Published online Jun 14, 2025. doi: 10.3748/wjg.v31.i22.105201
Revised: May 6, 2025
Accepted: June 3, 2025
Published online: June 14, 2025
Processing time: 57 Days and 18.7 Hours
The annual incidence of gastric cancer in elderly patients is increasing. Despite the continuous progress in treatment methods, the prognosis of elderly patients remains poor, and postoperative complications are frequent. Obesity is believed to be associated with the risk of gastric cancer and postoperative prognosis; however, the effect of visceral fat distribution on postoperative complications of gastric cancer in elderly patients remains unclear.
To explore the effect of visceral fat distribution on postoperative complications of gastric cancer in elderly patients.
A total of 163 elderly patients treated at the Affiliated Longyan First Hospital of Fujian Medical University after radical gastrectomy between January 2021 and January 2024 were enrolled. The patients' visceral and subcutaneous fat distributions were measured and divided into a high visceral fat area (VFA-H) group and a low visceral fat area (VFA-L) group, with a critical value of 100 cm2. The t-test and χ2 test were used to calculate and analyze the relationship between vis
Compared with the VFA-L group, the incidence of postoperative complications was higher in the VFA-H group (27.8% vs 6.4%, P < 0.001), and the operation time was longer (268.55 ± 63.41 vs 224.31 ± 51.89, P < 0.001). The amount of blood loss was more (163.77 ± 105.27 mL vs 127.93 ± 98.26 mL, P < 0.001). Logistic regression analysis showed that VFA [odds ratio (OR): 2.597, 95%CI: 1.479-4.853, P = 0.004], total fat area (OR: 1.655, 95%CI: 1.076-4.040, P = 0.013), and the visceral subcutaneous fat area ratio (OR: 2.046, 95%CI: 1.196-5.640, P = 0.008) were independent risk factors for postoperative complications.
This study showed that postoperative complications are closely related to fat distribution in elderly patients with gastric cancer undergoing gastrectomy. A high VFA is associated by a high incidence of postoperative complications.
Core Tip: Postoperative complications were closely related to visceral fat content in elderly patients with gastric cancer who underwent gastrectomy. High visceral fat area results in increased intraoperative blood loss, longer operation time, and a higher incidence of postoperative complications. Clinicians should pay close attention to the relevant indicators before surgery and improve the nutritional status of patients to minimize the risk of postoperative complications and improve prognosis.
- Citation: Li WF, Que CR, Xu DB, Li P. Impact of visceral fat distribution on postoperative complications in high-aged patients undergoing gastric cancer surgery: A cross-sectional study. World J Gastroenterol 2025; 31(22): 105201
- URL: https://www.wjgnet.com/1007-9327/full/v31/i22/105201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i22.105201
Gastric cancer is the fifth most common malignant tumor worldwide, the third leading cause of cancer-related deaths, and one of the major health problems facing mankind[1]. Its incidence is closely related to age, with about 65% of new cases in the United Kingdom occurring in patients over the age of 70[2]. Gastrectomy remains the definitive treatment for patients with potentially curable gastric cancer. However, the survival rate of elderly patients after surgery is also a concern. Recent studies have shown that the mortality rate within 30 days after radical surgery in elderly patients with gastric cancer remains very high, ranging from 4.1% to 7.4% in recent studies[3,4], and the 5-year overall and disease-specific survival rates after radical surgery are 51.5% and 58.3%, respectively[5]. Therefore, improving the prognosis of elderly patients is a priority. Moreover, reducing the incidence of complications in elderly patients with gastric cancer is crucial for improving short- and long-term outcomes[6].
As living standards have improved over the past few decades, the global prevalence of obesity has increased. The global average body mass index (BMI) increased by 0.4 kg/m2 per decade for men and 0.5 kg/m2 for women[7]. Surgeons have gradually recognized that extreme systemic obesity is a potential risk factor for various malignant tumors, surgical morbidity, and mortality[8]. A meta-analysis[9] showed that obesity is associated with an increased risk of stomach cancer, and the strength of this association increases with increasing BMI. However, other epidemiological studies on the association between excess body weight and cancer risk have yielded conflicting results[10,11]. Therefore, obesity alone is unlikely to fully explain the risk or distribution of stomach cancer. Adipose tissue is divided into visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT)[12]. Different fat distribution patterns may reflect a unique metabolic environment associated with disease development. Compared with SAT, VAT has more cells and blood vessels, accumulation of adipokines and immune cells that produce inflammatory mediators, and increased endocrine and metabolic activities, all of which cause chronic low-grade systemic inflammation[13]. A study has found that increased VAT is associated with reduced cancer survival in malignant melanoma[14], endometrial cancer[15], colorectal cancer[16], and postmenopausal breast cancer[17]. In addition, studies have found that visceral obesity is also associated with multiple hormones, such as insulin-like growth factor and adiponectin, which are known to affect cell division, cell death, and healing. Therefore, visceral obesity may alter the risk of cancer through these mechanisms[18].
Few studies have evaluated the association between the distribution of visceral obesity and postoperative complications of gastric cancer in older patients. Therefore, this study aimed to explore the effect of visceral fat distribution on postoperative complications in elderly patients with gastric cancer, and provide new ideas for improving the prognosis of patients with gastric cancer.
A total of 163 elderly patients treated in the Affiliated Longyan First Hospital of Fujian Medical University after radical gastrectomy from January 2021 to January 2024 were collected. The patients' visceral and subcutaneous fat distributions were measured and divided into a high visceral fat area (VFA-H) group and a low visceral fat area (VFA-L) group, with a critical value of 100 cm2. The t-test and χ2 test were used to calculate and analyze the relationship between VFA and complications. The independent risk factors for postoperative complications were analyzed using binary logistic regression analysis.
The inclusion criteria: (1) Age ≥ 60 years; (2) Patients undergoing laparoscopic total gastrectomy (LTG) combined with D2 Lymph node dissection for gastric adenocarcinoma confirmed by preoperative endoscopic biopsy or postoperative pathology, and undergoing postoperative pathological diagnosis confirming R0 resection; (3) The tumor stage was T1N0M0-T4aN0M0; (4) No other primary malignant tumor; and (5) Consented to participate in the trial and gave written informed consent.
The exclusion criteria: (1) Patients receiving neoadjuvant chemotherapy or radiotherapy that might affect the efficacy assessment; (2) Patients unable to undergo radical surgery for gastric cancer; (3) Patients with other malignant tumors or other organ failure; and (4) Patients who withdrew participation in the trial after signing the informed consent form. Based on the inclusion and exclusion criteria, 163 patients were included in the study.
This study was a retrospective cohort study. According to the previous literature investigation and clinical experience of the research team, the probability of postoperative complications was estimated, and the incidence was about 35%. The results were input into PASS 15 for sample size estimation, α was 0.05 (bilateral), and the sample loss rate of 10% was considered. Finally, n = 135 cases were identified. The general rule of logistic regression requires the ratio of the number of items to the sample size to be between 1:5 and 1:10. Therefore, the sample size of the participants in this study was 179 cases. There were 17 cases of loss to follow-up, and the final statistics included 163 cases. Written informed consent was obtained from all patients.
The general information questionnaire included demographic data (such as age, sex, and BMI) and clinical data, including whether there was a combination of hypertension, diabetes, hyperlipidemia, American Society of Anesthesiologists (ASA) score, visceral fat area (VFA), subcutaneous fat area (SFA), and blood pressure. SFA, muscle fat content, total fat area (TFA), relative VFA (rVFA), etc.
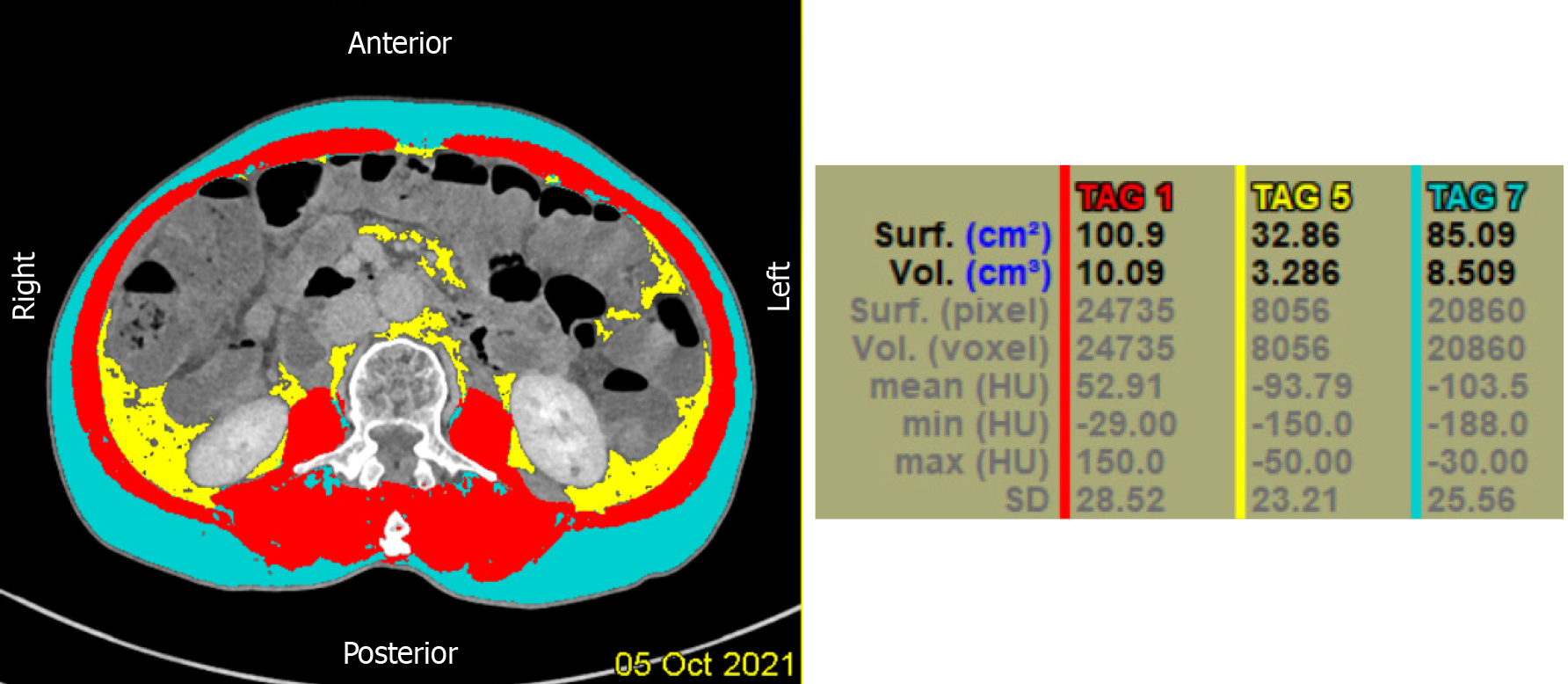
Quantitative computed tomography (QCT) combined with low-dose chest computed tomography (CT) can accurately measure the area and content of subcutaneous fat, visceral fat, and muscle fat without increasing the amount of radiation. Adipose tissue was determined using the Lightspeed VCT 64-row CT scanner (General Electric, United States) in accordance with conventional low-dose chest CT scanning specifications by setting attenuation levels in the range of -190 to -30 Hounsfield units. The scanning parameters were as follows: Tube voltage, set at 120 kV; tube current, 100 mA; scanning field of view 500 mm × 500 mm, layer thickness, 5 mm; and pitch, 0.984. The scanned images were uploaded to a QCT Pro workstation for image analysis using QCT Pro Version 6.1 software. The abdominal fat area was measured at the central level of the L2 vertebra using the "Tissue Composition" module, and the abdominal fat tissue was divided into subcutaneous fat and visceral fat using the blue threshold semi-automatic segmentation. The abdominal area of interest was mapped semi-automatically to identify the areas of subcutaneous and visceral fat. The "Measure Muscle Fat" module was used to measure the percentage of paravertebral muscle fat of the subjects. The regions with a relatively homogeneous central density of the psoas major muscle on both sides were selected in the horizontal cross-section of the endplate of the L2 vertebral body, and the areas of interest with each selected area of approximately 90-110 mm were measured. Finally, the mean values of the two were taken as the measurement results. Total fat area (TFA = VFA + SFA) and the visceral subcutaneous fat area ratio (VSR = VFA/SFA). In addition, patients were divided into two groups according to the VFA threshold recommended by the Japanese Association for the Study of Obesity[19]: VFA-L group, VFA < 100 cm2; and VFA-H group, VFA ≥ 100 cm2. The measurement method is shown in Figure 1.
The controlling nutritional status (CONUT score) was calculated using the latest preoperative results of serum albumin (ALB) level, serum total cholesterol (TC), and total lymphocyte count[20] (Table 1).
| Parameter | Malnutritional status | |||
| Normal | Mild | Moderate | Severe | |
| ALB (g/dL) | 3.5-4.5 | 3.0-3.49 | 2.5-2.9 | < 2.5 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocytes (/mm3) | ≥ 1600 | 1200-1599 | 800-1199 | < 800 |
| Score | 0 | 1 | 2 | 3 |
| Cholesterol (mg/dL) | ≥ 180 | 140-180 | 100-139 | < 100 |
| Score | 0 | 1 | 2 | 3 |
| Total | 0-1 | 2-4 | 5-8 | 9-12 |
Operative time, intraoperative blood loss, first exhaust time, oral recovery time, postoperative hospital stay, and postoperative complications were recorded as surgical outcomes. Serological indices mainly recorded ALB, hemoglobin, C-reactive protein (CRP), triglycerides (TG), TC, white blood cells, red blood cell, and total lymphocyte counts. Postoperative complications included anastomotic lesions (anastomotic fistula, anastomotic stenosis, and anastomotic hemorrhage), surgical incision infection or fat liquefaction, pulmonary infection, abdominal infection, intestinal obstruction, and others including pancreatic fistula, bacteremia, deep vein thrombosis, pulmonary embolism, and arrhythmia.
The results of each scale were input into the computer for score conversion, and SPSS 26 (IBM SPSS, United States) was used for statistical analysis. The measurement data were represented by mean and standard deviation, and the counting data were represented by frequency and percentage. The t-test and χ2 test were used for statistical analysis between groups. After collinearity diagnosis, the independent risk factors of postoperative complications and infection were analyzed using binary logistic regression analysis, and P < 0.05 was considered statistically significant.
The baseline characteristics of the patients are shown in Table 2. A total of 163 patients were included in this study, 22 of whom experienced postoperative complications. The mean age of the group without complications was 68.23 ± 7.94, and that of the group with complications was 69.12 ± 7.45. There were no significant differences in age, sex, history of hypertension, location of the primary tumor, or tumor-node-metastasis stage between the two groups (P > 0.05); however, there were significant differences in BMI, history of diabetes, history of hyperlipidemia, ASA score, VFA, SFA, TFA, and VSR between the two groups (P < 0.05).
| Item | Without complication | With complication | t/χ2 | P value |
| Age | 68.23 ± 7.94 | 69.12 ± 7.45 | -0.892 | 0.419 |
| Sex | ||||
| Male | 92 (65.2) | 15 (68.2) | 0.073 | 0.788 |
| Female | 49 (34.8) | 7 (31.8) | ||
| BMI (kg/m2) | 25.87 ± 5.19 | 29.14 ± 3.28 | 8.091 | 0.000 |
| Combined hypertension | ||||
| Yes | 34 (24.1) | 5 (22.7) | 0.020 | 0.887 |
| No | 107 (75.9) | 17 (77.3) | ||
| Combined diabetes | ||||
| Yes | 21 (14.9) | 9 (42.9) | 9.472 | 0.002 |
| No | 120 (85.1) | 12 (57.1) | ||
| Combined hyperlipidemia | ||||
| Yes | 16 (11.3) | 6 (27.3) | 4.134 | 0.042 |
| No | 125 (88.7) | 16 (72.7) | ||
| ASA score | ||||
| I | 61 (43.3) | 3 (13.6) | 7.004 | 0.008 |
| II | 80 (56.7) | 19 (86.4) | ||
| Primary tumor site | ||||
| Esophagogastric junction | 55 (39.0) | 9 (40.9) | 0.123 | 0.940 |
| Proximal stomach | 49 (34.8) | 8 (36.4) | ||
| Distal stomach | 37 (26.2) | 5 (22.7) | ||
| TNM stage | ||||
| I stage | 38 (27.0) | 5 (22.7) | 0.175 | 0.676 |
| II stage | 103 (73.0) | 17 (77.3) | ||
| VFA | 105.28 ± 37.61 | 151.98 ± 45.33 | 5.872 | 0.000 |
| SFA | 213.60 ± 57.18 | 247.35 ± 79.35 | 2.523 | 0.013 |
| TFA | 318.88 ± 47.40 | 399.33 ± 62.34 | 9.108 | 0.000 |
| VSR | 0.79 ± 0.41 | 1.07 ± 0.56 | 6.129 | 0.000 |
Patients were divided into two groups based on the VFA cutoff values: 109 in the VFA-L group and 54 in the VFA-H group. There were 7 and 15 complications in the VFA-L and VFA-H groups, respectively. There were statistically significant differences in the incidence of anastomotic lesions, surgical incision infection or fat liquefaction, and total complications between the two groups (P < 0.05), as shown in Table 3.
| Item | Anastomotic lesions | Surgical incision infection or fat liquefaction | Lung infection | Abdominal infection | Intestinal obstruction | Other | With complication |
| VFA-L | 1 (0.9) | 2 (1.8) | 1 (0.9) | 1 (0.9) | 1 (0.9) | 2 (1.8) | 7 (6.4) |
| VFA-H | 4 (7.4) | 5 (9.3) | 2 (3.7) | 2 (3.7) | 1 (1.9) | 3 (5.6) | 15 (27.8) |
| χ2 | 5.115 | 4.843 | 1.552 | 1.552 | 0.260 | 1.681 | 14.106 |
| P value | 0.024 | 0.028 | 0.213 | 0.213 | 0.610 | 0.195 | 0.000 |
The postoperative recovery indicators of patients in the VFA-H group included operation time (268.55 ± 63.41 minutes vs 224.31 ± 51.89 minutes) and intraoperative blood loss (163.77 ± 105.27 mL vs 127.93 ± 98.26 mL) were significantly greater than those in the VFA-L group (P < 0.05), as shown in Table 4.
| Item | Operation time (minute) | Intraoperative blood loss (mL) | First exhaust time (day) | Postoperative hospital stay (day) |
| VFA-L | 224.31 ± 51.89 | 127.93 ± 98.26 | 2.89 ± 1.13 | 10.13 ± 4.75 |
| VFA-H | 268.55 ± 63.41 | 163.77 ± 105.27 | 3.02 ± 1.88 | 11.28 ± 7.32 |
| t | 6.064 | 8.719 | 2.981 | 5.174 |
| P value | 0.000 | 0.000 | 0.109 | 0.552 |
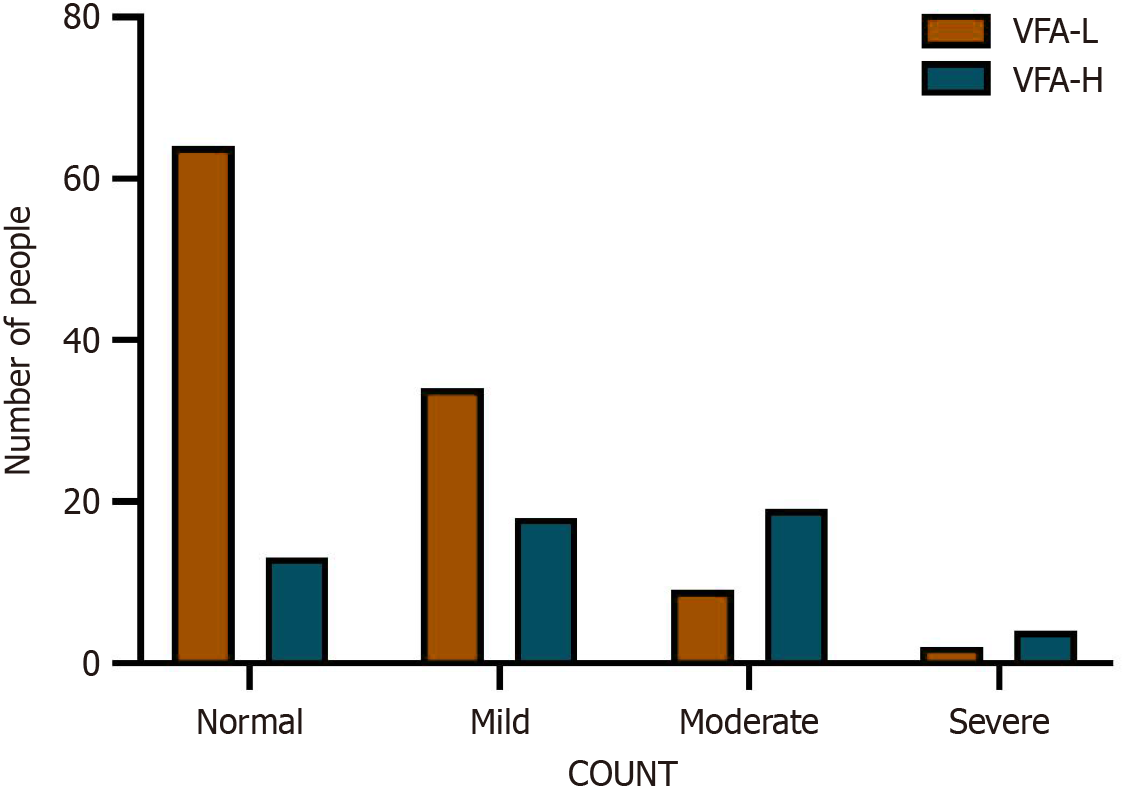
The t test showed that the ALB level in VFA-H group was significantly lower than that in the VFA-L group (33.12 ± 4.67 g/L vs 28.53 ± 3.92 g/L). CRP (6.14 ± 2.47 mg/L vs 13.58 ± 4.36 mg/L), TC (6.33 ± 2.35 mmol/L vs 4.71 ± 1.24 mmol/L), TG (2.78 ± 1.76 mmol/L vs 1.99 ± 1.01 mmol/L), and white blood cell (16.73 ± 5.27 × 109/L vs 11.89 ± 3.44 × 109/L) levels were significantly higher in the VFA-H group than in the VFA-L group (P < 0.05), and there was a significant difference in CONUT scores between the two groups (P < 0.001), as shown in Table 5 and Figure 2.
| Item | ALB (g/L) | Hb (g/L) | CRP (mg/L) | TC (mmol/ L) | TG (mmol/L) | WBC (109/L) |
| VFA-L | 33.12 ± 4.67 | 118.29 ± 39.61 | 6.14 ± 2.47 | 4.71 ± 1.24 | 1.99 ± 1.01 | 13.89 ± 3.44 |
| VFA-H | 28.53 ± 3.92 | 115.99 ± 41.23 | 13.58 ± 4.36 | 6.33 ± 2.35 | 2.78 ± 1.76 | 16.73 ± 5.27 |
| t | -3.378 | -0.276 | 4.416 | 2.434 | 2.136 | 3.192 |
| P value | 0.452 | 0.735 | 0.0007 | 0.031 | 0.045 | 0.057 |
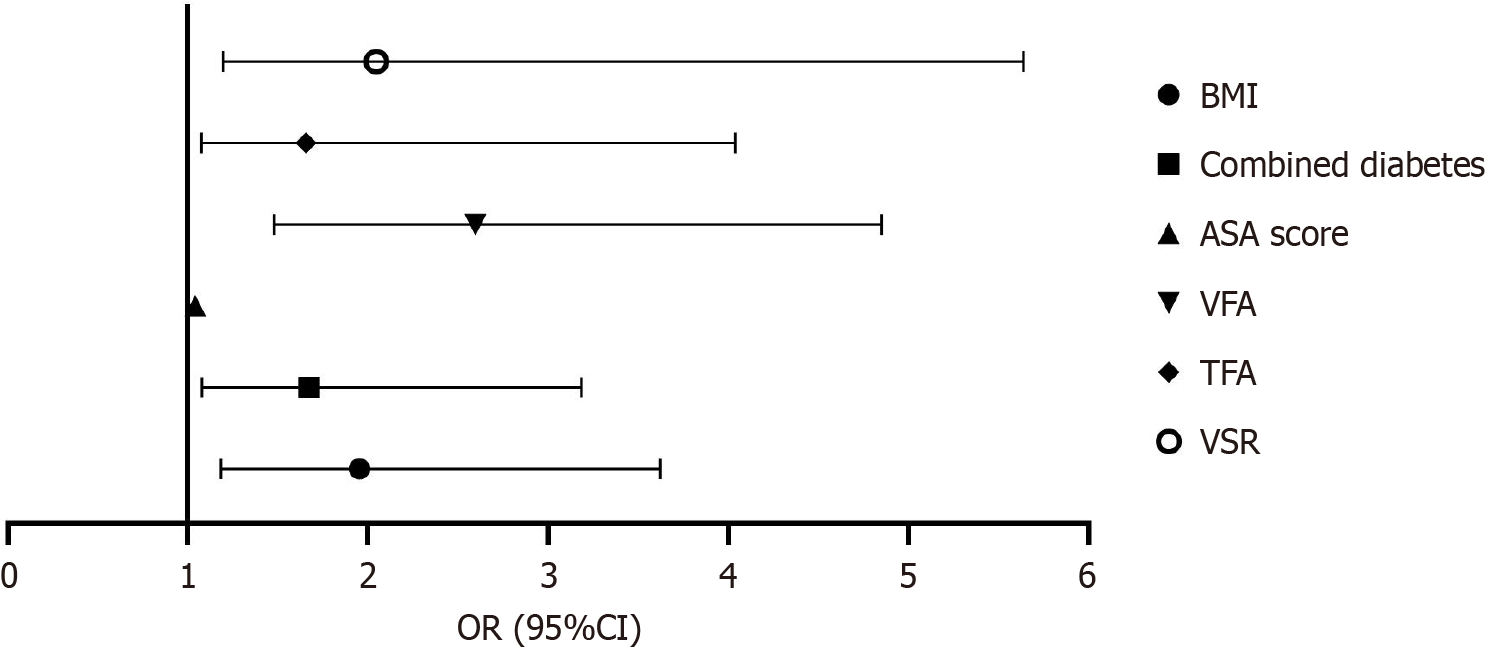
The binary logistic regression analysis showed that BMI, combined history of diabetes, ASA score, VFA, TFA, and VSR were independent risk factors for complications in elderly patients after gastric cancer surgery, as shown in Table 6 and Figure 3.
| Item | B | SE | Wald | P value | OR | 95%CI | |
| Upper | Lower | ||||||
| BMI (kg/m2) | 0.827 | 0.366 | 5.624 | 0.019 | 1.952 | 3.624 | 1.183 |
| Combined diabetes | 0.688 | 0.313 | 3.705 | 0.035 | 1.672 | 3.185 | 1.079 |
| Combined hyperlipidemia | 0.455 | 0.241 | 1.545 | 0.217 | 1.544 | 3.103 | 0.844 |
| ASA score | 0.040 | 0.019 | 4.635 | 0.031 | 1.041 | 1.080 | 1.004 |
| VFA | 0.951 | 0.342 | 8.531 | 0.004 | 2.597 | 4.853 | 1.479 |
| SFA | -0.341 | 0.169 | 2.121 | 0.142 | 0.773 | 1.074 | 0.529 |
| TFA | 0.857 | 0.306 | 6.133 | 0.013 | 1.655 | 4.040 | 1.076 |
| VSR | 0.868 | 0.325 | 7.879 | 0.008 | 2.046 | 5.640 | 1.196 |
Some previous retrospective studies have discussed the effect of visceral fat distribution on postoperative gastric cancer[21,22], mostly due to excessive abdominal fat affecting the surgical outcome; however, few studies have further defined the effect of visceral fat distribution on postoperative complications. Gastrectomy combined with D2 Lymphadenectomy is widely performed in East Asia. Due to the complexity and difficulty of radical surgery for gastric cancer (D2 lymph node dissection), a previous multicenter clinical study involving 17 Chinese medical centers reported that the incidence of serious complications after LTG was as high as 10.3%[23]. According to the results of high-quality RCTs in Japan and South Korea, the incidence of postoperative complications after gastrectomy ranges between 10%-30%[24,25]. Therefore, it is important to identify the complications as early as possible to improve the quality of life and prognosis of patients after surgery. This study retrospectively analyzed the distribution of visceral and subcutaneous fats to provide a relatively high level of evidence clarifying the influence of obesity on postoperative complications in elderly patients undergoing LTG.
A previous study showed that the incidence of postoperative complications in patients with a higher VFA was higher than that in patients with a lower VFA[22], and the results were approximately the same as those in this study. Tokunaga et al[26] found that the proportion of abdominal infections after gastrectomy was 9.6%, which was closely related to the VFA and total fat area. It has been suggested that patients with a high visceral fat content are more likely to develop abdominal infections after gastrectomy. These results were roughly the same as those of the present study, and there were statistically significant differences in the incidence of anastomotic lesions, surgical incision infection, fat liquefaction, and the incidence of total complications. This may be because malignant tumors alter glucose metabolism and induce peripheral insulin resistance due to long-term exposure to proinflammatory molecules[27]. Visceral and subcutaneous fat were independently and positively correlated with peripheral insulin resistance[28]. Diabetes has been proven to be highly associated with anastomotic fistula, infections, and other related conditions[29]. There is also evidence that visceral fat, unlike subcutaneous fat, may contribute to carcinogenesis by secreting pro-inflammatory cytokines and free fatty acids, especially in obese individuals[30,31]. Study has also found that pro-inflammatory cytokines are associated with tumor growth, immune escape and metastasis[32]. In conclusion, fat distribution may influence the occurrence of postoperative complications by affecting blood glucose levels and promoting tumor cell growth. However, this study also found that the operative time and intraoperative blood loss were significantly higher in the VFA-H group than in the VFA-L group. This may be because the excess visceral fat tissue makes the narrow cavity more crowded, increasing the difficulty of laparoscopically assisted gastrectomy. The regression results of this study confirm the above conclusions. In clinical practice, attention should be paid to assessing the fat distribution and nutritional status of patients and providing corresponding management measures to help reduce the occurrence of postoperative complications.
This study also found significant differences in the CONUT scores between the two groups. Lin et al[33] found that in elderly patients with gastric cancer after laparoscopic-assisted radical gastrectomy, the CONUT score is an independent risk factor for overall postoperative complications and can be used as a reliable indicator to judge short-term prognosis. The lower CONUT scores of patients in the VFA-L group may be due to lower ALB levels, as demonstrated in this study. However, whether this is related to the loss of muscle mass needs to be confirmed through further research. In addition, studies have shown that there is a shift in the distribution of VAT and an imbalance of pro-inflammatory adipocytokines in susceptible individuals. Visceral and intramuscular fat have been shown to secrete a large amount of pro-inflammatory cytokines[34], leading to the accumulation of pro-inflammatory immune cells, and the association between obesity and CRP is stronger[35,36]. A positive correlation between the VFA and CRP levels was also observed in this study.
In conclusion, visceral fat distribution has a significant effect on the occurrence of postoperative complications. Therefore, in clinical practice, VFA should be accurately evaluated by CT/magnetic resonance imaging before surgery. In Asian populations, with VFA ≥ 100 cm² as the high-risk threshold, metabolic intervention (such as high-protein diet, etc.) combined with aerobic resistance exercise is recommended to reduce VFA to a safe range within 3-6 months before surgery, while optimizing blood glucose and blood pressure. For patients undergoing short-term surgery, a low-calorie diet can be used to rapidly lose fat; however, branched-chain amino acids and vitamin D supplementation are required to maintain muscle mass. The intraoperative pneumoperitoneum pressure was controlled, and early enteral nutrition was provided after surgery. Finally, multidisciplinary collaboration can achieve simultaneous optimization of metabolism, nutrition, and muscle function to reduce the risk of complications.
Finally, this study has several limitations. First, because this was a retrospective, single-center, cross-sectional study, it was not possible to determine the causal relationship between visceral fat distribution and postoperative complications in gastric cancer. Second, the study population consisted of elderly patients who underwent radical gastrectomy at a single center; therefore, there was sampling bias. In the future, multi-center randomized controlled trials should be conducted to clarify the causal relationship between visceral fat distribution and postoperative complications of gastric cancer, reduce selection bias, and make the results more generalizable.
The results of this study show that postoperative complications are closely related to fat distribution in elderly patients with gastric cancer undergoing gastrectomy. A high VFA is characterized by large intraoperative blood loss, long operation time, and a high incidence of postoperative complications. Clinicians should pay close attention to relevant indicators before surgery to improve the nutritional status of patients to minimize the risk of postoperative complications and promote prognosis.
We would like to express our sincere gratitude to all those who contributed to the successful completion of this study. First, we would like to thank the patients who participated in this study, as well as their families, for their cooperation and trust. Without their willingness to participate, this research would not have been possible. We are deeply grateful to our colleagues at the Department of Gastrointestinal and Anus Surgery, The Affiliated Longyan First Hospital of Fujian Medical University, for their support and assistance throughout the research process. This work was supported by the resources and facilities provided by The Affiliated Longyan First Hospital of Fujian Medical University, Longyan, Fujian Province, China. Finally, we would like to thank the peer reviewers for their insightful feedback, which helped improve the quality of this manuscript.
| 1. | Aringhieri G, Di Salle G, Catanese S, Vivaldi C, Salani F, Vitali S, Caccese M, Vasile E, Genovesi V, Fornaro L, Tintori R, Balducci F, Cappelli C, Cioni D, Masi G, Neri E. Abdominal Visceral-to-Subcutaneous Fat Volume Ratio Predicts Survival and Response to First-Line Palliative Chemotherapy in Patients with Advanced Gastric Cancer. Cancers (Basel). 2023;15:5391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 2. | Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K. Gastric cancer in the elderly: an overview. Eur J Surg Oncol. 2010;36:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 3. | Nelen SD, Bosscha K, Lemmens VEPP, Hartgrink HH, Verhoeven RHA, de Wilt JHW; Dutch Upper Gastrointestinal Cancer Audit group. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg. 2018;105:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Norero E, Vega EA, Diaz C, Cavada G, Ceroni M, Martínez C, Briceño E, Araos F, Gonzalez P, Baez S, Vinuela E, Caracci M, Diaz A. Improvement in postoperative mortality in elective gastrectomy for gastric cancer: Analysis of predictive factors in 1066 patients from a single centre. Eur J Surg Oncol. 2017;43:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Jeong SA, Yook JH, Yoo MW, Kim BS, Lee IS, Kim S, Gong CS, Ko CS. Analysis of risk factors affecting long-term survival in elderly patients with advanced gastric cancer. Aging Clin Exp Res. 2023;35:2211-2218. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Arakawa H, Komatsu S, Kamiya H, Nishibeppu K, Ohashi T, Konishi H, Shiozaki A, Kubota T, Fujiwara H, Otsuji E. Differences of clinical features and outcomes between male and female elderly patients in gastric cancer. Sci Rep. 2023;13:17192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3177] [Cited by in RCA: 2970] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 8. | House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, Gonen M, Olson SH, Kurtz RC, Brennan MF, Allen PJ. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Engeland A, Tretli S, Bjørge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control. 2004;15:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Máchová L, Cízek L, Horáková D, Koutná J, Lorenc J, Janoutová G, Janout V. Association between obesity and cancer incidence in the population of the District Sumperk, Czech Republic. Onkologie. 2007;30:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tanaka T, Kishi S, Ninomiya K, Tomii D, Koseki K, Sato Y, Okuno T, Sato K, Koike H, Yahagi K, Komiyama K, Aoki J, Tanabe K. Impact of abdominal fat distribution, visceral fat, and subcutaneous fat on coronary plaque scores assessed by 320-row computed tomography coronary angiography. Atherosclerosis. 2019;287:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Eide AJ, Halle MK, Lura N, Fasmer KE, Wagner-Larsen K, Forsse D, Bertelsen BI, Salvesen Ø, Krakstad C, Haldorsen IS. Visceral fat percentage for prediction of outcome in uterine cervical cancer. Gynecol Oncol. 2023;176:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol. 2015;24:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 16. | Lee CS, Murphy DJ, McMahon C, Nolan B, Cullen G, Mulcahy H, Sheahan K, Barnes E, Fennelly D, Ryan EJ, Doherty GA. Visceral Adiposity is a Risk Factor for Poor Prognosis in Colorectal Cancer Patients Receiving Adjuvant Chemotherapy. J Gastrointest Cancer. 2015;46:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Pahk K, Joung C, Kim S. Visceral fat metabolic activity evaluated by preoperative (18)F-FDG PET/CT significantly affects axillary lymph node metastasis in postmenopausal luminal breast cancer. Sci Rep. 2020;10:1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Park SW, Lee HL, Ju YW, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS. Inverse association between visceral obesity and lymph node metastasis in gastric cancer. J Gastrointest Surg. 2015;19:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1455] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 20. | Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38-45. [PubMed] |
| 21. | Lv T, Beeharry MK, Zhu ZL. Impact of intra-peritoneal fat distribution on intra-operative bleeding volume with D2 lymphadenectomy in Chinese patients with gastric cancer. Asian J Surg. 2019;42:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Yang SJ, Li HR, Zhang WH, Liu K, Zhang DY, Sun LF, Chen XL, Zhao LY, Chen XZ, Yang K, Chen ZX, Zhou ZG, Hu JK. Visceral Fat Area (VFA) Superior to BMI for Predicting Postoperative Complications After Radical Gastrectomy: a Prospective Cohort Study. J Gastrointest Surg. 2020;24:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Hong QQ, Yan S, Zhao YL, Fan L, Yang L, Zhang WB, Liu H, Lin HX, Zhang J, Ye ZJ, Shen X, Cai LS, Zhang GW, Zhu JM, Ji G, Chen JP, Wang W, Li ZR, Zhu JT, Li GX, You J. Machine learning identifies the risk of complications after laparoscopic radical gastrectomy for gastric cancer. World J Gastroenterol. 2024;30:79-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (5)] |
| 24. | Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 25. | Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 289] [Article Influence: 32.1] [Reference Citation Analysis (1)] |
| 26. | Tokunaga M, Hiki N, Fukunaga T, Ogura T, Miyata S, Yamaguchi T. Effect of individual fat areas on early surgical outcomes after open gastrectomy for gastric cancer. Br J Surg. 2009;96:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2726] [Cited by in RCA: 3198] [Article Influence: 159.9] [Reference Citation Analysis (0)] |
| 28. | Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 639] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 29. | Tan DJH, Yaow CYL, Mok HT, Ng CH, Tai CH, Tham HY, Foo FJ, Chong CS. The influence of diabetes on postoperative complications following colorectal surgery. Tech Coloproctol. 2021;25:267-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 31. | Ongaro E, Buoro V, Cinausero M, Caccialanza R, Turri A, Fanotto V, Basile D, Vitale MG, Ermacora P, Cardellino GG, Nicoletti L, Fornaro L, Casadei-Gardini A, Aprile G. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer. 2017;20:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Jia XH, Feng GW, Wang ZL, Du Y, Shen C, Hui H, Peng D, Li ZJ, Kong DL, Tian J. Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects. Oncotarget. 2016;7:20934-20944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Lin J, Liang H, Zheng H, Li S, Liu H, Ge X. CONUT can be a predictor of postoperative complications after laparoscopic-assisted radical gastrectomy for elderly gastric cancer patients. Medicine (Baltimore). 2023;102:e35424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Kahn DE, Bergman BC. Keeping It Local in Metabolic Disease: Adipose Tissue Paracrine Signaling and Insulin Resistance. Diabetes. 2022;71:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 36. | Fall T, Hägg S, Ploner A, Mägi R, Fischer K, Draisma HH, Sarin AP, Benyamin B, Ladenvall C, Åkerlund M, Kals M, Esko T, Nelson CP, Kaakinen M, Huikari V, Mangino M, Meirhaeghe A, Kristiansson K, Nuotio ML, Kobl M, Grallert H, Dehghan A, Kuningas M, de Vries PS, de Bruijn RF, Willems SM, Heikkilä K, Silventoinen K, Pietiläinen KH, Legry V, Giedraitis V, Goumidi L, Syvänen AC, Strauch K, Koenig W, Lichtner P, Herder C, Palotie A, Menni C, Uitterlinden AG, Kuulasmaa K, Havulinna AS, Moreno LA, Gonzalez-Gross M, Evans A, Tregouet DA, Yarnell JW, Virtamo J, Ferrières J, Veronesi G, Perola M, Arveiler D, Brambilla P, Lind L, Kaprio J, Hofman A, Stricker BH, van Duijn CM, Ikram MA, Franco OH, Cottel D, Dallongeville J, Hall AS, Jula A, Tobin MD, Penninx BW, Peters A, Gieger C, Samani NJ, Montgomery GW, Whitfield JB, Martin NG, Groop L, Spector TD, Magnusson PK, Amouyel P, Boomsma DI, Nilsson PM, Järvelin MR, Lyssenko V, Metspalu A, Strachan DP, Salomaa V, Ripatti S, Pedersen NL, Prokopenko I, McCarthy MI, Ingelsson E; ENGAGE Consortium. Age- and sex-specific causal effects of adiposity on cardiovascular risk factors. Diabetes. 2015;64:1841-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/