Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3157
Peer-review started: February 27, 2023
First decision: March 28, 2023
Revised: April 7, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: May 28, 2023
Processing time: 87 Days and 23.5 Hours
It has been confirmed that three-dimensional (3D) imaging allows easier identification of bile duct anatomy and intraoperative guidance of endoscopic retrograde cholangiopancreatography (ERCP), which reduces the radiation dose and procedure time with improved safety. However, current 3D biliary imaging does not have good real-time fusion with intraoperative imaging, a process meant to overcome the influence of intraoperative respiratory motion and guide navigation. The present study explored the feasibility of real-time continuous image-guided ERCP.
To explore the feasibility of real-time continuous image-guided ERCP.
We selected 2 3D-printed abdominal biliary tract models with different structures to simulate different patients. The ERCP environment was simulated for the biliary phantom experiment to create a navigation system, which was further tested in patients. In addition, based on the estimation of the patient’s respiratory motion, preoperative 3D biliary imaging from computed tomography of 18 patients with cholelithiasis was registered and fused in real-time with 2D fluoroscopic sequence generated by the C-arm unit during ERCP.
Continuous image-guided ERCP was applied in the biliary phantom with a registration error of 0.46 mm ± 0.13 mm and a tracking error of 0.64 mm ± 0.24 mm. After estimating the respiratory motion, 3D/2D registration accurately transformed preoperative 3D biliary images to each image in the X-ray image sequence in real-time in 18 patients, with an average fusion rate of 88%.
Continuous image-guided ERCP may be an effective approach to assist the operator and reduce the use of X-ray and contrast agents.
Core Tip: Three-dimensional imaging allows easier identification of bile duct anatomy and intraoperative guidance of endoscopic retrograde cholangiopancreatography (ERCP). Continuous image-guided ERCP may be an effective means to assist the operator and reduce the use of X-ray and contrast agents.
- Citation: Zhang DY, Yang S, Geng HX, Yuan YJ, Ding CJ, Yang J, Li MY. Real-time continuous image guidance for endoscopic retrograde cholangiopancreatography based on 3D/2D registration and respiratory compensation. World J Gastroenterol 2023; 29(20): 3157-3167
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3157
Endoscopic retrograde cholangiopancreatography (ERCP) was first described by McCune et al[1] at George Washington University in 1968. Superselection of difficult bile ducts contributes to a higher incidence of adverse events, as well as increased radiation exposure for patients and endoscopists[2,3]. Recent studies have shown that even low doses of radiation may induce carcinogenic effects[4]. The radiation dose is one of the recommended quality indicators for ERCP[5,6]. Furthermore, injection of a contrast medium is considered a risk factor for post-ERCP pancreatitis and cholangitis[7]. Therefore, developing new technologies to enhance the efficacy and safety of ERCP is urgent.
Preoperative three-dimensional (3D) imaging is widely used for planning in interventional radiosurgery. Three-dimensional computed tomography (3D-CT) has been utilized in transcatheter arterial chemoembolization (TACE), and allows for better visualization of small vessels[8,9]. Transjugular intrahepatic portosystemic shunt (TIPS) combines preoperative 3D-CT images with images taken at the time of intervention, and has demonstrated a significant reduction in operation times and radiation doses[10]. Three-dimensional imaging is also beneficial in guiding therapeutic ERCP[11], as it allows easier identification of bile duct anatomy and guides intraoperative navigation, reducing necessary radiation dose and operation time with improved safety[11]. For example, biliary stent placement via ERCP in the setting of indeterminate biliary stricture, the X-ray exposure time was reduced from 8.1 min to 35.1 min. However, current 3D biliary imaging does not have good real-time fusion with intraoperative images.
Here, we explored the feasibility of real-time continuous image-guided ERCP, which overcomes the influence of intraoperative respiratory motion and provides an accurate navigation method. Preoperative 3D-CT bile duct sequences were registered and fused with real-time 2D images from the C-arm taken during ERCP; this strategy may improve the quality of ERCP.
This was a single-centered prospective observational study of patients receiving ERCP at a tertiary care hospital between July 2021 and March 2022. Twenty patients with cholelithiasis undergoing therapeutic ERCP were recruited. Only patients with a preoperative 3D-CT sequence of the bile duct were eligible. Dynamic 2D images of the bile duct generated by the fluoroscopic C-arm were obtained. Exclusion criteria were: age < 18 y, poor general health, inability to tolerate basic or intravenous anesthesia, uncontrollable coagulation disorder, anticoagulation therapy that could not be discontinued, altered anatomy (e.g., Billroth II gastrectomy or Roux-en-Y anastomosis), pregnancy, and intraoperative placement of a biliopancreatic stent. Written informed consent was obtained from all patients. The study was approved by the Ethics Committee of the Chinese People’s Liberation Army (PLA) General Hospital (No. S2021-415-01) and was conducted per the Declaration of Helsinki.
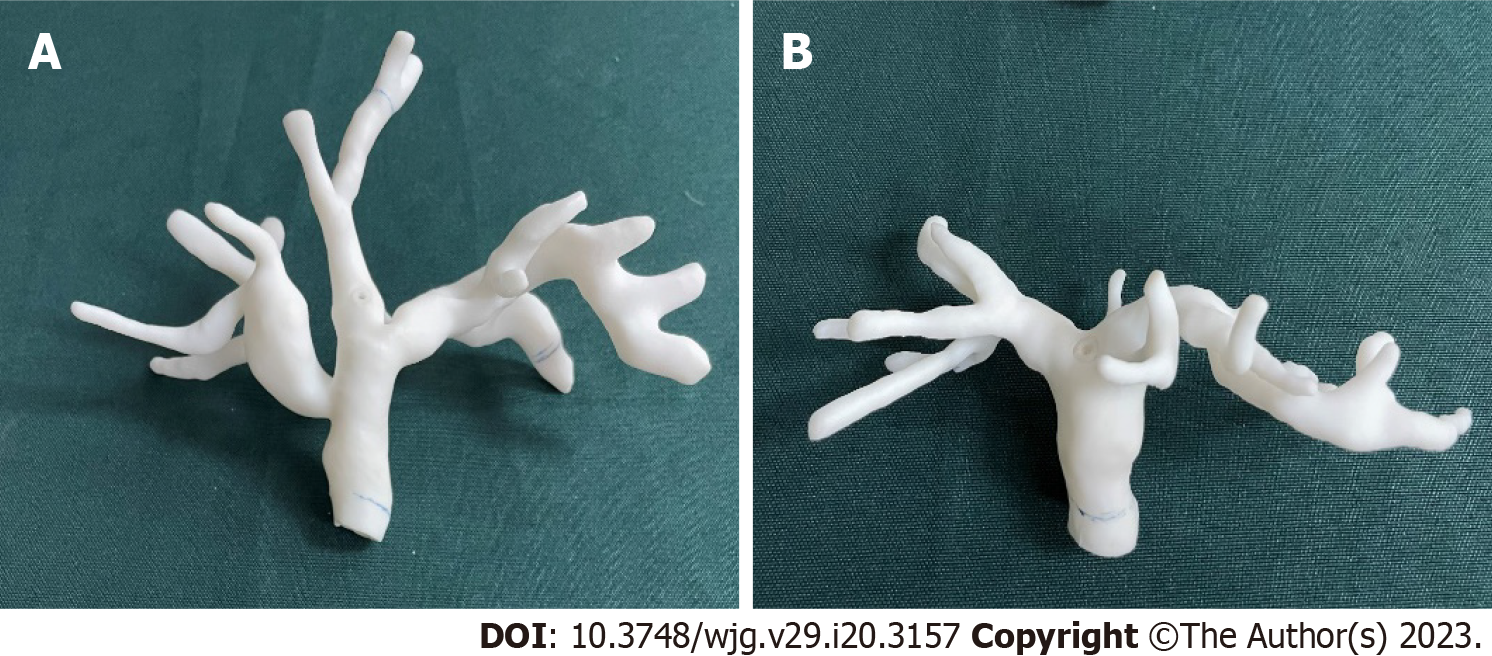
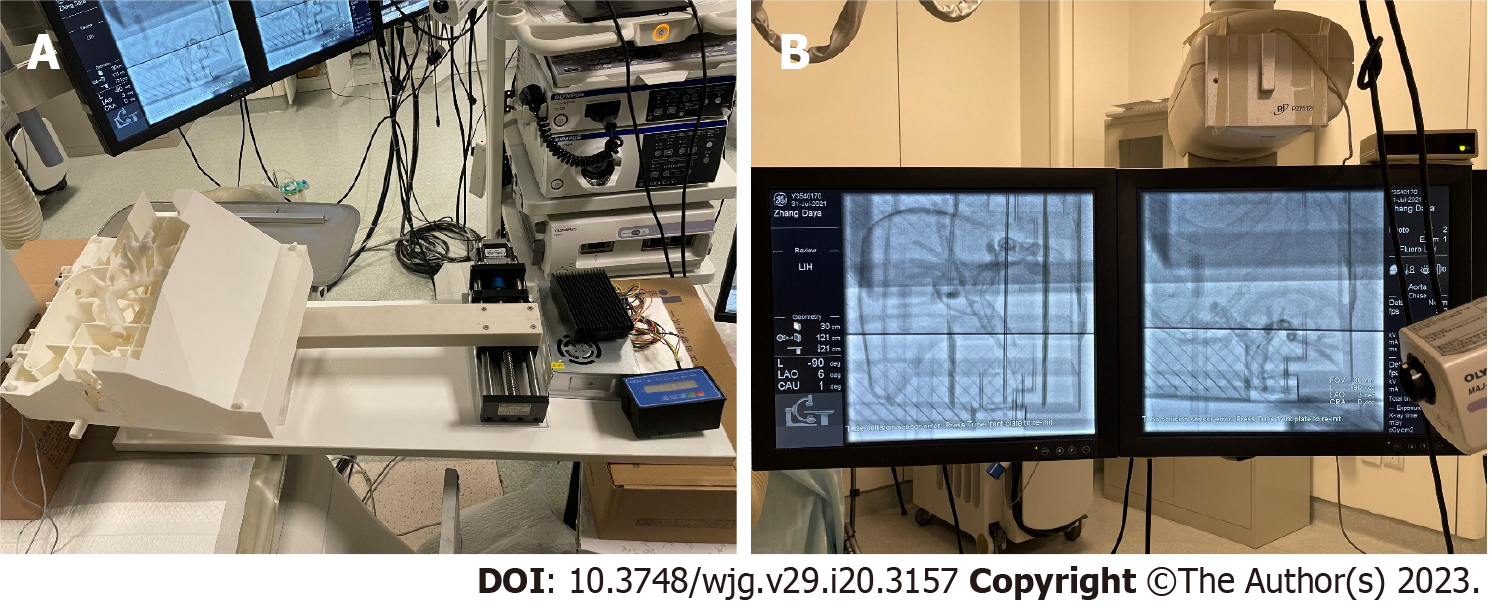
To verify the accuracy of our 3D/2D registration algorithm, we created 2 3D-printed biliary tract models with different structures to simulate different patients. Enhanced CT scan data of 2 patients with biliary dilatation were inputted into a 3D biomechanics research software tool (Visual 3D, Beijing, China). The operator manually segmented the contours of the biliary tract to acquire surface models. The acquired surface models were inputted into a Stratasys J750 3D printer (Markforged, Watertown, Massachusetts) and printed using resin (Figure 1). The printed phantom was life-sized and hollow, allowing sphincterotomes to enter. The phantom experiment was carried out in the PLA General Hospital operating room (Figure 2). Unlike in clinical experiments, model experiments could be repeated many times to simulate real intraoperative conditions to adjust the algorithm parameters.
An iopromide contrast medium (Ultravist 370; Bayer Healthcare, Leverkusen, Germany) was diluted to visualize the biliary tract. All CT scans were performed in the Imaging Department of PLA General Hospital (uCT 510; United Imaging Healthcare, Shanghai, China) with a slice thickness of 1.25 mm and a tube voltage of 120 kV. Patients were asked to remain in the end-inspiratory state for CT scan. To acquire the best alignment transformation, patients were asked to maintain the same pre-operative state at the beginning of surgery. Then, fluoroscopy was performed and the image was defined as the best matching frame. On the day of the ERCP procedure, the acquired CT scan data were imported into Mimics (Materialise, Belgium) to reconstruct the 3D volume-render models of the biliary tract, liver, gallbladder, and bone. All patients received intraoperative deep sedation. ERCP was performed using a standard endoscope (Olympus Duodenoscope TJF260). When the guidewire was successfully superselected in the bile duct and the contrast agent was injected into the duct, the C-arm system performed 1 consecutive respiratory cycle of fluoroscopy around the patient, producing approximately 20 images which were transmitted in real time to our image navigation system.
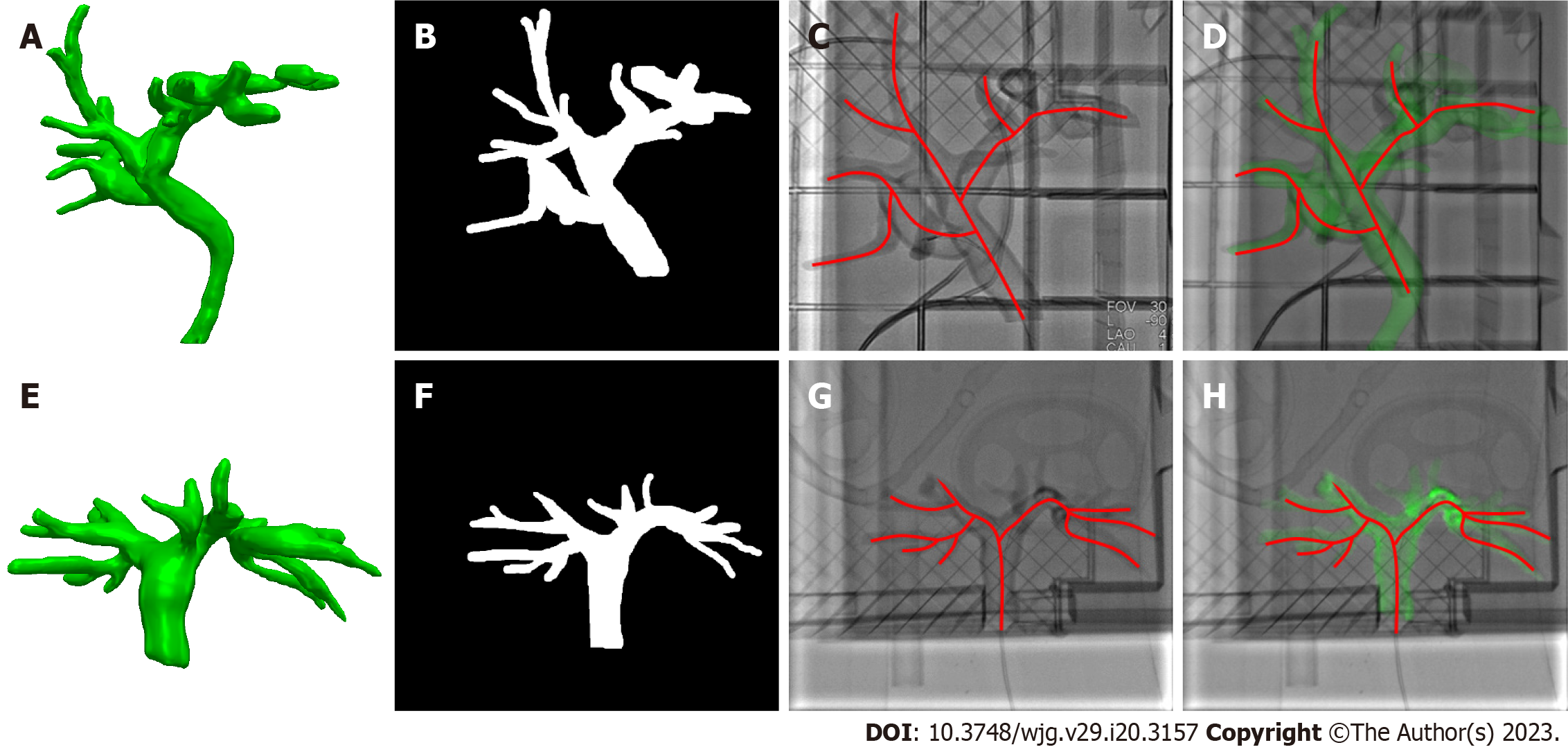
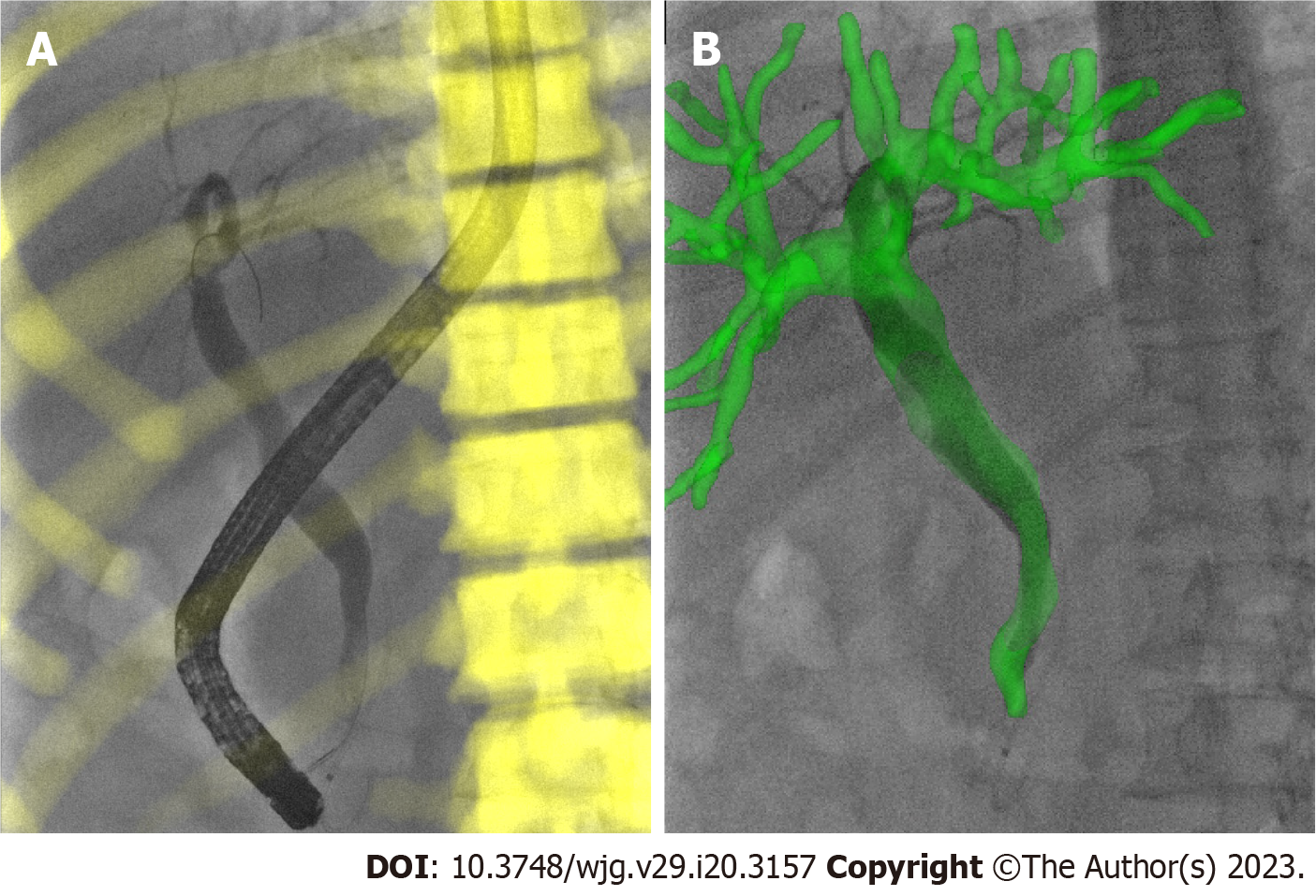
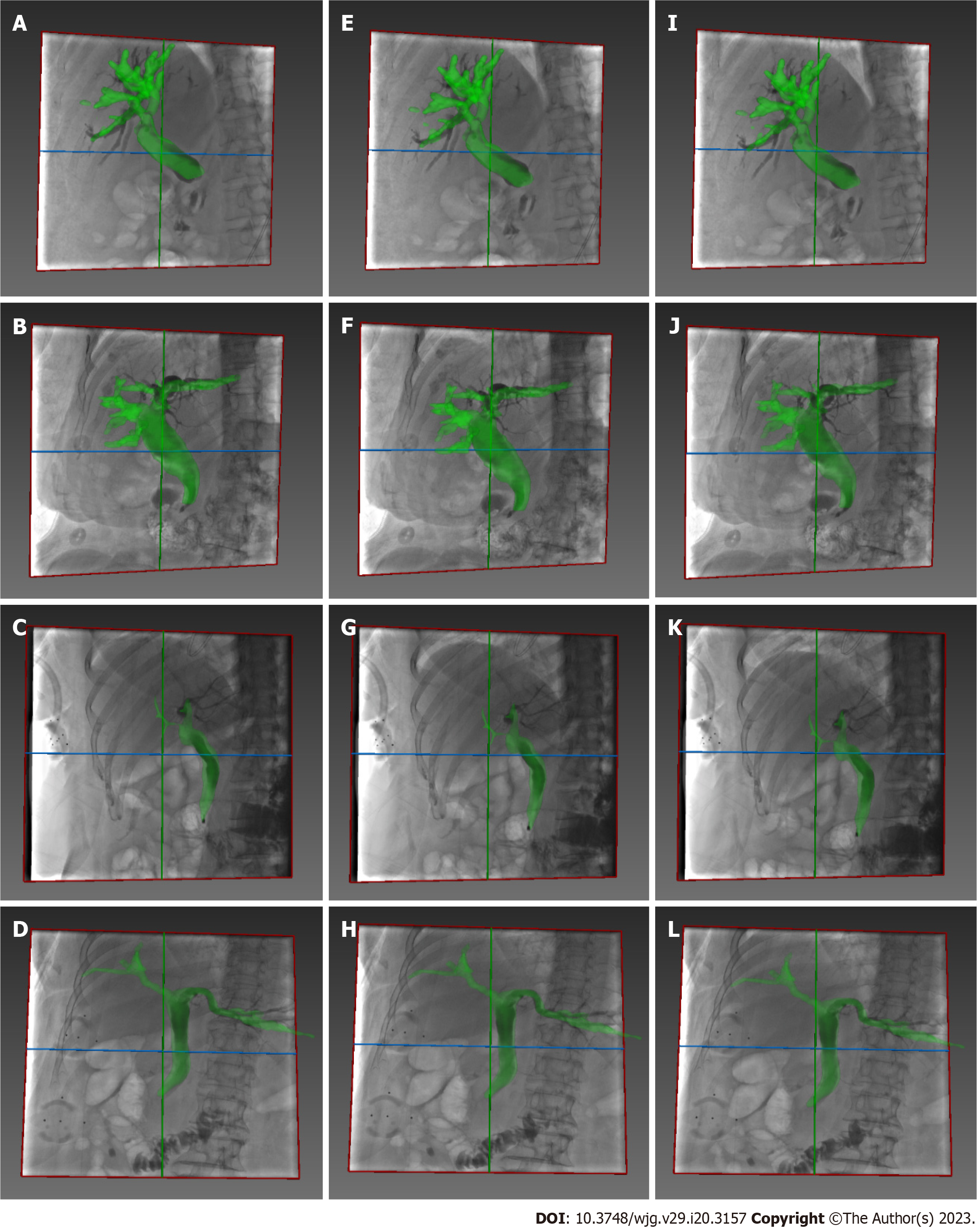
During the registration between CT and X-ray fluoroscopic images, the CT image was aligned with X-ray fluoroscopy through common features. For the biliary phantom, the registration fiducials were extracted from the CT and X-ray images, respectively, to get rigid transformation, by which the 3D biliary model segmented from CT was projected onto the X-ray fluoroscopic image to finish the overlap of biliary roadmapping. The biliary ducts were observed with X-ray images without contrast agents during model experimentation (Figure 3). After the 3D/2D registration was completed, the biliary ducts segmented from CT were projected onto the X-ray image, and the validity of the registration was judged according to the edge blending effect.
The bone, liver, gallbladder, and biliary tract were segmented by a 3D-UNet model based on pre-operative CT data (Figure 4). Afterward, the Medical Imaging Interaction Toolkit was used to extract 2D bone structures. Mimics software (Materialise, Leuven, Belgium) was used to extract 3D and 2D biliary centerlines for analysis. Finally, 3D/2D registration based on bone structures was performed to achieve effective fusion of the 3D biliary model and the best matching frame (Figure 3).
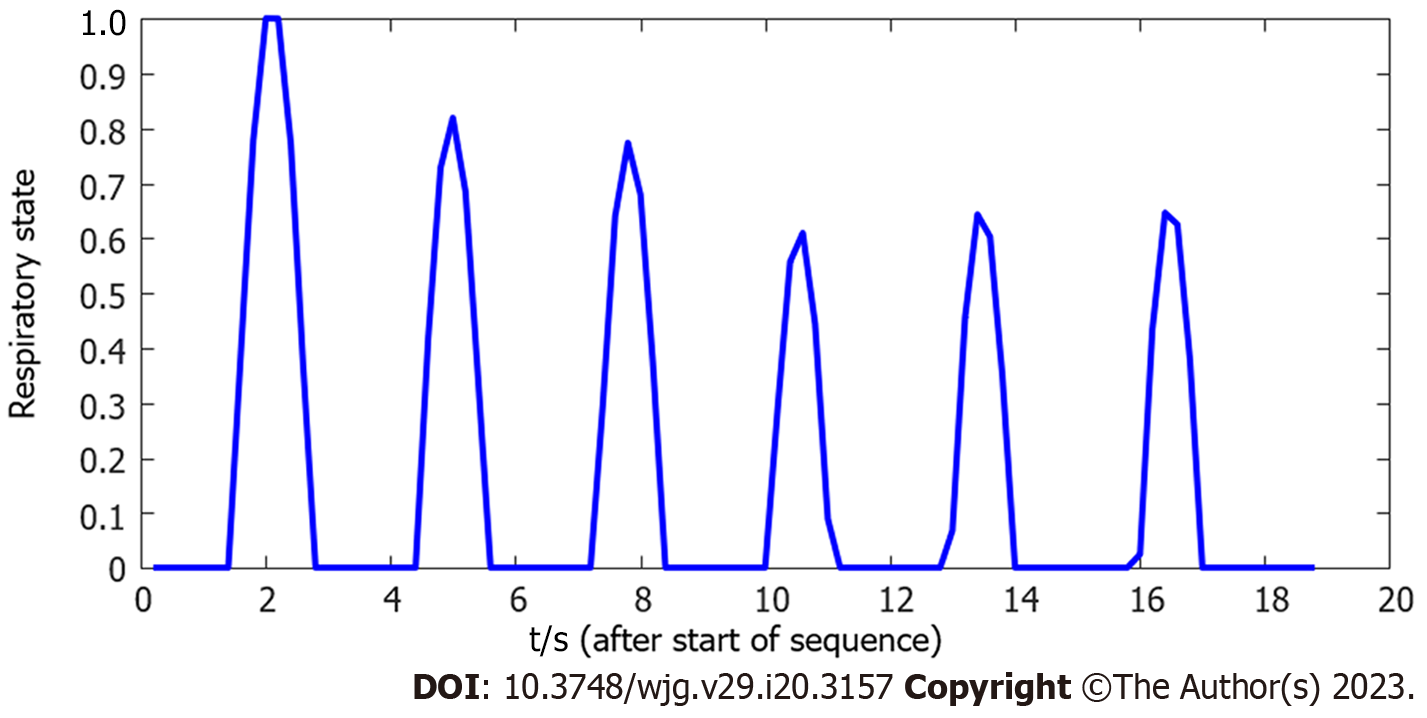
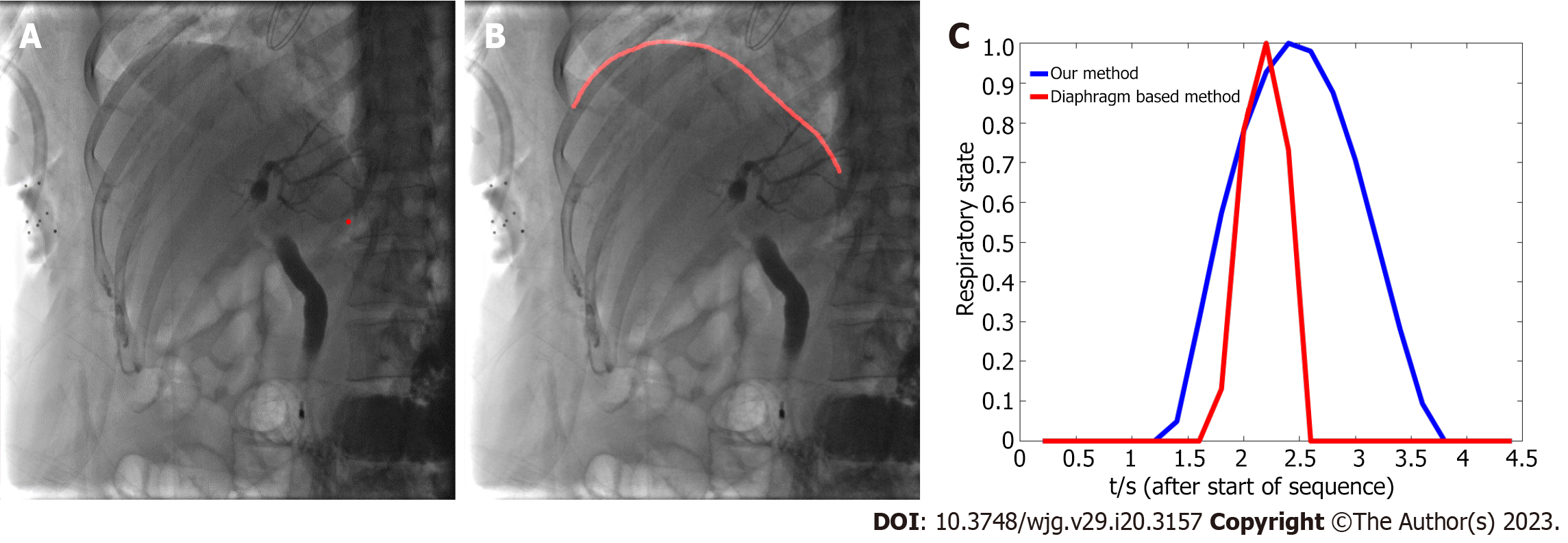
The respiratory motion of the patient during the operation affects fusion accuracy. Some researchers have estimated the patient’s respiratory state by extracting diaphragm data in real time[12-15]. The diaphragm-based method not only requires manual annotation, but also cannot guarantee real-time performance, thus prolonging the operation time and increasing X-ray radiation exposure. The method based on X-ray image centroid extraction was used in the current study to estimate the respiratory state[9] and monitor the motion change between frames through X-ray image gray distribution. The respiratory motion curve was acquired and used to complete continuous compensation for the fluoroscopy sequence, and real-time biliary roadmapping projected onto X-ray fluoroscopy was completed. This 3D/2D image registration technology is available in the current intraoperative imaging system takes approximately 60 s. Our method has a fast calculation speed and is convenient to operate, features that aid in real-time navigation.
Quantitative data included the registration error and the tracking error (TE). The registration error was calculated as the mean square error (MSE) between the projection of the 3D and 2D centerline. After finishing respiratory compensation and projecting, the mean MSE was set as the TE. Qualitative parameters included image quality and real-time fusion rates for both preoperative and intraoperative images.
Image-guided ERCP: Image quality was defined as “good” if the 3D-CT and fluoroscopic images of the bile duct filled by a contrast medium were visible. The outcome of real-time fusion rates was assessed by the degree to which the bile duct in the fluoroscopic mode matched perfectly with the 3D-CT biliary imaging.
Data were assessed for normality, and appropriate descriptive statistics were calculated and expressed as mean ± standard deviation (SD) and frequency (percentages). Data were analyzed using SPSS version 22 (IBM Corp., Armonk, NY, United States).
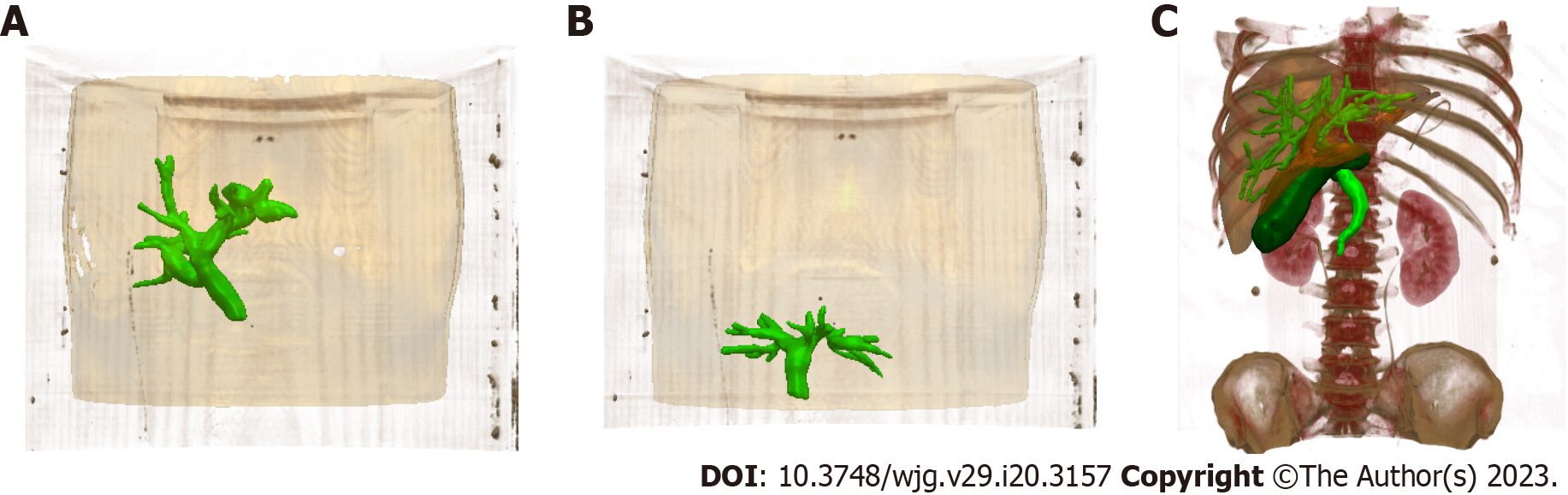
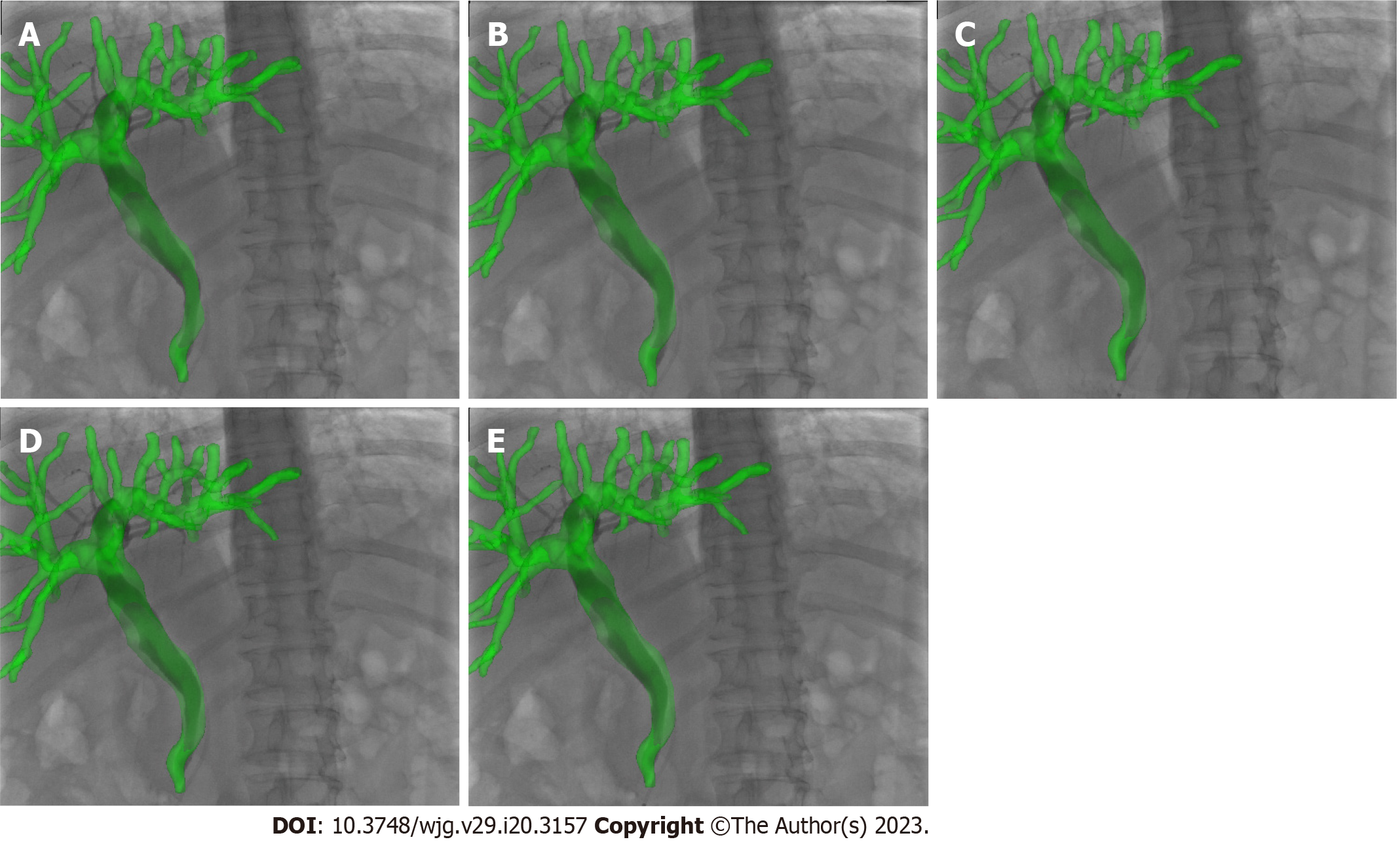
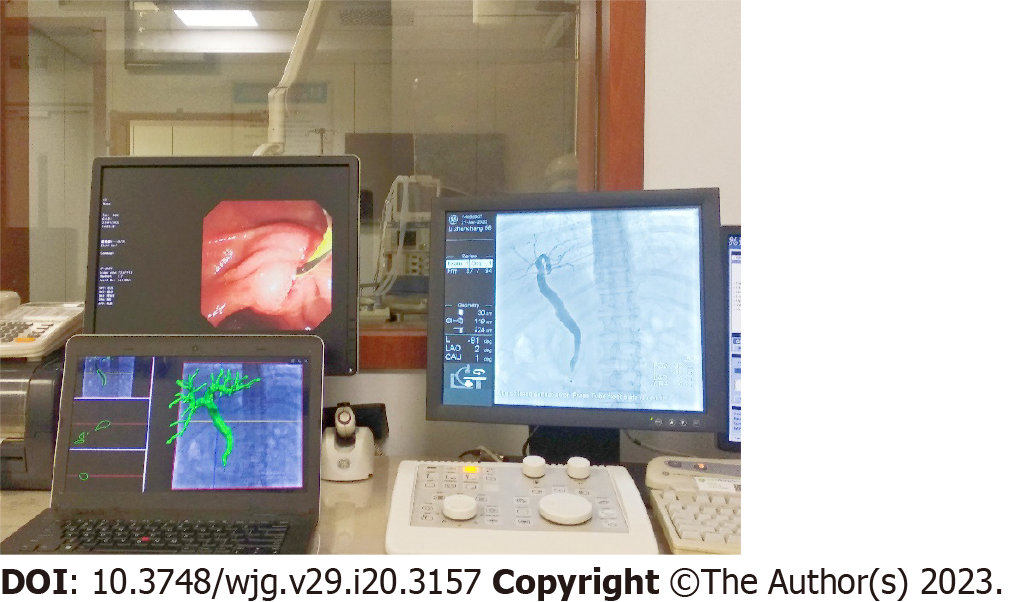
Image-guided ERCP had a mean registration error of 0.46 mm ± 0.13 mm and a TE of 0.64 mm ± 0.24 mm. For patients, the biliary model was projected onto the X-ray based on 3D/2D registration (Figure 5), and the respiratory motion curve was estimated through a continuous X-ray fluoroscopy sequence (Figure 6). Continuous biliary roadmapping guidance was acquired (Figure 7). Our navigation system was performed in the operating room (Figure 8), and it provided image-based guidance in multiple patients (Figure 9). Image quality was good in all 18 cases and CT to X-ray fluoroscopic image registration and fusion were technically feasible, with an average fusion rate of 88% (Supplementary material).
In the current study, continuous image guidance for ERCP based on 3D/2D registration was evaluated, and we found that this strategy may be an effective means of real-time localization of the bile duct.
The prognosis of hilar cholangiocarcinoma (HCCA) is extremely poor, and surgery is the only radical treatment modality. However, most patients present with an unresectable tumor or cholangitis, high bilirubin, and insufficient volume of the residual liver; as such, biliary drainage by ERCP is often best available treatment. Previous studies have shown the benefit of 3D imaging in guiding difficult ERCP procedures[11]. Preoperative 3D-CT, magnetic resonance cholangiopancreatography, magnetic resonance virtual endoscopy, or intraoperative 3D-CT of the cone beam have all provided adequate guidance for biliary stenting in patients with HCCA, but do not achieve real-time fusion of 3D images with 2D fluoroscopic images or display guidewire or sphincterotomes in three dimensions[16-19]. Our technology can obtain 3D models of the biliary duct, tumor, and hilar vessels through preoperative magnetic resonance imaging/CT segmentation. The registration between preoperative 3D models and intraoperative 2D X-ray images was completed based on the centerlines of the biliary ducts, and 3D models were projected onto X-ray images for display. Meanwhile, based on the respiratory compensation method, a continuous fusion between 3D models and the X-ray image sequences was achieved, and real-time guidance was realized through graphics processing unit acceleration. We believe that our method may provide physicians with more visual information during surgery and effectively improve the operability of the intervention.
The present study reports real-time fusion of preoperative 3D-CT images with the 2D fluoroscopic images during ERCP. The radiation dose required for the registration between 3D-CT and fluoroscopic image can be considered negligible compared with the total radiation dose for difficult ERCP procedures. In addition, image registration was completed in an average time of 1 min, with a minimal overall increase in total operation time. Therefore, with the real-time visual aid during ERCP, it is likely that less radiation, contrast medium, and operative time may be required during the navigation.
Furthermore, our methods estimated the respiratory state better than the classical diaphragm extraction method[17]. Since the diaphragm is incomplete or invisible during ERCP in some patients, the applicability and robustness of our method are better based on image gray distribution. Furthermore, this method can achieve a more accurate estimation of the respiratory state of the biliary tract, thus ensuring better fusion of 3D-CT and 2D X-ray images. The main reason for this is that the respiratory motion of the diaphragm can reflect well upon the upper edge of the liver, but cannot directly represent the motion of the biliary tract. Experimental results also proved that our method is more accurate for biliary motion estimation of patients under free breathing. Compared with the TE of the diaphragm-based method, based on our cholangiography image fusion results, TE was relatively low (1.68 mm ± 0.34 mm vs 0.64 mm ± 0.24 mm), providing more accurate guidance. As shown in Figure 10, the respiratory state estimation based on our method was smoother and closer to the true breathing curve, which ensures the stability of image-guided navigation. Moreover, our method is simpler and faster to compute and process intraoperative X-ray fluoroscopic image sequences in real-time. Conversely, the diaphragm-based method requires real-time segmentation of the diaphragm whether manual or network methods are used, which represents a complex task and ultimately leads to an increase in operation time and radiation dose.
There are further implications of our technology in the context of other minimally invasive procedures such as TACE, TIPS, and spine surgery[8-10,20]. Coronavirus disease-2019 (COVID-19) had devastating impacts on healthcare system operations. The pandemic disrupted normal workflow of spine surgeries, restricting and postponing elective procedures[20]. This disruption may have resulted in the prolonged impairment of patients who were forced to postpone their procedures. Minimally invasive surgery and perioperative telemedicine are inevitable trends in spine surgery after COVID-19. Spine surgery is usually completed under the guidance of X-ray images, and physicians need to conceive the 3D structure of the patient’s spine based on clinical experience. The spine is different from the soft liver and there is no respiratory interference; however, the similarity of the structure between the joints and the overall length makes surgery more difficult to perform only under the guidance of X-ray images. Meanwhile, the increase in operation time has also caused longer waiting periods for treatment. Our method can extract the 3D spine structure from preoperative CT and complete 3D/2D registration through the bone structure line, achieving the fusion of 3D information with intraoperative X-ray images. This technology could help physicians better localize lesions and enhance visual perception of depth. Fusion of 3D/2D imaging could also help physicians, especially those with insufficient clinical experience, to observe the patient’s spine structure more intuitively and improve surgical efficiency. In summary, the number of spinal surgeries increased following COVID-19 and has continued to rise; our technology may help the hospital to perform more operations and allow patients to receive timely and effective treatment.
COVID-19 has also been associated with an increased risk for ischemic and hemorrhagic strokes[21], conditions often treated with a vascular intervention procedure very similar to TACE that is achieved by embolizing the vessels connected to the aneurysm. Unlike liver vessels, intracranial vessels are not affected by respiratory motion and remain static with the use of X-ray images for guidance. However, intracranial vessels are generally thin and have multiple branching structures, and thus the risks associated with intracranial surgery are high. Surgeons require more clinical experience to ensure the accuracy of the surgery; otherwise, they may injure other blood vessels or tissues and cause secondary harm to the patient. Our technology can extract data regarding blood vessels connected to the lesion (e.g., aneurysm) before the surgery and guide the surgeon intraoperatively through high-precision registration and fusion. Given the risk of intracranial vascular intervention procedures, our technology could display the vessels around the catheter and render them in different colors to remind the surgeon to be cautious, thus benefiting the safety of the procedure. We believe that our technology may have potential clinical value in treating cerebrovascular diseases and could precisely treat ischemic and hemorrhagic strokes associated COVID-19.
Our image-guided ERCP strategy was fully validated in models that may be useful in cannulating specific biliary ducts in patients with complex biliopancreatic diseases such as HCCA. However, clinical studies are still needed to determine if this new approach could improve technical success while reducing radiation exposure and contrast agent administration during ERCP.
Three-dimensional (3D) imaging is beneficial in guiding therapeutic endoscopic retrograde cholangiopancreatography (ERCP), reducing the radiation dose and procedure time while increasing overall procedure safety. However, current 3D biliary imaging does not have good real-time fusion with intraoperative images.
We explored the feasibility of real-time continuous image-guided ERCP, which was shown to overcome the influence of intraoperative respiratory motion and provides an accurate navigation method.
To explore the feasibility of real-time continuous image-guided ERCP.
We simulated an ERCP environment using an experimental biliary phantom with the aim of designing a navigation system; this system was further tested in patients undergoing ERCP.
Continuous image-guided ERCP was applied in the biliary phantom with low registration and tracking errors. This 3D/2D registration accurately transformed preoperative 3D biliary images to each image in the X-ray sequence in real-time.
Continuous image-guided ERCP may be an effective approach to assist the operator and reduce the use of X-ray and contrast agents.
Clinical studies are needed to determine if this image-guided ERCP strategy could improve technical success while reducing radiation exposure and contrast agent administration during ERCP.
| 1. | McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 383] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Weiser R, Pencovich N, Mlynarsky L, Berliner-Senderey A, Lahat G, Santo E, Klausner JM, Nachmany I. Management of endoscopic retrograde cholangiopancreatography-related perforations: Experience of a tertiary center. Surgery. 2017;161:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Fung BM, Pitea TC, Tabibian JH. Difficult Biliary Cannulation in Endoscopic Retrograde Cholangiopancreatography: Definitions, Risk Factors, and Implications. Eur Med J Hepatol. 2021;9:64-72. [PubMed] |
| 4. | Hong JY, Han K, Jung JH, Kim JS. Association of Exposure to Diagnostic Low-Dose Ionizing Radiation With Risk of Cancer Among Youths in South Korea. JAMA Netw Open. 2019;2:e1910584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 5. | Adler DG, Lieb JG 2nd, Cohen J, Pike IM, Park WG, Rizk MK, Sawhney MS, Scheiman JM, Shaheen NJ, Sherman S, Wani S. Quality indicators for ERCP. Gastrointest Endosc. 2015;81:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Hayashi S, Takenaka M, Hosono M, Nishida T. Radiation exposure during image-guided endoscopic procedures: The next quality indicator for endoscopic retrograde cholangiopancreatography. World J Clin Cases. 2018;6:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 539] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 8. | Hammami H, Lalys F, Rolland Y, Petit A, Haigron P. Catheter navigation support for liver radioembolization guidance: feasibility of structure-driven intensity-based registration. Int J Comput Assist Radiol Surg. 2020;15:1881-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Wagner MG, Periyasamy S, Longhurst C, McLachlan MJ, Whitehead JF, Speidel MA, Laeseke PF. Real-time respiratory motion compensated roadmaps for hepatic arterial interventions. Med Phys. 2021;48:5661-5673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Meine TC, Dewald CLA, Becker LS, Mähringer-Kunz A, Maasoumy B, Maschke SK, Kirstein MM, Werncke T, Wacker FK, Meyer BC, Hinrichs JB. Correction to: Transjugular intrahepatic portosystemic shunt placement: portal vein puncture guided by 3D/2D image registration of contrast-enhanced multi-detector computed tomography and fluoroscopy. Abdom Radiol (NY). 2021;46:398. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Cao D, Chen SL, Li YM, Zheng YW, Ohkohchi N. Current trends in three-dimensional visualization and real-time navigation as well as robot-assisted technologies in hepatobiliary surgery. World J Gastrointest Surg. 2021;13:904-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 12. | Timinger H, Krueger S, Dietmayer K, Borgert J. Motion compensated coronary interventional navigation by means of diaphragm tracking and elastic motion models. Phys Med Biol. 2005;50:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Chen PY. A novel network module for medical devices. Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:1553-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | King AP, Boubertakh R, Rhode KS, Ma YL, Chinchapatnam P, Gao G, Tangcharoen T, Ginks M, Cooklin M, Gill JS, Hawkes DJ, Razavi RS, Schaeffter T. A subject-specific technique for respiratory motion correction in image-guided cardiac catheterisation procedures. Med Image Anal. 2009;13:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Shahshahani A, Laverdiere C, Bhadra S, Zilic Z. Ultrasound Sensors for Diaphragm Motion Tracking: An Application in Non-Invasive Respiratory Monitoring. Sensors (Basel). 2018;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Toki M, Tateishi H, Yoshida T, Gondo K, Watanabe S, Hisamatsu T. Utilization of a new technology of 3D biliary CT for ERCP-related procedures: a case report. BMC Gastroenterol. 2020;20:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Reuterwall M, Waldthaler A, Lubbe J, Kadesjö N, Pozzi Mucelli R, Del Chiaro M, Lohr M, Arnelo U. Bimodal ERCP, a new way of seeing things. Endosc Int Open. 2020;8:E368-E376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Azuma T, Yamaguchi K, Iida T, Oouhida J, Suzuki M. MR virtual endoscopy for biliary tract and pancreatic duct. Magn Reson Med Sci. 2007;6:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Weigt J, Pech M, Kandulski A, Malfertheiner P. Cone-beam computed tomography - adding a new dimension to ERCP. Endoscopy. 2015;47:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Lucke-Wold B, Cerillo JL, Becsey AN, Chernicki BP, Root KT. Minimally Invasive Procedures, Perioperative Telemedicine, and Decreased Hospital Stays Following Covid-19 Surgical Restrictions: Spinal Surgery. Arch Med Case Rep Case Study. 2022;6. [PubMed] |
| 21. | Small C, Mehkri Y, Panther E, Felisma P, Lucke-Wold B. Coronavirus Disease-2019 and Stroke: Pathophysiology and Management. Can J Neurol Sci. 2022;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Donato G, Italy; Lucke-Wold B, United States S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YX