outcomes that were comparable to those observed in vivo (Figure 1C and D). Exposure to hypoxia for 1 h and subsequent reoxygenation for 4 hours were therefore selected for subsequent experiments. These data suggested that NIPSNAP1 expression is significantly downregulated during hepatic I/R. Furthermore, double-labeling experiments confirmed that NIPSNAP1 is indeed localized in hepatocytes in vivo (Figure 1E).
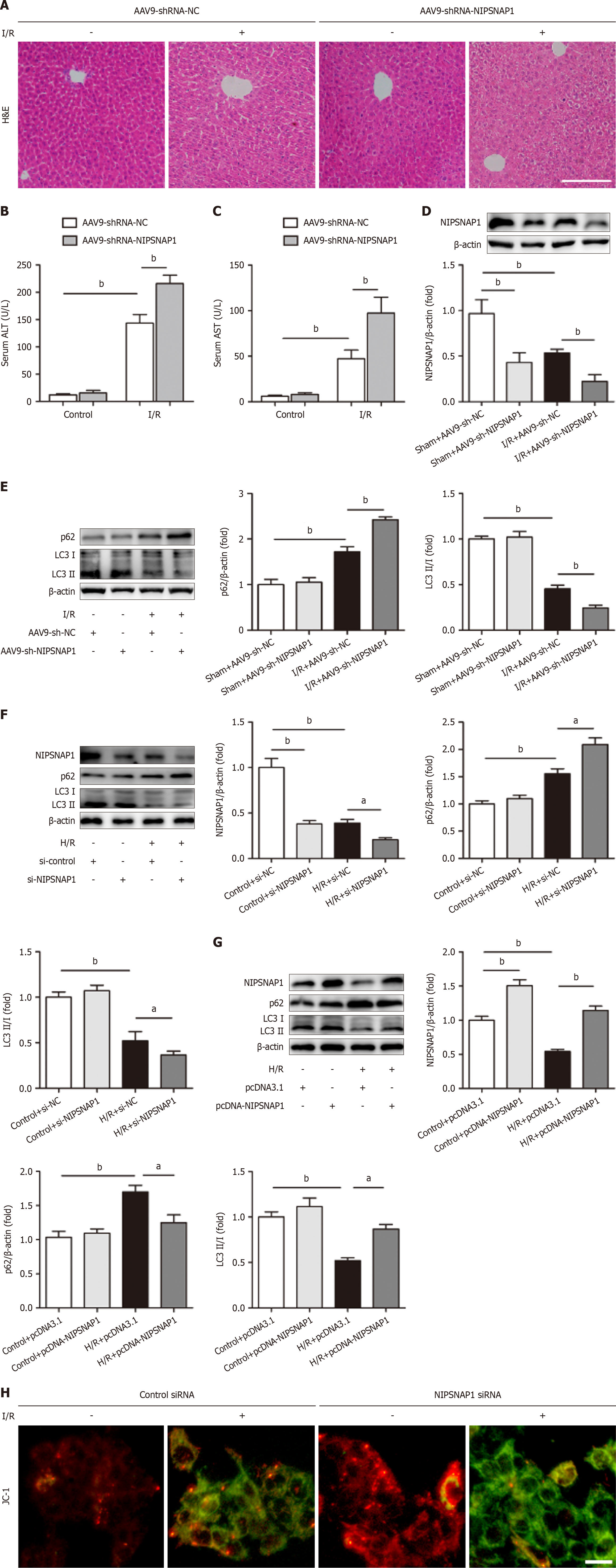
Previous research has shown that the mitochondrial matrix protein NIPSNAP1 plays an important role in mitophagy and acts as an “eat-me” signal[19]. To further explore the impact of NIPSNAP1 on hepatic I/R, AAV9-shRNA-NIPSNAP1 was injected into the mice. HE staining revealed that NIPSNAP1 knockdown in vivo resulted in more necrotic areas after hepatic I/R (Figure 2A). Moreover, the downregulation of NIPSNAP1 expression exacerbated the increases in the serum ALT and AST levels in animals subjected to hepatic I/R (Figure 2B and C). The 95% confidence intervals for each group were shown in Supplementary Table 4. We subsequently validated the knockdown efficiency and assessed changes in mitophagy-related indicators (Figure 2D). As expected, mitochondrial autophagic flux was impaired after reperfusion, as demonstrated by an increase in p62 protein expression and a significant decrease in MAP1LC3 (LC3) II/I protein expression compared with the sham group. These changes were reinforced by NIPSNAP1 knockdown (Figure 2E). The possible role of NIPSNAP1 in HepG2 cells exposed to H/R was then investigated. Consistent with the in vivo findings, after cells were transfected with NIPSNAP1 siRNA, the p62 protein level increased and the LC3II/I ratio decreased in response to H/R (Figure 2F). In contrast, NIPSNAP1 overexpression decreased p62 expression and increased LC3II/I expression (Figure 2G). Furthermore, as shown in Figure 2H, impaired mitophagy contributes to a decrease in the ΔΨm, which leads to an increase in the amount of green fluorescent JC-1 monomers. In this study, JC-1 was selected as the endpoint to assess the ΔΨm following NIPSNAP1 knockdown. Further validation of the experimental results will also be considered in conjunction with tetramethylrhodamine methyl ester/tetramethylrhodamine ethyl ester dyes to assess ΔΨm changes via prolonged live-cell imaging.
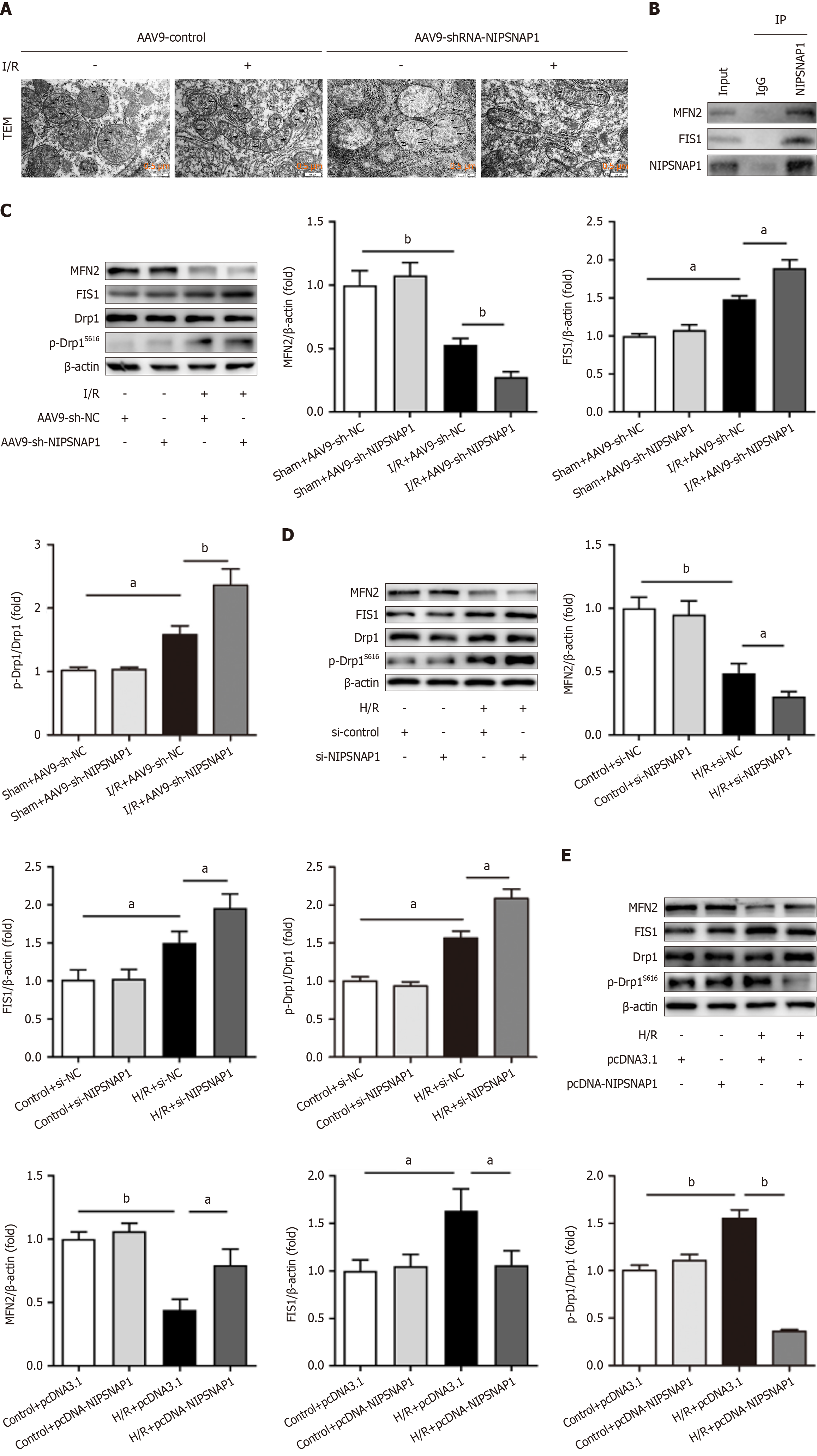
To further investigate the molecular mechanism by which NIPSNAP1 regulates mitochondrial homeostasis in hepatic I/R, NIPSNAP1 was knocked down in mice subjected to I/R and mitochondrial dynamics were observed. TEM images revealed swollen and small mitochondria and disorganized cristae in mice subjected to I/R. NIPSNAP1 knockdown significantly aggravated mitochondrial damage (Figure 3A). The results of the coimmunoprecipitation experiments suggested that NIPSNAP1 may interact with mitochondrial fission 1 protein (FIS1) and mitofusin 2 (MFN2) (Figure 3B). As shown in Figure 3C, NIPSNAP1 knockdown in vivo resulted in more severe disruption of mitochondrial dynamics, as evidenced by decreased MFN2 and increased FIS1 and phosphorylated dynamin-related protein 1 (p-Drp1) (Ser616) levels. Next, as shown in Figure 3D, after transfection with NIPSNAP1 siRNA and in response to H/R in vitro, the MFN2 protein levels decreased, and the FIS1 and p-Drp1 (Ser616) protein levels increased. However, NIPSNAP1 overexpression in vitro reversed these trends (Figure 3E). These results demonstrated that NIPSNAP1 can alleviate hepatic I/R injury by regulating mitochondrial dynamics.
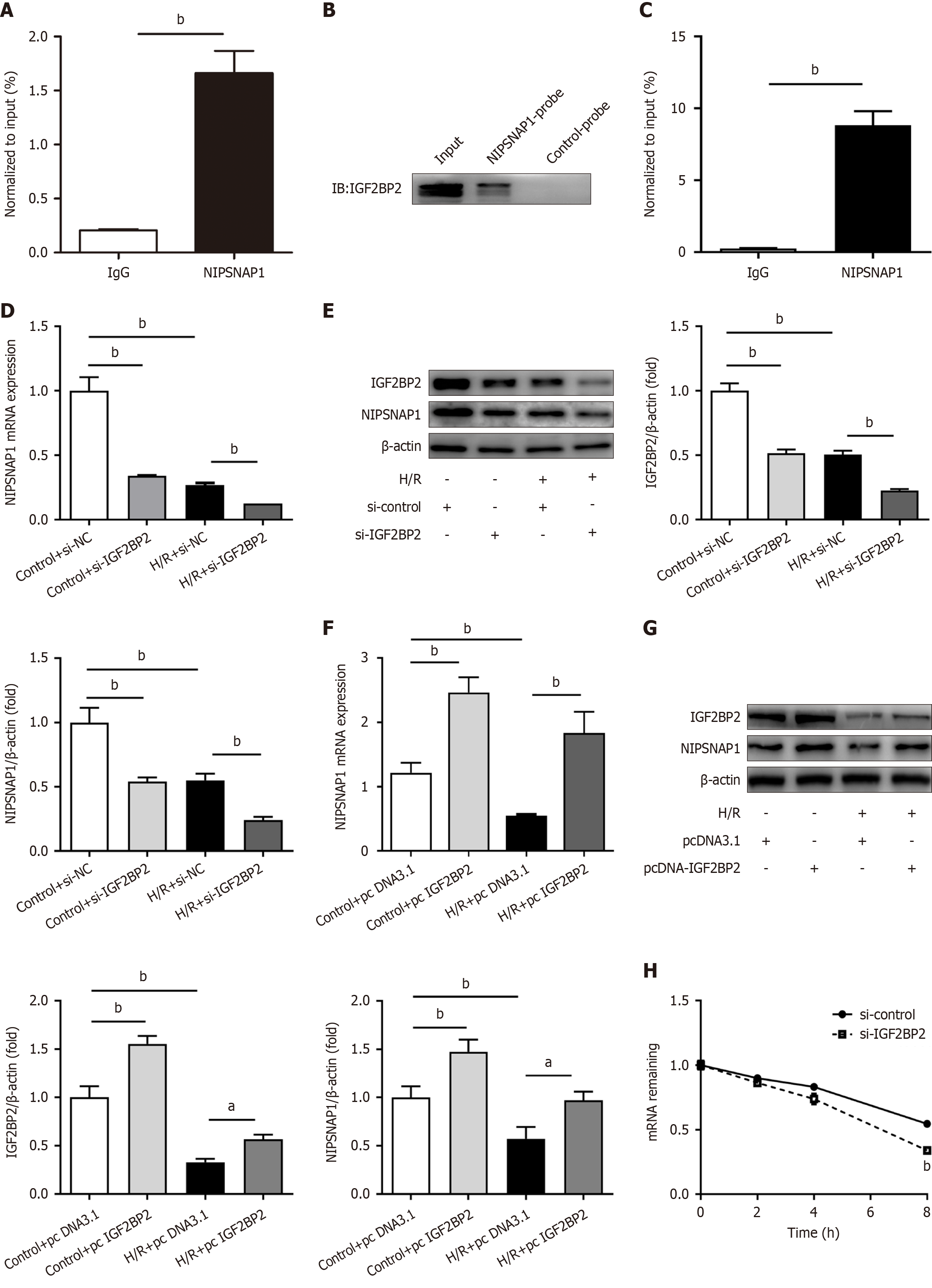
We further investigated the underlying processes upstream of NIPSNAP1, given its importance as a regulator of mitophagy and mitochondrial dynamics in hepatic I/R. The online bioinformatic tool sequence-based RNA adenosine methylation site predictor (http://www.cuilab.cn/sramp)[20] revealed multiple m6A modification sites in the NIPSNAP1 mRNA sequence (Supplementary Table 5). As shown in Figure 4A, MeRIPTM-qRT-PCR demonstrated that NIPSNAP1 mRNA was significantly enriched with m6A-specific antibodies in HepG2 cells.
In contrast to the ability of YT521-B domain family protein 2 to promote mRNA degradation, IGF2BPs influence gene expression output by increasing the stability of their target mRNAs in an m6A-dependent manner[12]. The RNA-seq and RNA stability profiles of the IGF2BP family (GSE90684) were retrieved and downloaded from the Gene Expression Omnibus[12]. Unlike after IGF2BP1 and IGF2BP3 knockdown, the expression of NIPSNAP1 significantly decreased after IGF2BP2 knockdown (Supplementary Table 6). We therefore speculated that IGF2BP2 may bind to NIPSNAP1 and modulate its stability, thereby regulating hepatic I/R injury.
The specific connection between IGF2BP2 and NIPSNAP1 was identified via an IGF2BP2 pull-down assay with biotinylated NIPSNAP1 mRNA (Figure 4B). Additionally, RIP assays verified the direct interaction between IGF2BP2 and NIPSNAP1 in HepG2 cells (Figure 4C). Next, we focused on the regulatory effect between IGF2BP2 and NIPSNAP1. NIPSNAP1 protein and mRNA expression significantly decreased after IGF2BP2 was knocked down in HepG2 cells (Figure 4D and E). In contrast, IGF2BP2 overexpression upregulated the expression of NIPSNAP1 mRNA and protein (Figure 4F and G). Furthermore, actinomycin D experiments revealed that depletion of IGF2BP2 increased the NIPSNAP1 RNA degradation rate and significantly decreased NIPSNAP1 mRNA stability (Figure 4H). In summary, these findings implied that IGF2BP2 serves as an upstream mediator of NIPSNAP1 and functions as an RNA stabilizer.
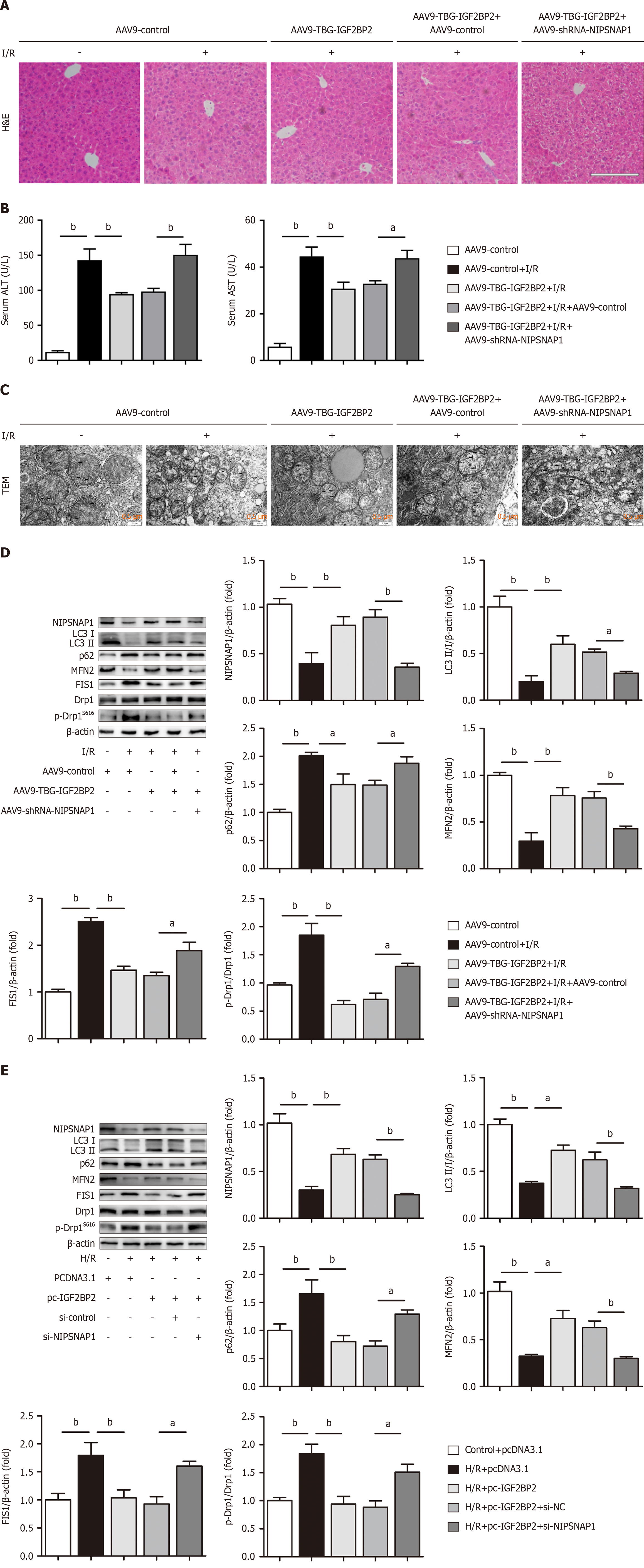
We subsequently performed rescue experiments to explore whether IGF2BP2 functions in hepatic I/R by modulating NIPSNAP1 expression. The link between IGF2BP2 and NIPSNAP1 was ascertained by NIPSNAP1 knockdown in conjunction with IGF2BP2 overexpression in mice subjected to hepatic I/R. As shown in Figure 5A, HE staining revealed that the decrease in the necrotic area promoted by IGF2BP2 overexpression was disrupted by NIPSNAP1 knockdown. Mice in which IGF2BP2 was overexpressed exhibited decreased AST and ALT levels, an effect that was disrupted by NIPSNAP1 silencing (Figure 5B, Supplementary Table 7). In addition, IGF2BP2 overexpression substantially rescued mitochondria with fragmented and disorganized cristae in response to I/R, whereas NIPSNAP1 knockdown diminished the effect of IGF2BP2 overexpression on mitochondria (Figure 5C). IGF2BP2 overexpression regulated mitophagy and mitochondrial dynamics through NIPSNAP1, as evidenced by increased NIPSNAP1, LC3II/I and MFN2 levels and decreased p62, FIS1 and p-Drp1/dynamin-related protein 1 levels. However, the protective effects of AAV9-TBG-IGF2BP2 on hepatic I/R were impaired by NIPSNAP1 knockdown (Figure 5D). These results were validated in HepG2 cells under H/R conditions (Figure 5E). Overall, we demonstrated that the influence of IGF2BP2 on mitophagy and mitochondrial dynamics in hepatic I/R injury was at least partly achieved through NIPSNAP1.
As the liver depends on the oxygen supply and mitochondrial function for energy production, liver function is inhibited in response to ischemia or hypoxia and impaired mitochondrial function[21]. Previous research has shown that mitochondrial dysfunction may be linked to the deleterious effects of intestinal, brain, hepatic, renal, and cardiac I/R injury[1,22,23]. Classically, excessive mitochondrial production of free radicals has received substantial attention as a major cause of organ damage during reperfusion injury[24]. Recent research, however, has demonstrated the presence of additional, more intricate players, such as mitochondrial malfunction, which is the critical cause of I/R injury[1,22,23]. Mitochondrial dysfunction has been implicated in I/R injury in many organs and tissues. First, mitophagy is impaired, which may not be sufficient to eliminate accumulated dysfunctional mitochondria. Second, the disruption of mitochondrial fusion and fission leads to impaired mitochondrial function such that the clearance of excess reactive oxygen species is insufficient, which results in cell apoptosis and necrosis as well as organ failure, among other effects. Considering that the estrogen in female animals has a complex influence on pathophysiological functions, male mice were used in our research. Nonetheless, we also detected decreased NIPSNAP1 and IGF2BP2 expression during hepatic I/R in both male and female mice (Figure 1, Supplementary Figure 1). In this study, we found that hepatic I/R damage in mice was exacerbated by liver-specific NIPSNAP1 knockdown, together with impaired mitophagy and imbalanced mitochondrial dynamics; the same results were observed in vitro. Conversely, NIPSNAP1 overexpression exerted the opposite effect. Thus, we propose that NIPSNAP1 might be an important target for mitophagy and mitochondrial dynamics and may participate in hepatic I/R.
NIPSNAP1 interacts with important autophagic proteins to control the autophagy of damaged mitochondria. Research has also shown that when the membrane potential decreases, NIPSNAP1 expression on the mitochondrial surface appears to increase. This accumulation plays a role in the increased recruitment of autophagic proteins[9]. Additionally, FUNDC1 regulates mitophagy through its capacity as a receptor in mitochondrial autophagy[25]. Intestinal I/R has been shown to lower NIPSNAP1/2 Levels, which may be connected to increased levels of phosphorylated FUNDC1[26]. According to what was shown in the BioGRID database (https://thebiogrid.org/), we were particularly interested in proteins related to mitochondrial dynamics, such as MFN2 and FIS1. As noted, we hypothesized that NIPSNAP1 plays a novel functional role in mitochondrial dynamics during hepatic I/R injury. Our data revealed that NIPSNAP1 knock
Numerous biological processes are governed by m6A modification, which controls the fate and function of mRNAs. m6A reader-mediated m6A modification has been linked to numerous human disorders and is also essential for controlling liver diseases. Among the m6A readers, we focused on the IGF2BP family in this study. Notably, bioinformatics analysis revealed that only IGF2BP2 silencing, and not IGF2BP1 or IGF2BP3 silencing, significantly downregulated the expression of NIPSNAP1. Previous studies have shown that IGF2BP2 affects a wide range of chronic liver diseases. Intriguingly, studies that address the role of IGF2BP2 in fatty liver have yielded conflicting results. Dai et al[31] reported that IGF2BP2-/- mice exhibit increased glucose tolerance and insulin sensitivity and are extremely resistant to diet-induced obesity and fatty liver disease. A recent publication also suggested that IGF2BP2 is involved in lipid metabolism[32]. However, IGF2BP2 knockout specifically in hepatocytes has been shown to result in the accumulation of triglycerides in the liver, as reported by Regué et al[17]. Moreover, IGF2BP2 expression has been shown to be increased in both TGF-β-activated hepatic stellate cells and carbon tetrachloride-induced liver fibrosis[16]; IGF2BP2 is also involved in nonalcoholic fatty liver disease[33] and has been shown to promote liver cancer[15]. In addition, IGF2BP2-mediated m6A modification is important in a variety of physiological and pathological settings. By preserving the stability of its target mRNA polycomb complex protein BMI-1, IGF2BP2 maintains mitochondrial homeostasis in hematopoietic stem cells. These findings suggest that IGF2BP2-mediated m6A modification is vital for hematopoietic stem cell maintenance and hematopoietic development[34]. Furthermore, IGF2BP2 has recently been reported to be involved in mitochondrial metabolic reprogramming by binding to hypoxia-inducible factor 1-alpha mRNA in a methyltransferase-like 3/m6A-dependent manner[35]. Our study subsequently revealed the relationship between IGF2BP2 and NIPSNAP1. A positive correlation was observed between the expression level of NIPSNAP1 and that of IGF2BP2. The most well-characterized role of IGF2BP2 is to promote mRNA stability via the recognition of m6A modifications, and herein, we demonstrated the important role of IGF2BP2 in stabilizing NIPSNAP1 mRNA. However, its specific molecular mechanism still requires further study, which may include detecting the change in NIPSNAP1 stability after mutating the binding site of IGF2BP2 that interacts with NIPSNAP1 mRNA, as well as exploring the involvement of other RNA-binding proteins and regulators that may synergize with IGF2BP2 to regulate the stability of NIPSNAP1 mRNA. As noted above, this study is the first to elucidate the role of IGF2BP2 in regulating NIPSNAP1 in acute liver injury.
In summary, these findings reveal that NIPSNAP1 not only controls the highly sensitive process of mitophagy but also plays a crucial role in the homeostasis of mitochondrial function, including mitochondrial dynamics. In future studies, we will increase the sample size and/or include additional clinical samples to further validate our findings. Many intriguing mechanistic questions still need to be addressed to fully characterize NIPSNAP1 and its broader implications. Moreover, NIPSNAP1 is a novel target of the m6A reader IGF2BP2, and targeting the IGF2BP2/NIPSNAP1 axis may be a new therapeutic strategy for the treatment of hepatic I/R.
| 1. | Go KL, Lee S, Zendejas I, Behrns KE, Kim JS. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. Biomed Res Int. 2015;2015:183469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Teodoro JS, Da Silva RT, Machado IF, Panisello-Roselló A, Roselló-Catafau J, Rolo AP, Palmeira CM. Shaping of Hepatic Ischemia/Reperfusion Events: The Crucial Role of Mitochondria. Cells. 2022;11:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Cannistrà M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, Naso A, Grande R, Serra R, Nardo B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg. 2016;33 Suppl 1:S57-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 4. | Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011;1813:1382-1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 1089] [Article Influence: 181.5] [Reference Citation Analysis (0)] |
| 6. | Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1502] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 7. | Seroussi E, Pan HQ, Kedra D, Roe BA, Dumanski JP. Characterization of the human NIPSNAP1 gene from 22q12: a member of a novel gene family. Gene. 1998;212:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Okamoto K, Ohashi M, Ohno K, Takeuchi A, Matsuoka E, Fujisato K, Minami T, Ito S, Okuda-Ashitaka E. Involvement of NIPSNAP1, a neuropeptide nocistatin-interacting protein, in inflammatory pain. Mol Pain. 2016;12:1744806916637699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Princely Abudu Y, Pankiv S, Mathai BJ, Håkon Lystad A, Bindesbøll C, Brenne HB, Yoke Wui Ng M, Thiede B, Yamamoto A, Mutugi Nthiga T, Lamark T, Esguerra CV, Johansen T, Simonsen A. NIPSNAP1 and NIPSNAP2 Act as "Eat Me" Signals for Mitophagy. Dev Cell. 2019;49:509-525.e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Li R, Wang Z, Wang Y, Sun R, Zou B, Tian X, Liu D, Zhao X, Zhou J, Zhao Y, Yao J. SIRT3 regulates mitophagy in liver fibrosis through deacetylation of PINK1/NIPSNAP1. J Cell Physiol. 2023;238:2090-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 11. | He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 1022] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 12. | Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, Hu YC, Hüttelmaier S, Skibbe JR, Su R, Deng X, Dong L, Sun M, Li C, Nachtergaele S, Wang Y, Hu C, Ferchen K, Greis KD, Jiang X, Wei M, Qu L, Guan JL, He C, Yang J, Chen J. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 928] [Cited by in RCA: 2253] [Article Influence: 281.6] [Reference Citation Analysis (0)] |
| 13. | Wang J, Chen L, Qiang P. The role of IGF2BP2, an m6A reader gene, in human metabolic diseases and cancers. Cancer Cell Int. 2021;21:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 14. | Hu X, Peng WX, Zhou H, Jiang J, Zhou X, Huang D, Mo YY, Yang L. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2020;27:1782-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 15. | Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu X, Wu Y, Li W, Xu Z, Lu Y, Tang Q, Wei H. IGF2BP2 Promotes Liver Cancer Growth Through an m6A-FEN1-Dependent Mechanism. Front Oncol. 2020;10:578816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Xu Z, He B, Jiang Y, Zhang M, Tian Y, Zhou N, Zhou Y, Chen M, Tang M, Gao J, Peng F. Igf2bp2 knockdown improves CCl(4)-induced liver fibrosis and TGF-β-activated mouse hepatic stellate cells by regulating Tgfbr1. Int Immunopharmacol. 2022;110:108987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Regué L, Minichiello L, Avruch J, Dai N. Liver-specific deletion of IGF2 mRNA binding protein-2/IMP2 reduces hepatic fatty acid oxidation and increases hepatic triglyceride accumulation. J Biol Chem. 2019;294:11944-11951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Spencer NY, Zhou W, Li Q, Zhang Y, Luo M, Yan Z, Lynch TJ, Abbott D, Banfi B, Engelhardt JF. Hepatocytes produce TNF-α following hypoxia-reoxygenation and liver ischemia-reperfusion in a NADPH oxidase- and c-Src-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2013;305:G84-G94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Mukherjee R, Dikic I. NIPSNAP Beacons in Mitophagy. Dev Cell. 2019;49:503-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Zhou Y, Zeng P, Li YH, Zhang Z, Cui Q. SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 760] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 21. | Teoh NC. Hepatic ischemia reperfusion injury: Contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection-the good, bad and deadly. J Gastroenterol Hepatol. 2011;26 Suppl 1:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Khan H, Kaur Grewal A, Gurjeet Singh T. Mitochondrial dynamics related neurovascular approaches in cerebral ischemic injury. Mitochondrion. 2022;66:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Yu W, Xu M, Zhang T, Zhang Q, Zou C. Mst1 promotes cardiac ischemia-reperfusion injury by inhibiting the ERK-CREB pathway and repressing FUNDC1-mediated mitophagy. J Physiol Sci. 2019;69:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 25. | Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2α. Basic Res Cardiol. 2018;113:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 26. | Li S, Zhou Y, Gu X, Zhang X, Jia Z. NLRX1/FUNDC1/NIPSNAP1-2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 2021;54:e12986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 27. | Ihenacho UK, Meacham KA, Harwig MC, Widlansky ME, Hill RB. Mitochondrial Fission Protein 1: Emerging Roles in Organellar Form and Function in Health and Disease. Front Endocrinol (Lausanne). 2021;12:660095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 28. | Suzuki M, Jeong SY, Karbowski M, Youle RJ, Tjandra N. The solution structure of human mitochondria fission protein Fis1 reveals a novel TPR-like helix bundle. J Mol Biol. 2003;334:445-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Gao E, Sun X, Thorne RF, Zhang XD, Li J, Shao F, Ma J, Wu M. NIPSNAP1 directs dual mechanisms to restrain senescence in cancer cells. J Transl Med. 2023;21:401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Liu Y, Qu Y, Cheng C, Tsai PY, Edwards K, Xue S, Pandit S, Eguchi S, Sanghera N, Barrow JJ. Nipsnap1-A regulatory factor required for long-term maintenance of non-shivering thermogenesis. Mol Metab. 2023;75:101770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Dai N, Zhao L, Wrighting D, Krämer D, Majithia A, Wang Y, Cracan V, Borges-Rivera D, Mootha VK, Nahrendorf M, Thorburn DR, Minichiello L, Altshuler D, Avruch J. IGF2BP2/IMP2-Deficient mice resist obesity through enhanced translation of Ucp1 mRNA and Other mRNAs encoding mitochondrial proteins. Cell Metab. 2015;21:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Shao J, Wang M, Zhang A, Liu Z, Jiang G, Tang T, Wang J, Jia X, Lai S. Interference of a mammalian circRNA regulates lipid metabolism reprogramming by targeting miR-24-3p/Igf2/PI3K-AKT-mTOR and Igf2bp2/Ucp1 axis. Cell Mol Life Sci. 2023;80:252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Thomas H. NAFLD: Loss of CD4(+) T cells in HCC. Nat Rev Gastroenterol Hepatol. 2016;13:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Yin R, Chang J, Li Y, Gao Z, Qiu Q, Wang Q, Han G, Chai J, Feng M, Wang P, Zhang T, Xie X, Hu J, Cheng Y, Guo C, Wang J, Gao K, Cui M, Li S, Zheng Y, Jiang W, Hu Y, Yang QY, Zhang H. Differential m(6)A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell. 2022;29:149-159.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 35. | Zhang H, Wu D, Wang Y, Guo K, Spencer CB, Ortoga L, Qu M, Shi Y, Shao Y, Wang Z, Cata JP, Miao C. METTL3-mediated N6-methyladenosine exacerbates ferroptosis via m6A-IGF2BP2-dependent mitochondrial metabolic reprogramming in sepsis-induced acute lung injury. Clin Transl Med. 2023;13:e1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/