Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3357
Peer-review started: January 29, 2021
First decision: April 5, 2021
Revised: April 14, 2021
Accepted: June 7, 2021
Article in press: June 7, 2021
Published online: June 21, 2021
Processing time: 140 Days and 3.6 Hours
New-onset prediabetes/diabetes after acute pancreatitis (NODAP) is the most common sequela of pancreatitis, and it differs from type 2 prediabetes/diabetes mellitus (T2DM).
To study the associations between circulating levels of pancreatic amylase, pancreatic lipase, chymotrypsin and fat phenotypes in NODAP, T2DM, and health.
Individuals with NODAP (n = 30), T2DM (n = 30), and sex-matched healthy individuals (n = 30) were included. Five fat phenotypes (intra-pancreatic fat, liver fat, skeletal muscle fat, visceral fat, and subcutaneous fat) were determined using the same magnetic resonance imaging protocol and scanner magnet strength for all participants. One-way analysis of covariance, linear regression analysis, and relative importance analysis were conducted.
Intra-pancreatic fat deposition (IPFD) was higher in NODAP (9.4% ± 1.8%) and T2DM (9.8% ± 1.1%) compared with healthy controls (7.8% ± 1.9%) after adjusting for covariates (P = 0.003). Similar findings were observed in regards to visceral fat volume (P = 0.005), but not subcutaneous fat volume, liver fat, or skeletal muscle fat. Both IPFD (β = -2.201, P = 0.023) and visceral fat volume (β = -0.004, P = 0.028) were significantly associated with circulating levels of pancreatic amylase in NODAP, but not in T2DM or healthy individuals. Of the five fat phenotypes, IPFD explained the highest amount of variance in pancreatic amylase concentration (R2 = 15.3% out of 41.2%). None of the phenotypes contributed meaning
Both NODAP and T2DM are characterized by increased IPFD and visceral fat volume. However, only NODAP is characterized by significant inverse associations between the two fat phenotypes and pancreatic amylase.
Core Tip: Intra-pancreatic fat deposition and visceral fat volume are significantly inversely associated with circulating levels of pancreatic amylase in individuals with new-onset prediabetes/diabetes after acute pancreatitis, but not in healthy individuals or those with type 2 prediabetes/diabetes mellitus.
- Citation: Ko J, Skudder-Hill L, Cho J, Bharmal SH, Petrov MS. Pancreatic enzymes and abdominal adipose tissue distribution in new-onset prediabetes/diabetes after acute pancreatitis. World J Gastroenterol 2021; 27(23): 3357-3371
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3357
Individuals after acute pancreatitis often develop metabolic sequelae such as post-pancreatitis diabetes, which accounts for 80% of cases of diabetes of the exocrine pancreas-the second most common type of adult-onset diabetes[1,2]. There is a circulating biomarker, involved in the regulation of exocrine pancreatic function, that distinguishes post-pancreatitis diabetes from type 2 diabetes[3]. Also, epidemiological data have shown that post-pancreatitis diabetes leads to worse clinical outcomes compared with type 2 diabetes. A population-based study found that individuals with post-pancreatitis diabetes were more likely to have poor glycemic control and to require more insulin than individuals with type 2 diabetes[4]. Another population-based study demonstrated that individuals with post-pancreatic diabetes (versus type 2 diabetes) were at a higher risk of mortality from cancer, gastrointestinal diseases, and infectious diseases, as well as hospitalization for chronic pulmonary disease, renal disease, and infectious disease[1]. The reasons for the above differences between post-pancreatic diabetes and type 2 diabetes are not fully understood but are worth investigating with a view to optimizing the management of both types of diabetes.
Excess deposition of body fat increases the risk of diabetes and has a deleterious effect on its clinical outcomes[5-9]. However, little evidence exists on the difference in excess body fat between post-pancreatitis diabetes and type 2 diabetes. A 2017 population-based study of people with new-onset diabetes showed that the proportion of individuals with obesity was higher in type 2 diabetes (48%) vs diabetes of the exocrine pancreas (35%)[4]. A 2020 population-based study of individuals with a history of clinically resolved acute pancreatitis demonstrated that the risk of new-onset diabetes was higher among individuals with normal body mass index (BMI) than those in the overall cohort (adjusted odds ratios of 3.1 and 2.1, correspondingly)[10]. BMI is a commonly used proxy for general adiposity but it may be suboptimal in quantifying excess abdominal fat[1,2]. Given that the effect of excess abdominal fat on metabolic functions depends on not only the degree of fat deposition but also its distribution[9,11-13], data on various abdominal fat phenotypes [determined with the use of magnetic resonance imaging (MRI)] are likely to provide useful insights[14-17]. For example, intra-pancreatic fat deposition (IPFD) and visceral fat volume were significantly higher in individuals with post-pancreatitis diabetes compared with healthy individuals[1]. Also, individuals with type 2 diabetes had a significantly higher IPFD compared with healthy individuals[18]. However, to date, no study has compared head-to-head abdominal fat phenotypes in post-pancreatitis diabetes vs type 2 diabetes. Also, individuals with metabolic disorders (including type 2 diabetes and obesity) not infrequently have within-normal but significantly lower circulating levels of pancreatic enzymes (amylase, lipase, and trypsin) compared with healthy individuals[19], and exocrine pancreatic dysfunction often develops after acute pancreatitis[2]. Hence, it is conceivable that the relationship between abdominal adipose tissue distribution and pancreatic enzymes may differ in post-pancreatitis diabetes vs type 2 diabetes.
The primary aim was to compare the differences in MRI-derived abdominal fat phenotypes between healthy individuals and the two types of diabetes. The secondary aim was to investigate the associations between abdominal fat phenotypes and circulating levels of pancreatic enzymes in the study groups.
The present case-control study was nested into prospective cohort study of individuals after an attack of acute pancreatitis (ARIES project) and was approved by the Health and Disability Ethics Committee (13/STH/182) of New Zealand. Individuals with new-onset prediabetes or diabetes after acute pancreatitis (NODAP) were randomly selected and 1:1 matched on sex with individuals with type 2 prediabetes or diabetes mellitus (T2DM) from the same cohort. Individuals with fasting plasma glucose (FPG) ≥ 100 mg/dL (≥ 5.6 mmol/L) and/or glycated hemoglobin A1c (HbA1c) ≥ 5.7% (39 mmol/mol) beyond three months after an attack of acute pancreatitis constituted the NODAP group, in line with the published recommendations[1]. Individuals with HbA1c ≥ 5.7% (39 mmol/mol) before, during hospitalization for AP, or within three months after it constituted the T2DM group. FPG ≥ 100 mg/dL (≥ 5.6 mmol/L) during hospitalization was not considered as an eligibility criterion for the T2DM group due to the possibility of stress-induced hyperglycemia during acute illness[1]. All cases were at least 18 years old, provided informed consent, had a primary diagnosis of mild acute pancreatitis established prospectively at the time of hospitalization, and met the American Diabetes Association criteria for prediabetes or diabetes[6].
Individuals were excluded from the study if they had a recurrent attack of acute pancreatitis within three months of the enrollment date, chronic pancreatitis, post-endoscopic retrograde cholangiopancreatography pancreatitis, pancreatic cyst, pancreatic lipomatosis or lipomatous pseudohypertrophy, congenital anomalies of the pancreas, hereditary pancreatitis, cystic fibrosis, malignancy, cognitive disability, received surgical, endoscopic or radiological interventions involving the pancreas, had metallic foreign body implantations, heart pacemakers, or other electronic device implantations, received steroid therapy, or were pregnant.
The control group included healthy volunteers who were 1:1 matched on sex with the two case groups. These participants were at least 18 years old, provided informed consent, had no personal and family history of diseases of the exocrine pancreas and diabetes, had no family history of cystic fibrosis or coeliac diseases, had no upper abdominal symptoms in the 12 mo preceding the study, had no history or evaluation for infectious or inflammatory diseases in the 6 mo preceding the study, and had no history of cancer.
Imaging protocol: Abdominal MRI for all participants was performed at the Centre of Advanced MRI (The University of Auckland) using 3.0 Tesla MAGNETOM Skyra scanner (Siemens, Erlangen, Germany). All participants underwent MRI wholly and exclusively for the purpose of the ARIES project. During the MRI, participants lied down in supine position and were asked to hold their breath during end-expiration. Axial T1-weighted volumetric interpolated breath-hold examination Dixon sequence was applied with the following parameters: true form abdomen shim mode; field of view, 420 mm; base resolution, 320; echo time, 1.27 ms, 2.5 ms; repetition time, 3.85 ms; flip angle, 9; pixel bandwidth, 920 Hz; slice thickness, 5 mm. All but liver fat phenotypes were quantified independently by two observers and average values of two independent MRI measurements were used for statistical analyses. The observers were blinded to the group allocation.
Intra-pancreatic fat: Intra-pancreatic fat was quantified using the 'MR-opsy' technique, as described in detail elsewhere[20]. In brief, two candidate slices with clear visualization of the pancreas were selected from a series of abdominal scans. Three regions of interest were placed in the head, body, and tail region of the pancreas for quantification of IPFD. Further, to prevent possible inclusion of non-parenchymal tissues within the selected area of interest, a thresholding range of 1%-20% was applied, as recommended[21]. The intra-pancreatic fat percentage was calculated as the average pancreatic fat fraction of both slices.
Liver fat: Single-voxel spectroscopy was used to quantify liver fat. A voxel (20 mm × 20 mm × 20 mm) was placed in the right lobe of the liver, away from the blood vessels and bile ducts and at least 10 mm away from the edge. Automated shimming was performed prior to signal acquisition to improve B0 homogeneity. Spectra were acquired using a free-breathing navigator-triggered spin echo acquisition with repetition time 3000 ms, echo time 33 ms, 50 averages. Acquisition duration was 853 ms. Both water-suppressed and non-water-suppressed spectra were taken, with the non-water-suppressed spectrum acting as a reference for liver fat quantification. Spectra were processed and analyzed using SIVIC software (University of California-San Francisco, California, United States)[22]. The magnetic resonance spectroscopy fat fraction was defined as fat fraction = area under fat peak/area under fat and water peaks × 100%.
Skeletal muscle fat: Total muscle area and intra-muscular fat area of erector spinae muscles were measured using a single axial slice at the lower endplate of L3 vertebra, as it had been demonstrated that the L3 level is optimal for determination of skeletal muscle fat[23]. The free-hand tool of ImageJ software (National Institutes of Health, United States) was used to outline the left and right erector spinae muscles followed by measurement of total pixel content[23,24]. Further, to calculate the intra-muscular fat area, the threshold-function of ImageJ was used to convert grayscale pixels into binary images, using global histogram-derived method. Care was taken not to include extra-muscular fat (i.e., beyond the fascial layer of the erector spinae muscles). Total muscle area and intra-muscular fat area were calculated by multiplying the selected total pixel content with pixel surface area. The ratio of fat-free cross-sectional muscle area to total cross-sectional muscle area was determined by subtracting intra-muscular fat area from the total muscle area and dividing this value by the total muscle area. Skeletal muscle fat percentage was defined as (1-fat-free cross-sectional muscle area to total cross-sectional muscle area ratio) × 100%.
Subcutaneous and visceral fat: Visceral fat volume and subcutaneous fat volume were quantified manually using ImageJ software. Identical fat-phase images (L2-L5) from the selected series were used for segmentation of visceral and subcutaneous fat compartments[25]. The threshold-function of ImageJ was used to convert grayscale pixels into binary images, using the global histogram-derived method[24]. Using the free-hand tool, visceral and subcutaneous fat regions were delineated from the abdominal musculature and measured separately. The non-adipose tissue, soft organs, and blood vessels were excluded from the measurement of visceral fat. The final step for all the above measurements involved summation of the pixel contents of all the slices in series and multiplied by the pixel area and slice thickness to obtain the total volume.
Venous blood samples were obtained from each participant after at least 8 h of fasting to assess pancreatic enzymes. These blood samples were centrifuged 4000 g for 5.5 min and plasma was separated into aliquots and stored at -80 ℃ until further use. The active form of pancreatic amylase was measured in plasma using the Reflotron® Plus reflectance photometer (Roche®, Basel, Switzerland) and results were expressed in U/L. The active forms of pancreatic lipase and chymotrypsin were measured using sandwich enzyme-linked immunosorbent assay (ELISA) kits. Pancreatic lipase was measured using the Cloud-Clone Corporation ELISA kit (Houston, Texas, United States) and results were expressed in ng/mL. The intra- and inter-assay variations of the assay were < 10% and < 12%, respectively. Chymotrypsin concentration was measured using the Cusabio ELISA kit (Wuhan, Hubei Province, China) and results were expressed in ng/mL. The intra- and inter-assay variations of the assay were < 8% and < 10%, respectively. Absorbance was detected at 450 nm. Concentrations in each sample were estimated using a standard curve.
Anthropometric data (height, weight, and waist circumference) of all study participants were recorded to calculate BMI and waist-height ratio. All measurements were taken over the light clothing of participants, and height and weight were measured in a standing position without shoes and headgear. Waist circumference was measured at the level of the umbilicus. Blood samples for lipids (triglycerides, total cholesterol, high-density lipoproteins cholesterol, and low-density lipoprotein cholesterol) were measured at LabPlus-a tertiary referral medical laboratory at Auckland City Hospital. The same laboratory measured HbA1c, using the boronate affinity chromatography assay (Trinity Biotech, Wicklow, Ireland) that is certified by the National Glycohaemoglobin Standardisation Program and standardized to the Diabetes Control and Complications Trial reference assay. Fasting insulin was measured using chemiluminescence sandwich immunoassay (Roche Diagnostics, Auckland, New Zealand).
A standardized questionnaire was administered at the time of the study. For information on the use of antidiabetic medications, participants were asked, 'Are you currently on antidiabetic mediation?'. If the answer was 'yes', they were classified as antidiabetic medication user, otherwise they were classified as non-user. For information on smoking status, participants were asked, 'Have you ever smoked cigarettes?'. If the answer was 'yes', they were classified as ever-smokers, otherwise, they were classified as never-smokers[26]. For information on alcohol consumption, participants were asked, 'On average, how much alcohol do you consume in a week?'. A reference diagram for drink volumes per unit was provided. The response to this question was presented as grams per week and used to determine the average amount of alcohol consumption[26].
All analyses were performed using SPSS for Windows Version 25 (SPSS Inc., Illinois, United States). A two-sided P < 0.05 was deemed to be statistically significant. The mean and standard deviation of the five studied abdominal fat phenotypes (i.e., intra-pancreatic fat, liver fat, skeletal muscle fat, visceral fat, and subcutaneous fat) in the three groups (NODAP, T2DM, and healthy controls) were compared using one-way analysis of variance (ANOVA).
To examine the differences in the five studied abdominal fat phenotypes between the three groups, one-way ANOVA and one-way analysis of covariance (ANCOVA) were conducted. ANCOVA enabled the reduction of within-group variance while adjusting for covariates. The following four models were constructed: (1) Unadjusted; (2) Adjusted for age and sex; (3) Adjusted for age, sex, triglycerides, and HbA1c; and (4) Adjusted for age, sex, triglycerides, HbA1c, BMI, use of antidiabetic medications, alcohol consumption, and smoking status. The Fisher's least significant difference method was used for post-hoc pair-wise comparisons.
To investigate the associations between the five fat phenotypes and pancreatic enzymes (pancreatic amylase, pancreatic lipase, and chymotrypsin) in each study group, linear regression analyses were conducted. In these analyses, each abdominal fat phenotype was entered as an independent variable and concentrations of pancreatic enzymes were treated as the dependent variable. In addition, relative importance of each abdominal fat phenotype in explaining the variance of pancreatic enzymes concentrations was determined in each study group. Using the 'relaimpo' package in R Studio Version3.6.1 (RStudio Inc., Massachusetts, United States), a multivariable linear regression model was constructed in each study group, including the five abdominal fat phenotypes as independent variables and pancreatic enzymes concentrations as the dependent variable[27]. The resulting individual R2 values of all the independent variables were obtained and plotted.
A total of 90 individuals were included (30 NODAP, 30 T2DM, and 30 healthy controls). The median time since the last attack of pancreatitis was 29 mo (interquartile range, 15.7-42.8 mo) and 29 mo (interquartile range, 21.1-36.2 mo) in the NODAP group and T2DM group, respectively. Other characteristics are presented in Table 1.
| Characteristic | Healthy controls (n = 30) | T2DM (n = 30) | NODAP (n = 30) | P value1 |
| Age (yr) | 50.0 (36.5-68.8) | 55.5 (41.8-66.3) | 58.5 (48.5-67.3) | 0.213 |
| Men, n (%) | 21 (70.0) | 21 (70.0) | 21 (70.0) | 1.000 |
| Body mass index (kg/m2) | 24.0 (21.8-28.1) | 30.3 (26.4-35.4) | 27.5 (24.1-32.7) | < 0.001 |
| Waist-height ratio | 0.5 (0.5-0.5) | 0.6 (0.5-0.6) | 0.6 (0.5-0.6) | < 0.001 |
| Triglycerides (mmol/L) | 1.0 (0.6-1.2) | 1.7 (1.3-3.7) | 2.2 (1.3-3.6) | 0.035 |
| Total cholesterol (mmol/L) | 4.5 (3.4-5.6) | 5.1 (3.9-5.8) | 5.1 (4.1-5.7) | 0.388 |
| HDL cholesterol (mmol/L) | 1.2 (0.8-1.8) | 1.2 (0.9-1.4) | 1.2 (1.1-1.4) | 0.680 |
| LDL cholesterol (mmol/L) | 2.7 (2.0-3.4) | 2.7 (1.6-3.3) | 2.6 (2.1-3.5) | 0.542 |
| Fasting insulin (mIU/L) | 11.8 (4.8-16.6) | 12.2 (8.2-17.2) | 13.3 (7.4-18.3) | 0.523 |
| HOMA-IR (mIU/L·mmol/L) | 2.8 (1.2-3.3) | 3.3 (2.1-5.1) | 3.3 (1.9-5.3) | 0.244 |
| Smoking status2 | 0 (0-1) | 0 (0-1) | 1 (0-1) | 0.016 |
| Alcohol consumption (g/wk) | 39 (4.5-96) | 12 (6-144) | 12 (0-108) | 0.279 |
| Amylase (U/L) | 29.0 (20.2-33.9) | 20.2 (14.0-31.5) | 28.7 (19.4-33.3) | 0.158 |
| Lipase (pg/mL) | 7.2 (5.5-8.7) | 7.6 (5.9-10.5) | 6.5 (5.5-8.6) | 0.340 |
| Chymotrypsin (U/L) | 6.2 (5.2-7.1) | 4.6 (2.9-6.7) | 5.9 (4.9-6.6) | 0.314 |
The intra-pancreatic fat percentage was 9.4 ± 1.8%, 9.8 ± 1.1%, and 7.8 ± 1.9% in the NODAP group, T2DM group, and healthy controls group, respectively. The difference between the three groups was statistically significant in both the unadjusted (P < 0.001) and all the adjusted models (P = 0.002 in model 2; P = 0.001 in model 3; P = 0.003 in model 4).
The liver fat percentage was 12.0 ± 9.7%, 11.3 ± 11.1%, and 9.3 ± 7.7% in the NODAP group, T2DM group, and healthy controls group, respectively. The difference between the three groups was not statistically significant in all the models.
The skeletal muscle fat percentage was 14.9 ± 6.1%, 15.5 ± 6.0%, and 14.1 ± 7.0% in the NODAP group, T2DM group, and healthy controls group, respectively. The difference between the three groups was not statistically significant in all the models.
The visceral fat volume was 2205.0 ± 1098.1 cm3, 2622.5 ± 1172.2 cm3, and 1208.8 ± 808.1cm3 in the NODAP group, T2DM group, and healthy controls group, respectively. The difference between the three groups was statistically significant in both the unadjusted (P < 0.001) and all the adjusted models (P = 0.010 in model 2; P = 0.001 in model 3; P = 0.005 in model 4).
The subcutaneous fat volume was 3011.4 ± 1432.2 cm3, 3463.0 ± 1323.7 cm3, and 2523.8 ± 1437.7 cm3 in the NODAP group, T2DM group, and healthy controls group, respectively. The difference between the three groups was statistically significant in the unadjusted (P = 0.013) and two adjusted models (P = 0.038 in model 3; P = 0.034 in model 4).
Results of all the pair-wise comparisons between the study groups are presented in Table 2.
| Fat phenotype | Overall1 | T2DM vs NODAP | T2DM vs healthy controls | NODAP vs healthy controls |
| Intra-pancreatic fat (%) | ||||
| Model 1 | < 0.001 | 0.290 | < 0.001 | 0.001 |
| Model 2 | 0.002 | 0.555 | 0.001 | 0.004 |
| Model 3 | 0.001 | 0.440 | 0.001 | 0.001 |
| Model 4 | 0.003 | 0.187 | 0.002 | 0.012 |
| Liver fat (%) | ||||
| Model 1 | 0.416 | 0.806 | 0.322 | 0.211 |
| Model 2 | 0.681 | 0.457 | 0.983 | 0.465 |
| Model 3 | 0.556 | 0.998 | 0.393 | 0.312 |
| Model 4 | 0.612 | 0.428 | 0.329 | 0.801 |
| Skeletal muscle fat (%) | ||||
| Model 1 | 0.348 | 0.148 | 0.516 | 0.420 |
| Model 2 | 0.329 | 0.137 | 0.455 | 0.489 |
| Model 3 | 0.585 | 0.319 | 0.692 | 0.544 |
| Model 4 | 0.477 | 0.243 | 0.551 | 0.460 |
| Visceral fat (cm3) | ||||
| Model 1 | < 0.001 | 0.093 | < 0.001 | 0.001 |
| Model 2 | 0.010 | 0.596 | 0.005 | 0.012 |
| Model 3 | 0.001 | 0.249 | < 0.001 | 0.003 |
| Model 4 | 0.005 | 0.787 | 0.012 | 0.003 |
| Subcutaneous fat (cm3) | ||||
| Model 1 | 0.013 | 0.214 | 0.003 | 0.082 |
| Model 2 | 0.740 | 0.488 | 0.508 | 0.987 |
| Model 3 | 0.038 | 0.245 | 0.012 | 0.081 |
| Model 4 | 0.034 | 0.063 | 0.009 | 0.301 |
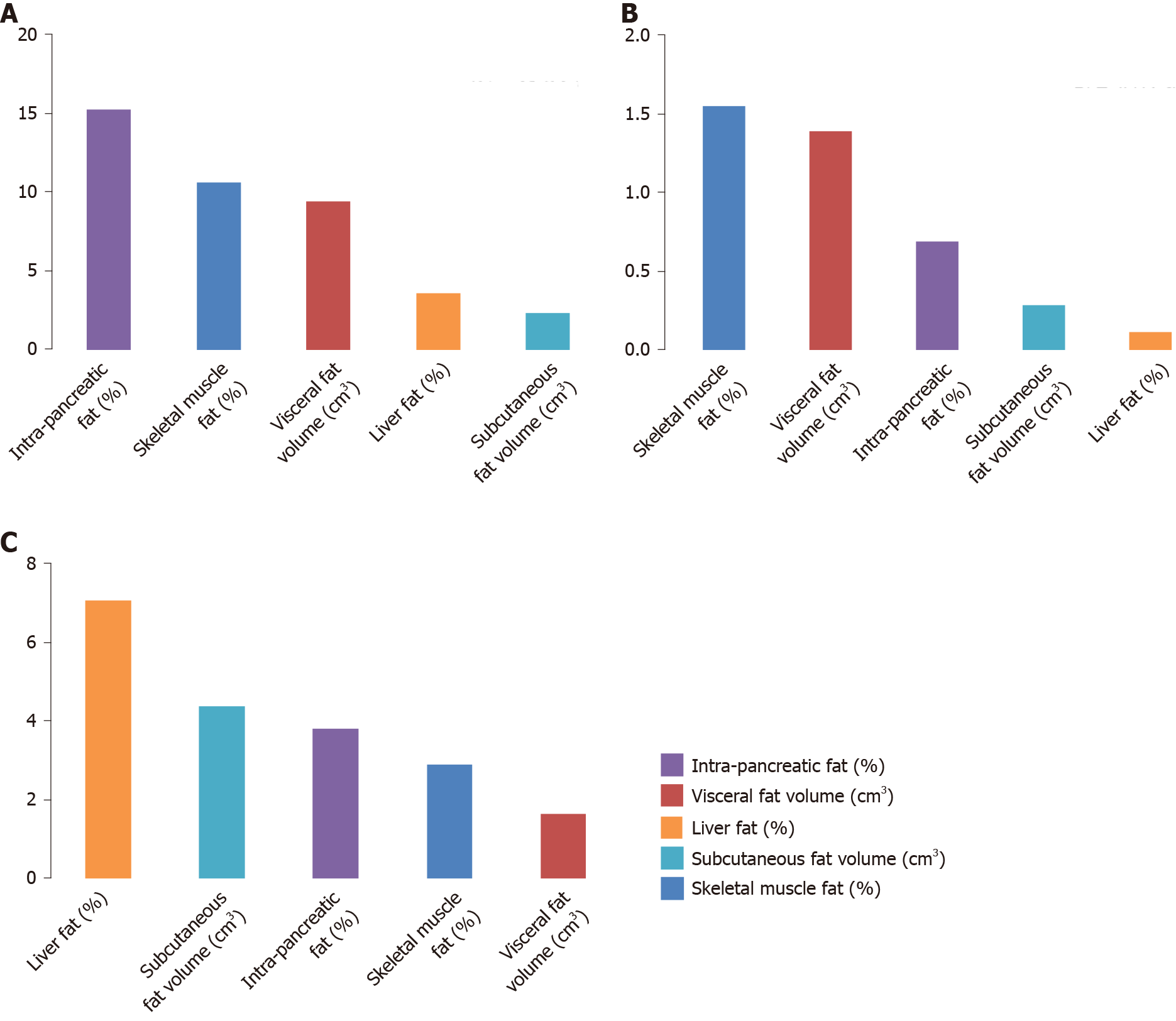
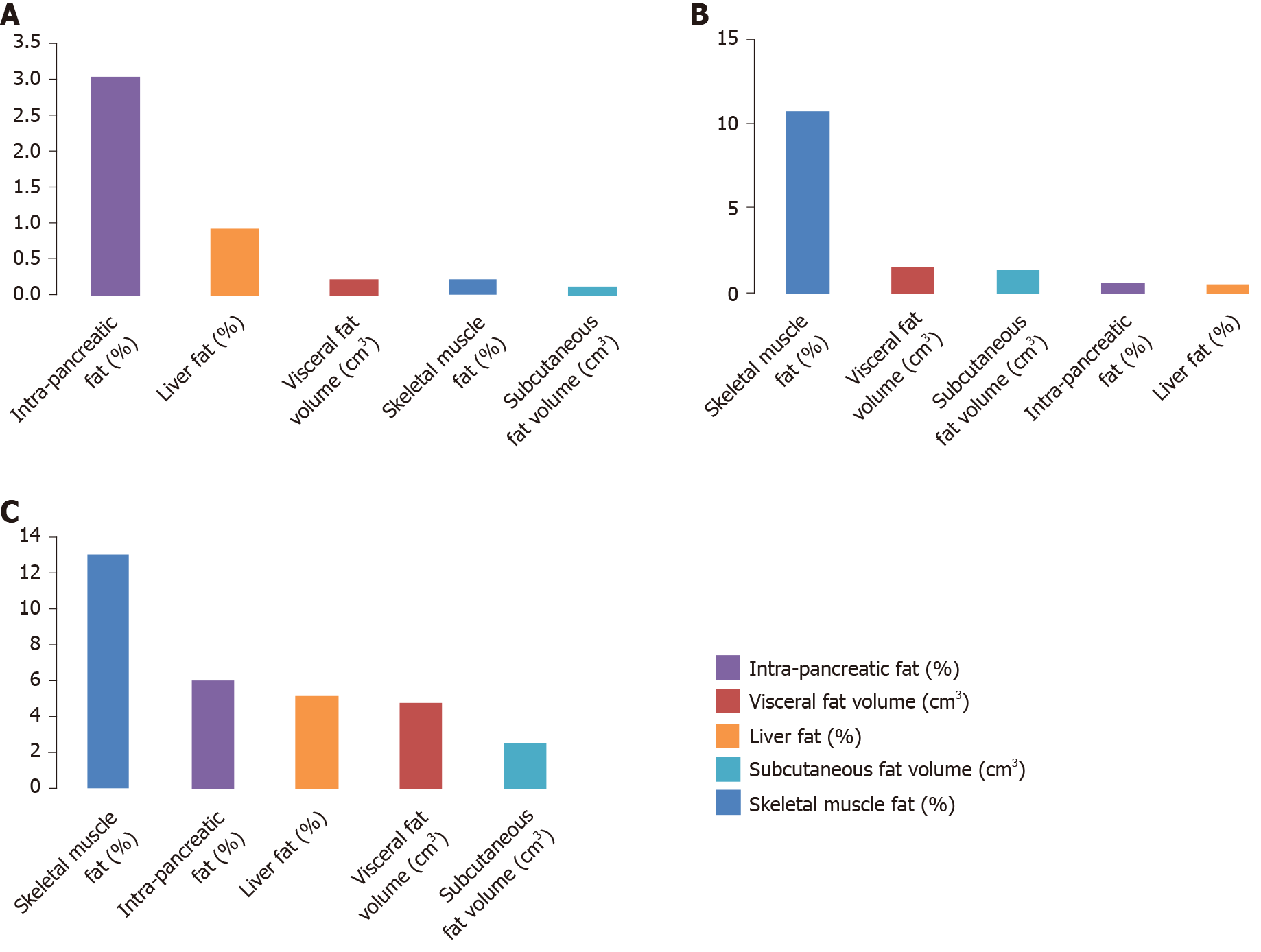
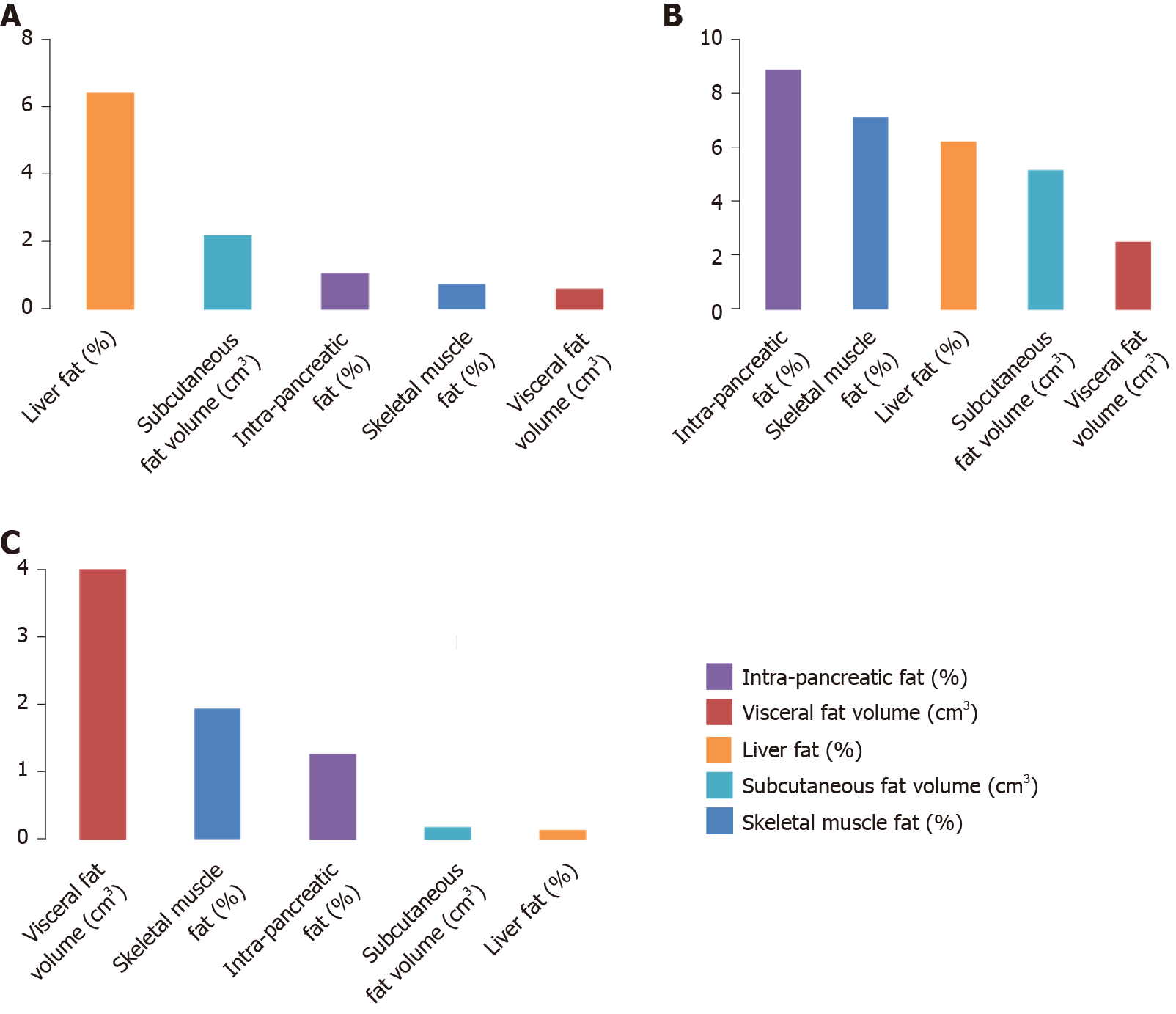
In the NODAP group, the five abdominal fat phenotypes altogether explained 41.2% of the variance in pancreatic amylase, 4.5% of the variance in pancreatic lipase, and 11.1% of the variance in chymotrypsin. Of the fat phenotypes studied, the variance in pancreatic amylase concentration was explained the most by IPFD (R2 = 15.3%) (Figure 1A); the variance in pancreatic lipase concentration was explained the most by IPFD (R2 = 3.0%) (Figure 2A); the variance in chymotrypsin concentration was explained the most by liver fat (R2 = 6.5%) (Figure 3A). IPFD and visceral fat volumes were significantly associated with pancreatic amylase concentration (β = -2.201, P = 0.023; and β = -0.004, P = 0.028, correspondingly). The other abdominal fat phenotypes were not significantly associated with the studied pancreatic enzymes (Table 3).
| Fat phenotype | Healthy | T2DM | NODAP | |||||||
| β | S.E. | P value | β | S.E. | P value | β | S.E. | P value | ||
| Intra-pancreatic fat (%) | ||||||||||
| Pancreatic amylase | -0.832 | 2.220 | 0.712 | 0.573 | 1.709 | 0.741 | -2.201 | 0.899 | 0.023 | |
| Pancreatic lipase | 0.258 | 0.421 | 0.546 | -0.631 | 0.397 | 0.876 | 1.343 | 1.462 | 0.367 | |
| Chymotrypsin | -0.137 | 0.179 | 0.452 | -0.579 | 0.610 | 0.343 | 0.164 | 0.270 | 0.550 | |
| Liver fat (%) | ||||||||||
| Pancreatic amylase | 0.685 | 0.444 | 0.140 | -0.022 | 0.157 | 0.890 | -0.493 | 0.331 | 0.151 | |
| Pancreatic lipase | -0.136 | 0.089 | 0.141 | -0.134 | 0.351 | 0.709 | -0.098 | 0.280 | 0.730 | |
| Chymotrypsin | 0.002 | 0.040 | 0.967 | -0.071 | 0.069 | 0.314 | 0.059 | 0.051 | 0.259 | |
| Skeletal muscle fat (%) | ||||||||||
| Pancreatic amylase | -0.570 | 0.595 | 0.351 | -0.241 | 0.474 | 0.619 | -0.185 | 0.125 | 0.151 | |
| Pancreatic lipase | 0.195 | 0.093 | 0.047 | -1.345 | 0.912 | 0.161 | -0.071 | 0.446 | 0.875 | |
| Chymotrypsin | -0.023 | 0.044 | 0.609 | -0.129 | 0.105 | 0.238 | -0.014 | 0.086 | 0.868 | |
| Visceral fat (cm3) | ||||||||||
| Pancreatic amylase | 0.002 | 0.005 | 0.664 | 0.000 | 0.002 | 0.841 | -0.004 | 0.002 | 0.028 | |
| Pancreatic lipase | -0.588 | 0.839 | 0.490 | -3.247 | 0.462 | 0.493 | 0.533 | 0.250 | 0.833 | |
| Chymotrypsin | -0.314 | 0.362 | 0.394 | 0.287 | 0.566 | 0.619 | 0.240 | 0.458 | 0.606 | |
| Subcutaneous fat (cm3) | ||||||||||
| Pancreatic amylase | 0.003 | 0.002 | 0.224 | 0.000 | 0.002 | 0.821 | -0.002 | 0.001 | 0.232 | |
| Pancreatic lipase | -0.573 | 0.482 | 0.246 | -2.032 | 3.444 | 0.564 | -0.036 | 1.931 | 0.985 | |
| Chymotrypsin | -0.030 | 0.215 | 0.890 | -0.527 | 0.534 | 0.338 | -0.091 | 0.350 | 0.797 | |
In the T2DM group, the five abdominal fat phenotypes altogether explained 4.0% of the variance in pancreatic amylase, 14.7% of the variance in pancreatic lipase, and 29.9% of the variance in chymotrypsin. Of the fat phenotypes studied, the variance in pancreatic amylase concentration was explained the most by skeletal muscle fat (R2 = 1.5%) (Figure 1B); the variance in pancreatic lipase concentration was explained the most by skeletal muscle fat (R2 = 10.7%) (Figure 2B); the variance in chymotrypsin concentration was explained the most by IPFD (R2 = 8.9%) (Figure 3B). None of the abdominal fat phenotypes were significantly associated with the studied pancreatic enzymes (Table 3).
In the healthy controls group, the five abdominal fat phenotypes altogether explained 17.8% of the variance in pancreatic amylase, 31.3% of the variance in pancreatic lipase, and 7.5% of the variance in chymotrypsin. Of the abdominal fat phenotypes studied, the variance in pancreatic amylase concentration was explained the most by liver fat (R2 = 7.1%) (Figure 1C); the variance in pancreatic lipase concentration was explained the most by skeletal muscle fat (R2 = 13.0%) (Figure 2C); the variance in chymotrypsin concentration was explained the most by visceral fat (R2 = 4.0%) (Figure 3C). Skeletal muscle fat percentage was significantly associated with pancreatic lipase concentration (β = 0.195, P = 0.047). The other abdominal fat phenotypes were not significantly associated with the studied pancreatic enzymes (Table 3).
In the present study, a uniformed MRI protocol on a single 3T scanner was used to comprehensively compare, for the first time, abdominal fat phenotypes in 90 matched individuals with NODAP, T2DM, and healthy controls. An important finding of the study was that both NODAP and T2DM were characterized by a significantly larger amount of intra-pancreatic fat and visceral fat (but not liver fat, skeletal muscle fat, or subcutaneous fat) compared with healthy controls, consistently in both unadjusted and all the adjusted analyses. In addition, IPFD and visceral fat volume were significantly inversely associated with circulating levels of pancreatic amylase in the NODAP group (but not the other two groups). The relative importance analyses revealed that the five studied abdominal fat phenotypes altogether explained 41% of the variance in pancreatic amylase concentration in individuals with NODAP. By contrast, only 4% and 13% of the variance in this pancreatic enzyme was explained by the five abdominal phenotypes in individuals with T2DM and healthy controls, respectively.
Abdominal fat phenotypes are often used to differentiate between type 1 diabetes and the much more common type 2 diabetes. Several studies have compared head-to-head these two types of diabetes and have agreed that individuals with type 1 diabetes typically have lower levels of adiposity (as evidenced by both BMI and visceral adiposity) than those with type 2 diabetes[28-30]. Moreover, a large study from the United Kingdom demonstrated that the prevalence of type 2 diabetes, but not type 1 diabetes, was significantly associated with BMI[29]. NODAP, another type of diabetes that is much less common than type 2 diabetes, is often misdiagnosed as type 2 diabetes[4]. Specifically, a 2017 population-based study of new-onset diabetes demonstrated that 93% cases of new-onset diabetes after pancreatitis were misclassified as type 2 diabetes (and 4% as type 1 diabetes)[4]. In the present study, the NODAP group did not significantly differ from the T2DM group in terms of visceral adiposity, which was significantly higher in the two case groups in comparison with the healthy control group. Although we did not specifically measure insulin sensitivity in the present study, our earlier study showed that individuals with NODAP were characterized by decreased insulin sensitivity compared with healthy controls[31]. The findings of the two studies are complementary as increased visceral adiposity is known to be more strongly linked with decreased insulin sensitivity than subcutan
IPFD has recently emerged as another fat phenotype strongly linked with insulin sensitivity[14,31,32]. This is epitomized in the 'twin cycle hypothesis' that posits that type 2 diabetes is caused by excess fat deposition in the pancreas (and liver). During chronic positive caloric balance, β-cells enter a ‘survival mode’ and fail to function adequately in the pancreas because of the fat-induced metabolic stress[33]. Studies from a primary care-based weight management program in the United Kingdom found that the reduction in fat depositions in the pancreas (and liver) in the first few years after diabetes onset can normalize hepatic insulin responsiveness and possibly trigger β-cell re-differentiation[34]. Collectively, these changes can lead to normalization of blood glucose levels and reversal of biochemical diabetes status[33-35]. Further, the link between IPFD and insulin traits, specifically in NODAP, was investigated in a 2019 study. It showed that a fasted state index of insulin sensitivity (specifically, Raynaud index) was significantly inversely associated with IPFD in individuals with NODAP and it explained 20% of the variance in IPFD, which was the highest among the nearly 30 body composition variables and insulin traits investigated[31]. The present study bridges the gap in the literature by showing, for the first time, that IPFD is similarly increased in NODAP and T2DM as compared with healthy controls.
The other novel finding in the present study was that, although high IPFD and visceral fat volume characterized both NODAP and T2DM, the two fat phenotypes were significantly inversely associated with circulating levels of pancreatic amylase in the NODAP group only. Further, IPFD contributed the most to the variance in circulating levels of pancreatic amylase (R2 = 15.0%). By contrast, there was only a small contribution of IPFD to the variance in circulating levels of pancreatic amylase in the healthy controls group (R2 = 3.8%) and the T2DM group (R2 = 0.7%). The exact mechanism underlying the above findings is yet to be elucidated but we believe it may relate to a more prominent role of exocrine pancreatic dysfunction in NODAP vs T2DM. A 2015 study demonstrated that MRI-derived IPFD was inversely associated with serum lipase activity (pancreatic amylase and other pancreatic enzymes were not studied) in the general population, suggesting that increased IPFD is associated with reduced pancreatic acinar cell mass[36]. A subsequent add-on study of 1458 participants with available fecal elastase measurements showed that MRI-derived IPFD was significantly inversely associated with exocrine pancreatic function (defined based on fecal elastase levels), in both crude analysis and after adjustment for age, sex, and BMI[37]. Interestingly, other fat phenotypes (subcutaneous fat, visceral fat, and liver fat) were not significantly associated with exocrine pancreatic function in that study. Another study of fecal elastase and MRI-derived IPFD included 56 individuals without diabetes or pancreatitis and showed that individuals with excess IPFD had a significantly higher frequency of exocrine pancreatic dysfunction than controls[38].
Amylase is the main enzyme responsible for the hydrolysis of carbohydrates[39] and, hence, its observed involvement in the pathogenesis of NODAP may have practical implications for the dietary management of this disorder. In most human populations, starch is the primary source of carbohydrates[40-42]. A 2012 study found that individuals with low amylase activity had higher postprandial plasma glucose concentrations after starch ingestion than individuals with high amylase activity[43]. A 2016 metabolomic study showed that utilization of glucose in the body for energy was attenuated in individuals with low serum amylase who have an energy dependence on fats rather than carbohydrates[44]. Based on the above findings, it is likely that individuals with NODAP have a different glycemic response in comparison with individuals with T2DM. Glycemic load reflects the quantity and quality of carbohydrates in the diet[40,45]. Because individuals with NODAP may not be fully adapted to a diet rich in carbohydrates, they may benefit from the determination of glycemic load for foods that are high in starch. The differential association between amylase and fat phenotypes in NODAP vs T2DM may also influence response to weight-loss dietary interventions and this needs to be taken into account in the design of future randomized controlled trials[41].
Several limitations have to be acknowledged. First, genetic factors were not analyzed in the present study. Some genes (e.g., the α-amylase gene cluster) are highly relevant to the present research. The α-amylase cluster comprises 2 pancreatic amylase genes (AMY2A and AMY2B), 3 salivary amylase genes (AMY1A, AMY1B, and
IPFD and visceral fat were significantly increased in individuals with NODAP and T2DM (in comparison with healthy controls). However, only individuals with NODAP were characterized by significant inverse associations between the two abdominal fat phenotypes and circulating levels of pancreatic amylase. Pancreatic amylase may have implications for the pathogenesis and management of NODAP and, hence, the role of this pancreatic enzyme in NODAP warrants purposely designed investigations in the future.
Abdominal adipose tissue distribution is an important factor in the pathogenesis of diabetes in general and new-onset diabetes after acute pancreatitis in particular.
The role of pancreatic enzymes in the pathogenesis of new-onset diabetes after acute pancreatitis is unknown.
The objective was to compare head-to-head abdominal adipose tissue distribution in new-onset prediabetes or diabetes after acute pancreatitis (NODAP), type 2 prediabetes or diabetes, and healthy controls.
The design was a case-control study. Intra-pancreatic fat, liver fat, skeletal muscle fat, visceral fat, and subcutaneous fat were quantified in a blinded fashion with the use of magnetic resonance imaging. Circulating levels of pancreatic amylase, pancreatic lipase, and chymotrypsin were determined.
The intra-pancreatic fat percentage was 9.4 ± 1.8%, 9.8 ± 1.1%, and 7.8 ± 1.9% in NODAP, type 2 prediabetes or diabetes, and healthy controls, respectively (P < 0.001). The visceral fat volume was 2205 ± 1098 cm3, 2622 ± 1172 cm3, and 1209 ± 808 cm3 in NODAP, type 2 prediabetes or diabetes, and healthy controls, respectively (P < 0.001). The other fat phenotypes did not differ between the groups. The amount of intra-pancreatic fat and visceral fat was significantly associated with circulating levels of pancreatic amylase in NODAP (but not type 2 prediabetes or diabetes or healthy controls).
Excess intra-pancreatic fat deposition is a key factor in the pathogenesis of new-onset diabetes after acute pancreatitis. There is a significant inverse relationship between circulating levels of pancreatic amylase and intra-pancreatic fat.
Human studies on the role of pancreatic amylase in new-onset diabetes after acute pancreatitis are warranted.
| 1. | Petrov MS. Post-pancreatitis diabetes mellitus: prime time for secondary disease. Eur J Endocrinol. 2021;184:R137-R149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 586] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 3. | Bharmal SH, Cho J, Stuart CE, Alarcon Ramos GC, Ko J, Petrov MS. Oxyntomodulin may distinguish new-onset diabetes after acute pancreatitis from type 2 diabetes. Clin Transl Gastroenterol. 2020;11:e00132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, Gatenby PAC, Jones SA, de Lusignan S. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care. 2017;40:1486-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Han TS, Al-Gindan YY, Govan L, Hankey CR, Lean MEJ. Associations of BMI, waist circumference, body fat, and skeletal muscle with type 2 diabetes in adults. Acta Diabetol. 2019;56:947-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2233] [Article Influence: 372.2] [Reference Citation Analysis (0)] |
| 7. | Gudipaty L, Rosenfeld NK, Fuller CS, Cuchel M, Rickels MR. Different β-cell secretory phenotype in non-obese compared to obese early type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 9. | Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol. 2013;12:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Bendor CD, Bardugo A, Zucker I, Cukierman-Yaffe T, Lutski M, Derazne E, Shohat T, Mosenzon O, Tzur D, Sapir A, Pinhas-Hamiel O, Kibbey RG, Raz I, Afek A, Gerstein HC, Tirosh A, Twig G. Childhood pancreatitis and risk for incident diabetes in adulthood. Diabetes Care. 2020;43:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Roh E, Kim KM, Park KS, Kim YJ, Chun EJ, Choi SH, Jang HC, Lim S. Comparison of pancreatic volume and fat amount linked with glucose homeostasis between healthy Caucasians and Koreans. Diabetes Obes Metab. 2018;20:2642-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Wang CY, Ou HY, Chen MF, Chang TC, Chang CJ. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc. 2014;3:e000297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 779] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 14. | Sakai NS, Taylor SA, Chouhan MD. Obesity, metabolic disease and the pancreas-quantitative imaging of pancreatic fat. Br J Radiol. 2018;91:20180267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Acharya C, Navina S, Singh VP. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Komada H, Sakaguchi K, Hirota Y, Sou A, Nakamura T, Kyotani K, Kawamitsu H, Sugimura K, Okuno Y, Ogawa W. Pancreatic fat content assessed by 1H magnetic resonance spectroscopy is correlated with insulin resistance, but not with insulin secretion, in Japanese individuals with normal glucose tolerance. J Diabetes Investig. 2018;9:505-511. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wong VW, Wong GL, Yeung DK, Abrigo JM, Kong AP, Chan RS, Chim AM, Shen J, Ho CS, Woo J, Chu WC, Chan HL. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol. 2014;109:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Nadarajah C, Fananapazir G, Cui E, Gichoya J, Thayalan N, Asare-Sawiri M, Menias CO, Sandrasegaran K. Association of pancreatic fat content with type II diabetes mellitus. Clin Radiol. 2020;75:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Ko J, Cho J, Petrov MS. Low serum amylase, lipase, and trypsin as biomarkers of metabolic disorders: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2020;159:107974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Al-Mrabeh A, Hollingsworth KG, Steven S, Taylor R. Morphology of the pancreas in type 2 diabetes: effect of weight loss with or without normalisation of insulin secretory capacity. Diabetologia. 2016;59:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Al-Mrabeh A, Hollingsworth KG, Steven S, Tiniakos D, Taylor R. Quantification of intrapancreatic fat in type 2 diabetes by MRI. PLoS One. 2017;12:e0174660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Crane JC, Olson MP, Nelson SJ. SIVIC: open-source, standards-based software for DICOM MR spectroscopy workflows. Int J Biomed Imaging. 2013;2013:169526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Kiefer LS, Fabian J, Lorbeer R, Machann J, Storz C, Kraus MS, Wintermeyer E, Schlett C, Roemer F, Nikolaou K, Peters A, Bamberg F. Inter- and intra-observer variability of an anatomical landmark-based, manual segmentation method by MRI for the assessment of skeletal muscle fat content and area in subjects from the general population. Br J Radiol. 2018;91:20180019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34536] [Cited by in RCA: 40156] [Article Influence: 2868.3] [Reference Citation Analysis (0)] |
| 25. | Irlbeck T, Massaro JM, Bamberg F, O'Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond). 2010;34:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lo SK, DeMeo MT, Steinberg WM, Kochman ML, Etemad B, Forsmark CE, Elinoff B, Greer JB, O'Connell M, Lamb J, Barmada MM; North American Pancreatic Study Group. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008;8:520-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Grömping U. Relative importance for linear regression in R: The package relaimpo. J Stat Softw. 2006;17:1-27. |
| 28. | Dubé MC, Joanisse DR, Prud'homme D, Lemieux S, Bouchard C, Pérusse L, Lavoie C, Weisnagel SJ. Muscle adiposity and body fat distribution in type 1 and type 2 diabetes: varying relationships according to diabetes type. Int J Obes (Lond). 2006;30:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Song SH. Complication characteristics between young-onset type 2 vs type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015;3:e000044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Zhu A, Cui B, Dang H, Yao D, Yu H, Jia H, Hu Z, Zhang X. Correlation of abdominal fat distribution with different types of diabetes in a Chinese population. J Diabetes Res. 2013;2013:651462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Singh RG, Nguyen NN, DeSouza SV, Pendharkar SA, Petrov MS. Comprehensive analysis of body composition and insulin traits associated with intra-pancreatic fat deposition in healthy individuals and people with new-onset prediabetes/diabetes after acute pancreatitis. Diabetes Obes Metab. 2019;21:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Rattarasarn C. Dysregulated lipid storage and its relationship with insulin resistance and cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. Adipocyte. 2018;7:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 34. | Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1314] [Article Influence: 164.3] [Reference Citation Analysis (3)] |
| 35. | Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Welsh P, Kean S, Ford I, McConnachie A, Messow CM, Sattar N, Taylor R. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 614] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 36. | Kühn JP, Berthold F, Mayerle J, Völzke H, Reeder SB, Rathmann W, Lerch MM, Hosten N, Hegenscheid K, Meffert PJ. Pancreatic steatosis demonstrated at MR imaging in the general population: clinical relevance. Radiology. 2015;276:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Kromrey ML, Friedrich N, Hoffmann RT, Bülow R, Völzke H, Weiss FU, Lerch MM, Motosugi U, Kühn JP. Pancreatic steatosis is associated with impaired exocrine pancreatic function. Invest Radiol. 2019;54:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Tahtacı M, Algın O, Karakan T, Yürekli ÖT, Alışık M, Köseoğlu H, Metin MR, Bolat AD, Erel Ö, Ersoy O. Can pancreatic steatosis affect exocrine functions of pancreas? Turk J Gastroenterol. 2018;29:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Cummings JH, Stephen AM. Carbohydrate terminology and classification. Eur J Clin Nutr. 2007;61 Suppl 1:S5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Shahdadian F, Saneei P, Milajerdi A, Esmaillzadeh A. Dietary glycemic index, glycemic load, and risk of mortality from all causes and cardiovascular diseases: a systematic review and dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2019;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Heianza Y, Sun D, Wang T, Huang T, Bray GA, Sacks FM, Qi L. Starch digestion-related amylase genetic variant affects 2-year changes in adiposity in response to weight-loss diets: the POUNDS Lost Trial. Diabetes. 2017;66:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 1051] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 43. | Mandel AL, Breslin PA. High endogeneous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J Nutr. 2012;142:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 44. | Arredouani A, Stocchero M, Culeddu N, Moustafa JE, Tichet J, Balkau B, Brousseau T, Manca M, Falchi M. Metabolomic profile of low-copy number carriers at the salivary α-amylase gene suggests a metabolic shift toward lipid-based energy production. Diabetes. 2016;65:3362-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Jenkins DJ, Dehghan M, Mente A, Bangdiwala SI, Rangarajan S, Srichaikul K, Mohan V, Avezum A, Díaz R, Rosengren A, Lanas F, Lopez-Jaramillo P, Li W, Oguz A, Khatib R, Poirier P, Mohammadifard N, Pepe A, Alhabib KF, Chifamba J, Yusufali AH, Iqbal R, Yeates K, Yusoff K, Ismail N, Teo K, Swaminathan S, Liu X, Zatońska K, Yusuf R, Yusuf S; PURE Study Investigators. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 46. | Santos JL, Saus E, Smalley SV, Cataldo LR, Alberti G, Parada J, Gratacòs M, Estivill X. Copy number polymorphism of the salivary amylase gene: implications in human nutrition research. J Nutrigenet Nutrigenomics. 2012;5:117-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Falchi M, El-Sayed Moustafa JS, Takousis P, Pesce F, Bonnefond A, Andersson-Assarsson JC, Sudmant PH, Dorajoo R, Al-Shafai MN, Bottolo L, Ozdemir E, So HC, Davies RW, Patrice A, Dent R, Mangino M, Hysi PG, Dechaume A, Huyvaert M, Skinner J, Pigeyre M, Caiazzo R, Raverdy V, Vaillant E, Field S, Balkau B, Marre M, Visvikis-Siest S, Weill J, Poulain-Godefroy O, Jacobson P, Sjostrom L, Hammond CJ, Deloukas P, Sham PC, McPherson R, Lee J, Tai ES, Sladek R, Carlsson LM, Walley A, Eichler EE, Pattou F, Spector TD, Froguel P. Low copy number of the salivary amylase gene predisposes to obesity. Nat Genet. 2014;46:492-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 48. | Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, Schlesinger S. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 49. | Galani C, Schneider H. Prevention and treatment of obesity with lifestyle interventions: review and meta-analysis. Int J Public Health. 2007;52:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Tamura Y. Ectopic fat, insulin resistance and metabolic disease in non-obese Asians: investigating metabolic gradation. Endocr J. 2019;66:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: New Zealand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fonteh Fru PN S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ