Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3158
Peer-review started: January 22, 2021
First decision: February 28, 2021
Revised: March 3, 2021
Accepted: May 21, 2021
Article in press: May 21, 2021
Published online: June 21, 2021
Processing time: 146 Days and 11.8 Hours
Pancreatic cancer is one of the dreaded malignancies for both the patient and the clinician. The five-year survival rate of pancreatic adenocarcinoma (PDA) is as low as 2% despite multimodality treatment even in the best hands. As per the Global Cancer Observatory of the International Agency for Research in Cancer estimates of pancreatic cancer, by 2040, a 61.7% increase is expected in the total number of cases globally. With the widespread availability of next-generation sequencing, the entire genome of the tumors is being sequenced regularly, providing insight into their pathogenesis. As invasive PDA arises from pancreatic intraepithelial neoplasia and mucinous neoplasm and intraductal papillary neoplasm, screening for them can be beneficial as the disease is curable with resection at an early stage. Routine preoperative biliary drainage has no role in patients suffering from PDA with obstructive jaundice. If performed, metallic stents are preferred over plastic ones. Minimally invasive procedures are preferred to open procedures as they have less morbidity. The duct-to-mucosa technique for pancreaticojejunostomy is presently widely practiced. The role of intraperitoneal drains after surgery for PDA is controversial. Neoadjuvant chemoradiotherapy has been proven to have a significant role both in locally advanced as well as in resectable PDA. Many new regimens and drugs have been added in the arsenal of chemoradiotherapy for metastatic disease. The roles of immunotherapy and gene therapy in PDA are being investigated. This review article is intended to improve the understanding of the readers with respect to the latest updates of PDA, which may help to trigger new research ideas and make better management decisions.
Core Tip: Pancreatic cancer is one of the dreaded malignancies for both patients and clinicians. This narrative review highlights the newer trends and achievements in the epidemiology, etiopathogenesis, screening, diagnosis and management of pancreatic adenocarcinoma (PDA). It is intended to improve the readers’ understanding of the latest updates of PDA, which may help to trigger new research ideas and make better management decisions. Newer screening and diagnostic techniques will help in diagnosing the patients in early stages and prognosticate them better. The newer discoveries in drugs and management protocols will help increase the survival and quality of life of the patients.
- Citation: Gupta N, Yelamanchi R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J Gastroenterol 2021; 27(23): 3158-3181
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3158
Pancreatic cancer is one of the dreaded malignancies for both patients and clinicians. For patients, it is associated with a poor survival rate and decreased quality of life due to local invasion and complications, and for the clinician, it is challenging to diagnose at an early stage and treat.
Pancreatic cancer is the third leading cause of cancer deaths in the United States of America and the seventh leading cause worldwide as per the 2018 GLOBACON data[1,2]. It may arise from either the exocrine or the endocrine pancreas with the former being far more common than the latter. Pancreatic adenocarcinoma (PDA) and its subtypes constitute more than 90% of pancreatic tumors[3]. Most patients with PDA present at an advanced stage, which makes curative treatment virtually impossible[4]. The five-year survival rate of PDA is as low as 2% despite multimodality treatment even in the best hands[5]. The high mortality and morbidity associated with PDA have stimulated researchers all over the world to intensify the search for better diagnostic and treatment protocols.
Clinically, patients with PDA present to the healthcare facility with symptoms only in the advanced stage[6]. Early lesions have a good prognosis but are clinically silent. Diagnosis of an early-stage PDA is rare as there are no effective and reliable screening tools or investigations at present[7]. Surgery is the mainstay of treatment if therapy is planned with curative intention in PDA patients[8]. Patients with PDA from the surgical point of view are classified into resectable, borderline resectable, unresectable and metastatic disease categories at diagnosis. Chemotherapy and radiotherapy remain the backbone of the treatment for PDA with almost all patients requiring some form of chemoradiotherapy for curative or palliative purposes[9-11].
In view of the above facts, in the present narrative review, the authors have reviewed the latest trends in the epidemiology, diagnosis and management of PDA based on the published English literature so far. We have searched the literature using the keyword “PDA”. This review article is intended to improve the understanding of the readers regarding the latest updates of PDA, which may help to trigger new research ideas and promote better management decisions.
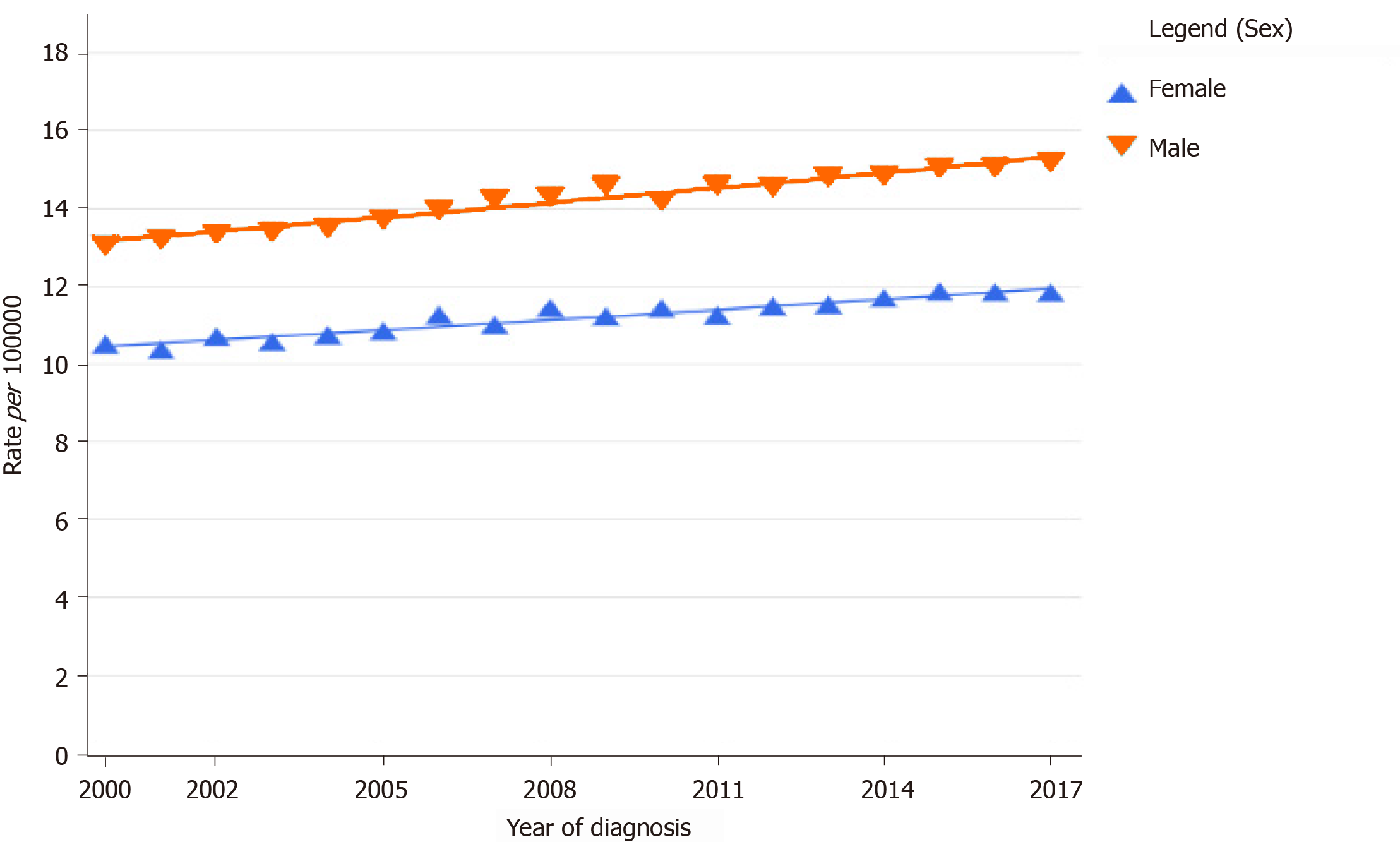
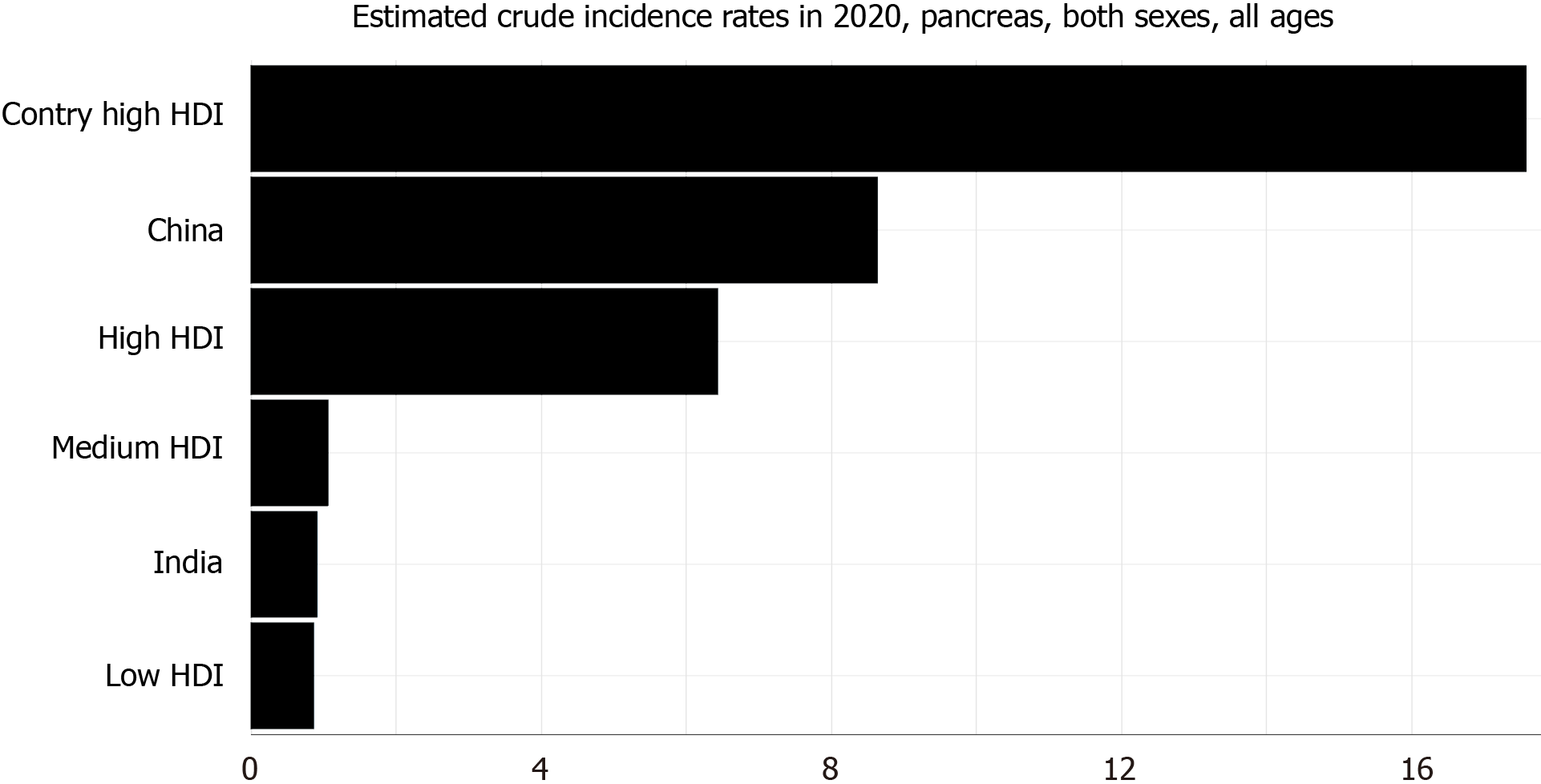
In the last decade, there has been a steady increase in the incidence and mortality of PDA across the globe for both males and females (Figure 1)[12,13]. Its incidence was higher among males when compared to females and continues to be so[1,12-14]. PDA constitutes about 2.5% of the total cancers diagnosed worldwide. The 2020 estimated crude incidence rate and age-standardized rate (ASR) of pancreatic cancer are 6.4% and 4.9%, respectively[1]. The ASR of pancreatic cancer is the highest in North America (8%) and Europe (7.8%) and lowest in Africa (2.3%)[1]. The incidence rate is also very high in countries with a high human development index (HDI) when compared to countries with low HDI (Figure 2)[1]. This difference can be attributed to the variation in the tobacco smoking, alcohol consumption and obesity rates among different countries[15-18].
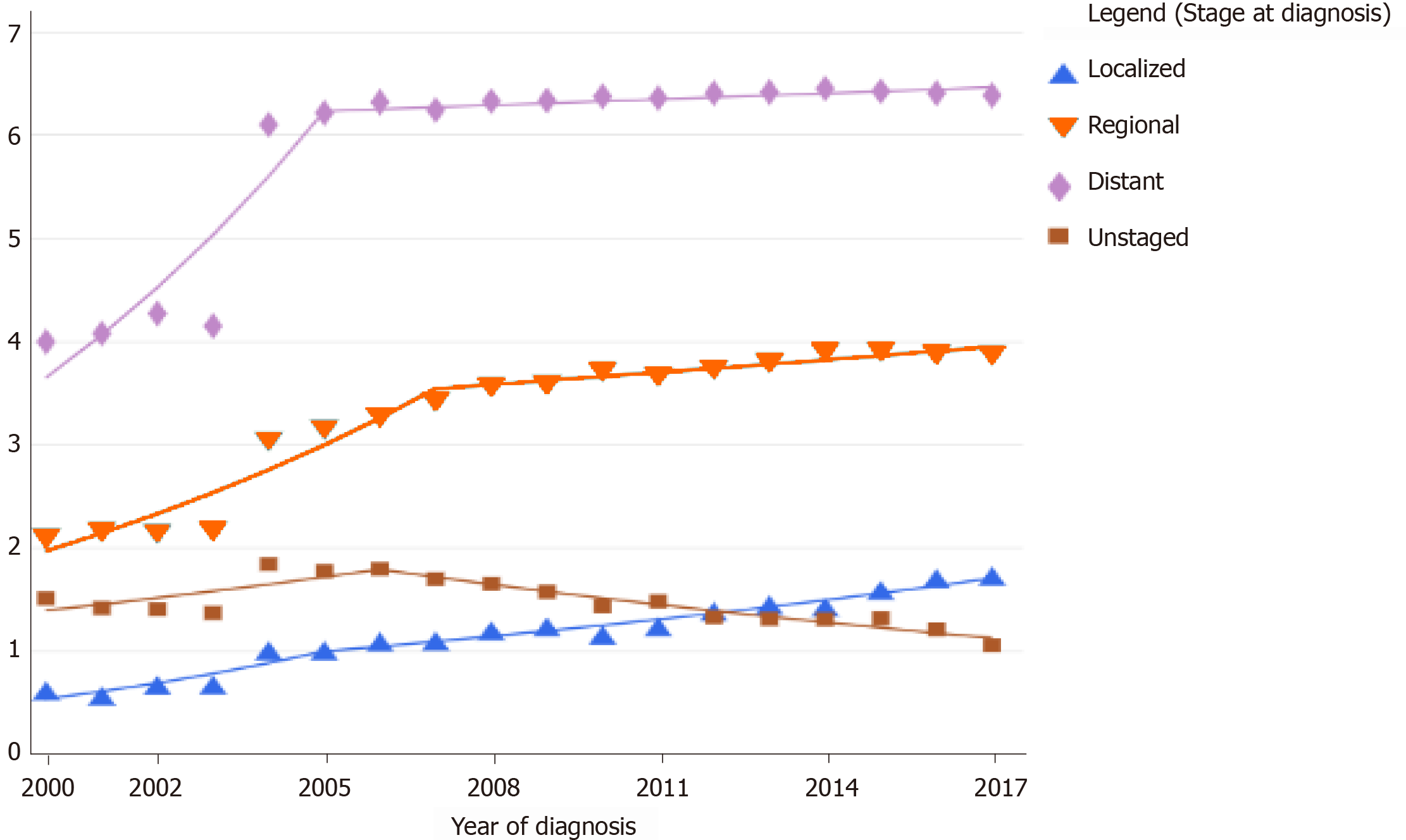
The Surveillance, Epidemiology, and End Results Program of the National Cancer Institute reveals an age-specific trend in the increase of the incidence rate of pancreatic cancer in the age groups of 20-29 years and > 80 years in the United States[19]. The increase also varied with the stage of the disease at diagnosis. The last decade has shown a higher increase in the age-adjusted incidence rates of early and localized pancreatic cancer when compared to advanced disease. The age-adjusted incidence rate of metastatic disease has declined[12] (Figure 3). When the American Joint Committee on Cancer Tumor-Node-Metastasis staging was compared with the age-adjusted incidence rate, there was an increase in the stage I and II patients and a simultaneous decline in the stage III and IV patients at diagnosis, indicating that the evolving techniques of screening and diagnosis of pancreatic cancer are showing some result[14].
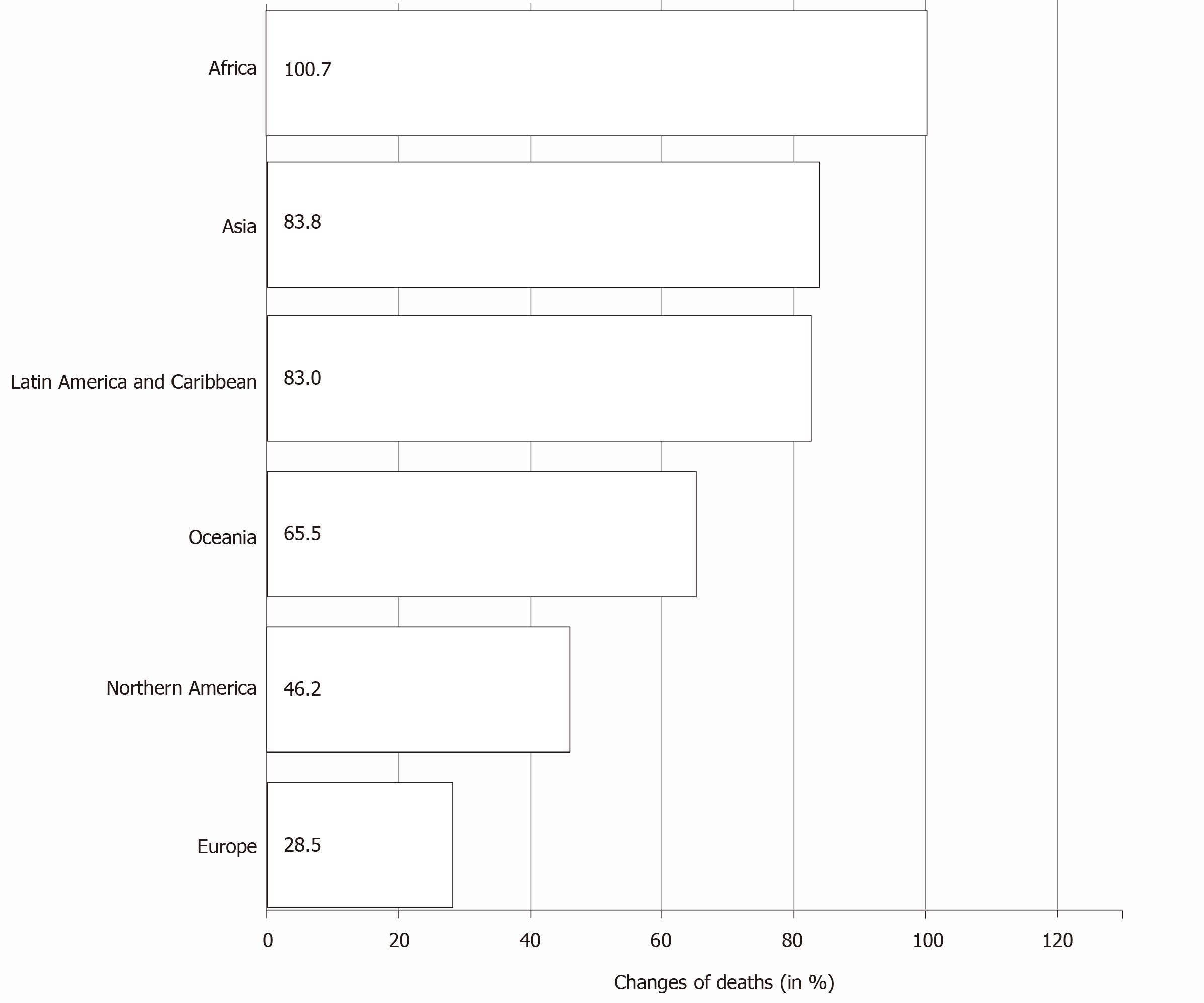
As per the Global Cancer Observatory of the International Agency for Research in Cancer estimates of pancreatic cancer, by 2040, a 61.7% increase is expected in the total number of cases globally. The most notable trend expected is the rapid increase in the number of cases from Africa (an increase of 100.1%) followed by Asia (81.5%). The expected increase from Europe and North America is less (Table 1)[1]. The mortality rates also follow the incidence rates with the expected increase in the mortality due to pancreatic cancer highest in Africa (Figure 4). There is an expected regional variation in the incidence among male and female sex in various continents[1,13]. The temporal trends of incidence and mortality of pancreatic cancer in various continents could be attributed to the temporal trends in tobacco smoking[15,20,21]. Anti-tobacco measures, which are being strongly advocated in developed countries, could be one of the main reasons for declining incidence rates[22]. On the other hand, the rise in the incidence and mortality rates in the developing and under-developed countries is a cause of concern and may be attributed to the lifestyle changes adopted as well as the impact of socioeconomic conditions.
| Population | Number of new cases | Change in number of cases | Change in number of cases due to population | |
| 2020 | 2040 | |||
| Africa | 17070 | 34165 | + 100.1% | + 100.1% |
| Asia | 233701 | 424138 | + 81.5% | + 81.5% |
| Europe | 140116 | 178438 | + 27.4% | + 27.4% |
| Latin America and Caribbean | 37352 | 67836 | + 81.6% | + 81.6% |
| Northern America | 62643 | 89124 | + 42.3% | + 42.3% |
| Oceania | 4891 | 7933 | + 62.2% | + 62.2% |
| Totals | 495773 | 801634 | + 61.7% | + 61.7% |
Based on the present and expected trends of pancreatic cancer, there must be a widespread endorsement of healthy lifestyle practices and strong anti-tobacco laws. If not, the developing countries will have to bear the major brunt of the disease in the near future as the diagnostic and treatment services are still under-developed in these countries.
PDA is recently being called a genetic disease. With the widespread availability of next-generation sequencing, the entire genome of the tumors is being sequenced regularly, providing insight into its pathogenesis. PDA has about 60 genetic alterations per tumor. The most important finding differentiating PDA from other cancers is the heterogeneity of the genome of each patient, meaning that each patient has a tumor with a specific genomic signature[23,24]. Four predominant genes have been identified in PDA. They are K-ras, CDKN2A, TP53 and SMAD4. There are many other genes identified, but they are mutated at a lower frequency (less than 10%)[23].
The K-ras oncogene is the most common gene mutation in PDA with an incidence of more than 90%[25]. Due to its high frequency of mutation, it is believed that the tumor pathogenesis revolves predominantly around the molecular pathways regulated by this gene and forms the basis for research on K-ras inhibitors. However, K-ras inhibitors were associated with high in-vivo toxicity[26]. CDKN2A gene is involved in the regulation of RB1 and plays an important role in the G1/S checkpoint inhibition of the cell cycle[27]. TP53 is the predominant DNA repair pathway gene and induces cell cycle arrest at G1 or G2 checkpoint[28]. SMAD4 gene is a part of the transforming growth factor β pathway and regulates the G1/S checkpoint of cell cycle[28]. The group of genes involved in the genome maintenance DNA repair pathway (BRCA2, PALB2, FANCC, FANCG) constitute less than 10% of the mutated genes in PDA but are important as tumors deficient in these genes can be targeted with DNA damaging agents and poly ADP-ribose pathway inhibitor therapy[23,24,29]. Other gene mutations are involved in the regulation of pathways such as Ras, cell cycle regulators, WNT pathway and NOTCH pathway[23,24].
Invasive PDA arises from pancreatic intraepithelial neoplasia (PanIN). The lesions of PanIN progress from PanIN1 to PanIN3 by acquiring progressive mutations as shown in Table 2[30]. PanINs are also associated with lobulocentric acinar atrophy and local pancreatic inflammation secondary to obstruction of small duct secretions[31]. The time required from the onset of PanIN till progression to invasive carcinoma is about 10 years suggesting a significant lead time if PanIN are screened and detected early. The proteins expressed by PanIN and invasive carcinoma are similar, which can be used for screening these lesions[32-35]. The high-grade PanINs express mucins such as MUC1, MUC4, MUC5AC, and MUC6[36-38]. These can be used for screening and in the treatment of PanIN[39,40].
| PanIN-1 | PanIN-2 | PanIN-3 | |
| Histology | Columnar epithelial cells with basally oriented uniform and round nuclei | More nuclear changes such as loss of nuclear polarity, pleomorphism, hyperchromasia and nuclear pseudostratification | Cribriform pattern, budding cells into lumen and nuclear changes |
| Genetic changes | K-Ras mutations | CDKN2A mutations | TP53 loss |
| Telomere shortening | SMAD4 loss | ||
| BRCA2 loss |
Based on the transcriptome analysis, PDA is divided into molecular subtypes[41]. The three subtypes named initially were classical, quasimesenchymal and exocrine-like, which have been modified as progenitor, squamous and aberrantly differentiated endocrine exocrine types, respectively, by the International Cancer Genome Consortium study[41]. An immunogenic subtype was also identified. The squamous subtype was associated with a poor prognosis similar to the basal subtype carcinomas of other organs and has a poor response to chemoradiotherapy and also exhibits TP53 mutations more frequently[41]. Recently, RNA-based sequencing of PDA revealed the heterogeneity of the tumor at the molecular level[42]. RNA-based subgrouping may add further insights into the pathogenesis and tumor progression. The desmoplastic stroma is the predominant component of the PDA. Recent transcriptome analysis of the stromal cells revealed two subtypes similar to PDA cells–normal subtype and activated subtype[43]. The normal subtype resembles pancreatic stellate cells, and the inflammatory subtype has immunogenic signatures. The gene expression is also different for the two subtypes leading to the inference of the role of stromal and neoplastic cell interaction in determining the tumor heterogeneity[44].
The neoplastic cells of PDA are acclimatized to survive in a microenvironment of depleted oxygen and nutrients due to the poor vascularity and intense desmoplastic stroma of the tumor. The K-ras mutation up-regulates several metabolic pathways such as glucose uptake and glycolysis[23]. Apart from the metabolic adaptations, cells survive by autophagy, mitophagy and macropinocytosis stimulated by the K-ras gene[24]. Genetic or pharmacological inhibition of autophagy leads to decreased tumor growth as seen in mouse models. Co-targeting with mitogen-activated protein kinase kinase/extracellular signal-regulated kinase inhibitors is an area of intense research in the treatment of pancreatic cancer[45,46]. Furthermore, the deregulation of these metabolic pathways is one of the reasons for resistance to chemotherapy in PDA[47-49].
Risk factors for PDA can be divided into modifiable and non-modifiable. Smoking has been proven beyond doubt to be the main modifiable risk factor. The risk is approximately twice in smokers, and they are at risk even after smoking cessation for about 20 years[50,51]. Alcohol is an additional risk factor in smokers but not in non-smokers[52]. Obesity is clearly associated with an increased risk of PDA as well as mortality[53]. Dietary factors such as consumption of red meat and processed foods are associated with an increased risk of PDA while consumption of fresh fruits and folate is protective[54,55]. Occupational exposure to nickel, cadmium and chlorinated biphenyls is associated with an increased risk[56].
Among the nonmodifiable risk factors, male sex, increasing age and African-American ethnicity are associated with an increased risk[13]. Genetic factors play an important role as 10% of the PDAs have a family history[57,58]. Mutations in the genes such as BRCA2, PALB2, STK11, CDKN2A, APC, Lynch syndrome genes, ATM, FANCC and FANCG are responsible for the familial causes of PDA[59]. Chronic pancreatitis is a risk factor for PDA, particularly chronic pancreatitis due to hereditary pancreatitis (PRSS1/SPINK1 gene mutation)[60]. Diabetes type 1 and 2, particularly recent-onset diabetes, are associated with an increased risk of PDA. However, the causal association was not proved and is a matter of debate as diabetes may be a manifes
As invasive PDA arises from PanIN and from mucinous neoplasm and intraductal papillary neoplasm (IPMN) screening for these lesions can be beneficial as the disease can be cured with resection at an early stage. However, till now there is no approved and reliable screening test for PDA[66,67]. PDA is a cancer of comparatively low prevalence but with high mortality. Due to the non-availability of any standard, economical and reliable screening test, screening the entire general population for PDA is not possible. However, patients with a family history of pancreatic cancer have an increased risk of PDA and benefit from screening[66]. Screening is particularly recommended for those with at least two first-degree relatives with PDA or in patients with known familial syndromes. Even though IPMNs are visible on conventional imaging, PanIN are very small lesions of size less than 5 mm and are not identified on routine imaging studies[66]. Hence, we need to rely on biomarkers alone or in combination with imaging studies for screening.
The most commonly used biomarker is carbohydrate antigen (CA) 19-9, which is sialylated Lewis blood group antigen on MUC1 expressed by neoplastic cells of PDA and also by the normal cells of the pancreaticobiliary system, stomach, colon, endometrium and salivary glands[68]. It is elevated in only 65% of resectable pancreatic cancers and hence of low sensitivity[69]. It is also elevated in benign diseases of the biliary tract, biliary obstruction and also in malignancies arising from other organs, and hence it is also less specific[69,70]. CA19-9 cannot be used as a tumor marker in populations who do not express Lewis antigen (4%-15%)[66,69,70]. Therefore, it is primarily used to assess the response to treatment and in the follow-up of patients diagnosed with PDA[69,70]. Carcinoembryonic antigen (CEA) in the pancreatic juice can also be used to screen pancreatic cancer with reasonable accuracy[71,72]. However, the main limiting factor of CEA is the low sensitivity although specificity is high[71,72].
PAM4 is an anti MUC1 antibody, which is directed specifically against an epitope of MUC1 secreted by the pancreatic cancer cells absent in normal pancreas and other tissues and therefore found to be more specific and sensitive than CA19-9 in differentiating pancreatitis and pancreatic cancer[73]. Patients with advanced disease had higher values of PAM4 compared to early stages[74]. PAM4 can also be used to screen early lesions of PDA such as PanINs and IPMNs as the expression begins at an early stage of the disease and continues throughout[75]. The role of several new biomarkers such as CA494, CA50, CA242, CEA -related cell adhesion molecule 1, CAM 17.1-Ab, parathyroid hormone-related oncoprotein and serum beta-human chorionic gonadotropin in diagnosing early pancreatic lesions is growingly evident[70,76-80]. In a meta-analysis of seven studies evaluating the role of tumor M2-pyruvate kinase in screening pancreatic cancer, the conclusion was that the efficacy of tumor M2-pyruvate kinase was similar to CA19-9[81]. SPan-1 is also one of the markers studied for the diagnosis of exocrine pancreatic cancer, but it did not improve the rates when combined with CA19-9[82].
Various genetic and epigenetic mutations can be detected in the pancreatic juice obtained by endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreticography[83]. K-ras mutations and TP53 mutations identified in the pancreatic juice are associated with low specificity and sensitivity even though they are mutated in most of the PDAs[84,85]. DNA methylation abnormalities of a panel of genes were associated with a sensitivity of 82% and a specificity of 100% in identifying pancreatic cancer in a study[86]. Identification of mitochondrial mutations in the pancreatic juice is also being studied in diagnosing pancreatic cancer[87]. The main drawback of the above-mentioned tests is the requirement of invasive intervention to obtain the sample. Micro-RNAs are being intensely investigated in diagnosing various human cancers including pancreatic cancer[88]. Circulating tumor cells are also present in 47% of patients with PDA and are also associated with early lesions.
Multi-detector computerized tomography (MDCT) is presently the gold standard for the diagnosis of pancreatic lesions[89,90]. It is the virtual eye of a surgeon to assess the resectability of the tumor and also to accurately stage the disease[91,92]. The major drawback is the low sensitivity of MDCT in identifying lesions less than 2 cm and negligible sensitivity for identifying the pre-invasive lesions. Also, the routine use of MDCT for screening purposes in high-risk patients may increase the risk of radiation-induced secondary tumors. Hence, MDCT is preferably not used for the purpose of screening high-risk individuals and is comparatively inferior to EUS for the same[93]. Magnetic resonance imaging (MRI) is a non-ionizing investigation, which can image the entire abdomen as opposed to EUS. MRCP can provide very accurate details of the biliary and pancreatic ductal system and can identify small cystic lesions such as IPMNs. In fact, MRCP is proven to be superior to MDCT in detecting these lesions[93,94]. In a prospective study evaluating the role of MRI in the screening of patients with P16 mutations, MRI was able to identify early lesions[95].
EUS plays an important role in the screening and diagnosis of PDA. EUS was shown to be superior to MDCT, MRI and positron emission tomography (PET) in detecting small lesions and lymph node involvement[96]. It was able to detect twice the number of lesions compared to MDCT and MRI when used for screening[94]. It can also be used for biopsying a suspected lesion. The major drawback is the invasiveness of the procedure and its operator dependence. It may also be associated with rare but severe complications such as iatrogenic gastrointestinal perforation. EUS is usually performed in sequence with biomarker tests (in those with elevated CA19-9) or after a basic imaging test rather than as a first option[66,97].
PET scan is one of the valuable investigations in PDA with good sensitivity but an average specificity. In two meta-analyses from Tang et al[98] and Wu et al[99], the pooled sensitivities were 90.1% and 87%, respectively, and pooled specificities were 80.1% and 83%, respectively[98,99]. The low specificity is because of the inability of the PET scan to differentiate between inflammatory lesions and neoplastic lesions[100]. The role of the PET scan in determining the T stage is limited and is surpassed by MDCT[101]. Even though the PET scan was able to pick up positive lymph nodes in other malignancies, the sensitivity of PET in accurately determining the N stage of PDA is limited[100,102]. However, a PET scan is invaluable in diagnosing metastatic PDA, which can alter the management of the patient[103].
Surgical resection of PDA either pancreaticoduodenectomy (PD) or distal pancreatectomy (DP) with splenectomy is associated with high morbidity and mortality. Hence, thorough preoperative preparation is essential to avoid complications. The preoperative preparation is similar to any other major surgery except for two unique complications encountered in PDA: Obstructive jaundice and nutritional deficiencies. Obstructive jaundice is common in patients with carcinoma involving the head of the pancreas.
There were many studies evaluating the role of preoperative biliary drainage (PBD) vs surgery alone. Preoperative drainage is associated with improvement in the general condition of the patient and liver function. However, it is associated with significant side effects such as the introduction of infection into the biliary tree[104]. Furthermore, preoperative drainage will make the surgery challenging due to inflammation and fibrosis induced by the common bile duct (CBD) stents and decreased diameter of the CBD making anastomosis difficult. In a meta-analysis by Sewnath et al[105], the lack of benefit of PBD over direct surgery in patients of periampullary carcinoma with obstructive jaundice was proven[105], further confirmed by a Cochrane review and in a meta-analysis by Wang et al[106] and Fang et al[107]. It was proven in various studies that PBD and delayed surgery to improve the general condition were not associated with any improvement in survival[108]. The only indications accepted for PBD before surgery at present include patients with cholangitis, poor general condition and poor performance status precluding surgery and in whom neoadjuvant treatment is planned. PBD can be undertaken with the help of CBD stents or percutaneous transhepatic biliary drainage. Among the CBD stents, metallic or plastic ones can be selected. In a meta-analysis of five studies by Crippa et al[109], the rate of re-intervention and post-operative biliary fistula was shown to be lower in the case of metallic stents compared to plastic stents[109]. Hence, at present, the available evidence supports the use of metallic stents over plastic stents, in both unresectable PDA and for PBD.
With the mortality and morbidity rates of pancreatic surgery improving over several decades, focus has been shifted to performing the surgeries using minimally invasive techniques. In a meta-analysis by Venkat et al[110] evaluating laparoscopic DP with open technique, they inferred that the laparoscopic technique was associated with lower blood loss, shorter hospital stay and lower overall postoperative complications compared to the open technique with no difference in the margin status and operating time[110]. Similar results were obtained in the meta-analysis by Jin et al[111].
The results from studies comparing laparoscopic PD (LPD) and open PD (OPD) are also encouraging. A summary of the outcomes of few recent studies in this regard is presented in Table 3. LPD was associated with lower intra-operative blood loss, faster recovery and shorter hospital stay with similar rate of post-operative complications and oncological outcomes[112-118]. However, in most of the studies, LPD was associated with greater operating times compared to OPD[112-118]. Robotic surgery has the added advantage of increased degrees of freedom and better images without motion artifacts compared to conventional laparoscopic surgery. In a retrospective study by Nassour et al[119], 428 minimally invasive PD surgeries were analyzed, and the 30-d complication rate was found to be the same between robotic and laparoscopic groups[119]. In a meta-analysis of 44 studies by Kamarajah et al[120] comparing robotic and conventional laparoscopic PD, the conclusion was that the robotic group was associated with lower conversion rates compared to the laparoscopic group[120]. No significant difference was noted in the operating times and blood loss between the two groups. The robotic surgery group had a shorter hospital stay compared to the laparoscopic group.
| Ref. | Study | Comparison | Outcome |
| Nickel et al[112], 2020 | Meta-analysis of 3 RCTs | LPD and OPD | 90-d mortality, post-operative complications and oncological outcomes were similar in both groups |
| Blood loss was less for LPD | |||
| Operating time was more for LPD | |||
| Yoo et al[113], 2020 | Retrospective cohort study 359 patients | LPD and OPD | Post-operative complications and hospital stay were shorter for LPD |
| Operative time was longer for LPD | |||
| Recurrence free outcomes andoverall survival rates were similar | |||
| Chen et al[114], 2020 | Meta-analysis of 6 cohort studies | LPD and OPD for PDA | Number of lymph nodes harvested, number of positive lymph nodes, rate of adjuvant therapy, time to adjuvant therapy, 1 yr survival and 2 yr survival are same for both the groups |
| Zhou et al[115], 2019 | Retrospective cohort study | LPD and OPD | Overall complications and survival were similar between the two groups |
| Chen et al[116], 2018 | Retrospective cohort study of 102 patients | LPD and OPD | Intra-operative blood loss, post-operative recovery and hospital stay were shorter for LPD |
| Operative time was longer for LPD | |||
| Post operative complications were similar in both the groups | |||
| Dang et al[117], 2020 | Retrospective cohort study | LPD and OPD | Intra-operative blood loss, operating time and hospital stay for shorter for LPD |
| 30 d and 90 d mortality rates were better for LPD | |||
| Long term survival rates were similar | |||
| Palanivelu et al[118], 2017 | RCT of 68 patients with periampullary carcinoma | LPD and OPD | Intra-operative blood loss and hospital stay for shorter for LPD |
| Operative time was longer for LPD | |||
| Post-operative complications were similar in both the groups |
With the expertise of vascular reconstructions, venous involvement is no longer an absolute contraindication for resection of PDA with the criteria for resectability being updated regularly[121]. Venous reconstructions are widely practiced, and the present limiting factor seems to be arterial involvement. Hence, the approach has been shifted to the artery-first approach to determine the resectability of the tumor at the initial phase of the surgery itself. These include the posterior approach, medial uncinate approach, inferior infracolic approach, left posterior approach, inferior supracolic approach and superior approaches, which have become popular[122]. In a meta-analysis of 22 studies by Yu et al[123], PD combined with portal-superior mesenteric vein synchronous resection (PSMVR) was found to be associated with similar post-operative morbidity and mortality compared to the group without resection[123]. However, only the group with R0 resection had a significant improvement in survival[123]. In a similar meta-analysis by Bell et al[124], it was concluded that PSMVR was associated with a higher R1 rate, poor 5-year survival rate and was not cost effective[124]. In a meta-analysis of 26 studies by Mollberg et al[125] comparing pancreatectomy with arterial resection (AR) and without AR, pancreatectomy with AR was associated with an increased peri-operative mortality and poor 1-year and 2-year survival[125].
Post-operative pancreatic fistula (POPF) is one of the most dreaded complications of PD[126]. Many methods have been advocated to reduce its incidence, beginning with the type of pancreaticoenteric anastomosis. Several randomized control trial (RCTs) and meta-analysis have shown that pancreaticogastrostomy was associated with less incidence of POPF[127-129]. However, pancreatojejunostomy (PJ) is the mostly widely practiced technique because it is more physiological and is associated with lower long-term complications than pancreaticogastrostomy[130]. Several techniques of PJ have been compared in trials for the incidence of POPF. Among the duct-to-mucosa anastomoses, the Blumgart technique was found to be better compared to the Cattell-Warren technique[131]. Both the Blumgart and Kakita techniques were associated with similar results in many studies[132,133].
The continuous suturing technique was associated with a lower incidence of POPF compared to the interrupted suturing technique in several studies[134,135]. It is hypothesized that continuous sutures lead to a uniform distribution of tension along the suture line compared to intermittent sutures. A brief interest was sparked in the invagination techniques after the introduction of the jejunal eversion with binding PJ technique by Peng et al[136] and the end-to-side invagination technique by Berger et al[137], which showed better results with respect to POPF compared to the duct-to-mucosa technique[136,137]. However, the results were not reproduced in other trials[138]. In a study by Kojima et al[139], it was concluded that the Blumgart technique of PJ when combined with the tight dressings of the wound and drain sites (complete packing method) was associated with less incidence of POPF[139].
In a move to reduce the incidence of POPF, stenting of the PJ was evaluated in various trials. Stenting is hypothesized to prevent the pancreatic enzymes from coming in contact with the anastomosis, thereby promoting healing of the anastomosis. Studies comparing internal stents and no stents revealed no significant difference in the rate of POPF[140-142]. In trials comparing external stenting and no stenting, few trials have demonstrated the benefit of external stenting[142-145]. However, these stents are associated with complications such as tube-related complications, digestive enzyme loss and possible peritonitis during tube removal[141]. Moreover, no difference was observed between the internal and external stents in preventing the POPF[146]. Most of the high-volume centers do not stent the PJ at present.
In the case of DP, stump closure using hand-sewn or stapler techniques was evaluated with respect to POPF. There was no significant difference in the POPF rates between the two techniques, and the stapler technique is commonly used by most surgeons[147,148]. In a meta-analysis evaluating bare metallic staplers and reinforced staplers using bioabsorbable materials, the superiority of the reinforced staplers was not proven even though the rate of POPF was less in reinforced staplers[149]. Pancreaticoenteric anastomosis of the distal stump has been shown to reduce the rate of POPF but increased the rate of post–operative hemorrhage[150,151].
Topical application of fibrin sealants over pancreatic anastomosis has no effect on POPF incidence in various studies[152,153]. Similarly, omental wrapping around the pancreatic anastomosis has no effect on the POPF or post-operative hemorrhage[154]. Covering the distal pancreatic stump with a teres ligament patch has shown to reduce the rate of reoperations and readmissions compared to simple closure even though the rate of POPF was not significantly different between the two groups in a randomized control trial[155].
The role of prophylactic intraperitoneal drains following pancreatectomy is controversial. In case of DP, there is no role of prophylactic intraperitoneal drains as concluded in various studies[156,157]. Moreover, prophylactic drain placement increases hospital stay. The PANDRA trial randomized 395 patients with PD with or without drains and concluded that there was no need for routine prophylactic intraperitoneal drainage[158]. However, other studies have reported increased mortality in patients who underwent PD without drains[159,160]. However, in low-risk patients, drains can be safely avoided[160]. If drains are placed, they must be removed as early as possible once their purpose is served as their prolonged placement may lead to intra-abdominal infections and increase the risk of POPF[161].
The role of somatostatin analogues in preventing POPF is unclear. Earlier RCTs revealed the efficacy of octreotide in preventing POPF. However, newer RCTs proved that there is no role of octreotide in preventing POPF[162-164]. Pasireotide was found to reduce the rate of POPF in one RCT by Allen et al[165], but not in other RCTs[165-167]. At present, the use of somatostatin analogues cannot be recommended due to inconsistent results in clinical trials.
Borderline and locally advanced lesions are started on chemotherapy ± chemoradiation as per the present guidelines[168,169]. The current preferred regimen is 5-Flurouracil, irinotecan and oxaliplatin (FOLFIRINOX) ± chemoradiation, if the patient has good performance status[169-172]. Alternatively, gemcitabine and nab-paclitaxel ± chemoradiation can also be used[170-172]. In patients with known mutations in the BRCA gene, substituting paclitaxel with cisplatin may provide added benefit[170]. Patients with poor performance status can be started on single-agent chemotherapy or provided with palliative care. The likelihood of resection depends upon the response to neoadjuvant therapy. As per the latest studies, the response should be measured by falling CA19-9 Levels and absence of disease progression while on neoadjuvant therapy rather than by assessing the radiological regression[173-176].
Most of the patients with PDA in the long term, even after complete resection, develop distant metastasis. This points to the notion of micrometastasis even in a resectable localized cancer at the time of presentation. Also, due to the morbidity associated with surgery, a significant proportion of patients are unable to receive adjuvant therapy or have a delay. The encouraging results of neoadjuvant and perioperative chemoradiation therapies in esophageal, gastric and rectal carcinoma have stimulated research on the role of neoadjuvant therapy in resectable lesions of PDA. It also improves the rate of R0 resections. Multiple trials have been conducted to assess the role of neoadjuvant chemotherapy and chemoradiation in resectable PDA (Table 4). The results of many of the latest trials support neoadjuvant chemotherapy therapy in resectable PDA[177,178]. The results of ongoing trails such as NEPAFOX, NorPACT-1, NEOPAC and NCT02562716 are eagerly awaited[179-183]. The role of neoadjuvant chemoradiotherapy in resectable PDA is proved in several trials[184-186].
| Ref. | Type of study | Type of neoadjuvant therapy | Drugs | Results |
| Tajima et al[177], 2012 | Retrospective pilot study | Chemotherapy | Gemcitabine and S1 | The 3 yr survival rates of NACT group (55.6%) was higher than control group (29.6%) |
| O’Reilly et al[178], 2014 | Phase II trial non randomized | Chemotherapy | Gemcitabine and oxalipaltin | Resectability was 71% |
| Overall survival was 21.7 mo | ||||
| Motoi et al[179], 2013 | RCT- NACT vs direct surgery | Chemotherapy | Gemcitabine and S1 | Results awaited |
| Scott et al[180], 2017 | RCT- NACT vs direct surgery | Chemotherapy | FOLFIRINOX | Results awaited |
| Labori et al[181], 2017 | RCT- NACT vs direct surgery | Chemotherapy | FOLFIRINOX | Results awaited |
| Heinrich et al[182], 2011 | RCT- NACT vs direct surgery | Chemotherapy | Gemcitabine and oxalipaltin | Results awaited |
| Sohal et al[183], 2017 | RCT-FOLFIRINOX vs GnP | Chemotherapy | FOLFIRINOX vs Gemcitabine and nab paclitaxel | Results awaited |
| Turrini et al[184], 2009 | Prospective study | Chemoradiotherapy | 5-Flurouracil and cisplatin with radiotherpay | Respectability rate is 82.6% |
| Median overall survival for resected patients is 23 mo | ||||
| Golcher et al[185], 2015 | RCT- NACRT vs direct surgery | Chemoradiotherapy | Gemcitabine and Cisplatin with radiotherpay | R0 resection rate (52%) and median overall survival after tumor resection (27 mo) was greater NACRT arm |
| Okano et al[186], 2017 | Prospective study | Chemoradiotherapy | S-1 with radiotherapy | 1-yr and 2-yr survival rates are 91% and 83% in resectable group |
The trials of GITSG and EORTC 40891 have clearly proven the role of adjuvant chemoradiotherapy in PDA[187,188]. The ESPAC-1 trial has shown the beneficial effects of chemotherapy over chemoradiotherapy[189]. Even though the opinion on the role of adjuvant chemoradiotherapy is different among practitioners in Europe and the United States of America, most of them have a common opinion on the role of adjuvant chemotherapy. The CONKO-1 trial after showcasing the efficacy of gemcitabine in adjuvant chemotherapy has shifted the adjuvant chemotherapy regimens from 5-flurouracil (5-FU) to gemcitabine-based regimens[190]. The ESPAC-3 trial has shown no significant difference between the 5-FU and gemcitabine regimen[191]. The ESPAC-4 trial has proved the increased efficacy of the gemcitabine and capecitabine combination over gemcitabine alone and is now the recommended regimen for adjuvant therapy[192]. The success of the FOLFIRINOX regimen in the metastatic setting has led to the evaluation of its role in the adjuvant setting. The PRODIGE 24 trial has proven the efficacy of FOLFIRINOX over gemcitabine, but its use is limited to patients with a good performance status[193]. In the Japanese trial JASPAC-01, S1 was proven superior to gemcitabine, but it is still not widely used outside Japan[194]. The CONKO-05 and CONKO-06 trials did not prove the efficacy of gemcitabine + erlotinib and gemcitabine +sorafenib, respectively, over gemcitabine alone[195,196].
Combination chemotherapy regimens are now being recommended over single agent gemcitabine for patients with metastatic disease with good performance status. The ACCORD trial has laid the ground for the FOLFIRINOX regimen as the preferred regimen over gemcitabine[197]. Several new second-line regimens were added to the arsenal after the success of the MPACT and NAPOLI-1 trials[198,199]. The trial comparing extracellular matrix degrader pegvorhyaluronidase alfa (PEGPH20) was stopped as its primary end-point was not met[200]. The results of the clinical trial AVENGER 50 comparing modified FOLFIRINOX with or without CPI-613 are awaited[201].
Studies on the role of immunotherapy in cancers are encouraging, but that is not the case with PDA[202]. The highly desmoplastic stroma and absence of any effector cells in the tumor microenvironment seem to be the predominant reason for the failure of immunotherapy[203]. Even though pembrolizumab is approved in patients with microsatellite instability, the latest results from the KEYNOTE-158 study are disappointing[204]. Two new targeted therapy drugs have been approved. Larotrec
Gene therapy for pancreatic cancer is being widely studied. A number of clinical trials and ongoing studies have been conducted in this regard[209]. Even though the results of gene therapy in phase 1 trials are encouraging, the same is not being replicated in the phase 2 studies in comparison with standardized treatment. The role of miRNAs such as miR-4516 is being studied, which may be the future target for therapies[210]. The role of intra-operative chemotherapy in PDA is being studied in the combiCaRe trial[211].
This narrative review highlights the newer trends and achievements in the epidemiology, etiopathogenesis, screening, diagnosis and management of PDA. The newer trends in epidemiology will help us to predict the population and countries at risk in the near future. The newer concepts in the field of etiopathogenesis will help us to understand this stubborn disease better. Newer screening and diagnostic techniques will help in diagnosing the patients at an early stage and prognosticate better. The newer discoveries in drugs and management protocols will help the physicians and surgeons increase the survival and quality of life of the patients.
| 1. | International Agency for Research on Cancer. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer. [cited 22 December 2020]. Available from: https://gco.iarc.fr/today. |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56638] [Article Influence: 7079.8] [Reference Citation Analysis (134)] |
| 3. | Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Luchini C, Capelli P, Scarpa A. Pancreatic Ductal Adenocarcinoma and Its Variants. Surg Pathol Clin. 2016;9:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1337] [Article Influence: 167.1] [Reference Citation Analysis (45)] |
| 6. | Vareedayah AA, Alkaade S, Taylor JR. Pancreatic Adenocarcinoma. Mo Med. 2018;115:230-235. [PubMed] |
| 7. | Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Buanes TA. Role of surgery in pancreatic cancer. World J Gastroenterol. 2017;23:3765-3770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | White RR, Reddy S, Tyler DS. The role of chemoradiation therapy in locally advanced pancreatic cancer. HPB (Oxford). 2005;7:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Pathy S, Chander S. Chemoradiotherapy in pancreatic carcinoma. Indian J Med Paediatr Oncol. 2009;30:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, El-Rayes BF, Wang-Gillam A, Lacy J, Hosein PJ, Moorcraft SY, Conroy T, Hohla F, Allen P, Taieb J, Hong TS, Shridhar R, Chau I, van Eijck CH, Koerkamp BG. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 714] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 12. | SEER*Explorer. An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. [cited 14 September 2020]. Available from: https://seer.cancer.gov/explorer/. |
| 13. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1610] [Article Influence: 230.0] [Reference Citation Analysis (1)] |
| 14. | Pei X, Song F, Wang Z. Emerging incidence trends and application of curative treatments of pancreatic cancer in the USA. Medicine (Baltimore). 2019;98:e17175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 16. | Willett WC. Diet and cancer. Oncologist. 2000;5:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 17. | Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, English DR, Folsom AR, Freudenheim JL, Fuchs CS, Giles GG, Giovannucci E, Horn-Ross PL, Larsson SC, Leitzmann M, Männistö S, Marshall JR, Miller AB, Patel AV, Rohan TE, Stolzenberg-Solomon RZ, Verhage BA, Virtamo J, Willcox BJ, Wolk A, Ziegler RG, Smith-Warner SA. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129:1708-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Jarosz M, Sekuła W, Rychlik E. Influence of diet and tobacco smoking on pancreatic cancer incidence in poland in 1960-2008. Gastroenterol Res Pract. 2012;2012:682156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Wu W, He X, Yang L, Wang Q, Bian X, Ye J, Li Y, Li L. Rising trends in pancreatic cancer incidence and mortality in 2000-2014. Clin Epidemiol. 2018;10:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105 Suppl 2:S77-S81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 466] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (2)] |
| 21. | West R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol Health. 2017;32:1018-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 381] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 22. | Kuroczycki-Saniutycz S, Grzeszczuk A, Zwierz ZW, Kołodziejczyk P, Szczesiul J, Zalewska-Szajda B, Ościłowicz K, Waszkiewicz N, Zwierz K, Szajda SD. Prevention of pancreatic cancer. Contemp Oncol (Pozn). 2017;21:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 24. | Yao W, Maitra A, Ying H. Recent insights into the biology of pancreatic cancer. EBioMedicine. 2020;53:102655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 641] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 26. | Bollag G, Zhang C. Drug discovery: Pocket of opportunity. Nature. 2013;503:475-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Scully KM, Lahmy R, Signaevskaia L, Sasik R, Medal R, Kim H, French R, James B, Wu Y, Lowy AM, Itkin-Ansari P. E47 Governs the MYC-CDKN1B/p27KIP1-RB Network to Growth Arrest PDA Cells Independent of CDKN2A/p16INK4A and Wild-Type p53. Cell Mol Gastroenterol Hepatol. 2018;6:181-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Tatarian T, Winter JM. Genetics of Pancreatic Cancer and Its Implications on Therapy. Surg Clin North Am. 2016;96:1207-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Das S, Cardin D. Targeting DNA Damage Repair Pathways in Pancreatic Adenocarcinoma. Curr Treat Options Oncol. 2020;21:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306-316. [PubMed] |
| 31. | Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [PubMed] |
| 32. | Liu BB, Wang WH. Survivin and pancreatic cancer. World J Clin Oncol. 2011;2:164-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Wilentz RE. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Ohuchida K, Mizumoto K, Ohhashi S, Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. S100A11, a putative tumor suppressor gene, is overexpressed in pancreatic carcinogenesis. Clin Cancer Res. 2006;12:5417-5422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Ohuchida K, Mizumoto K, Egami T, Yamaguchi H, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. S100P is an early developmental marker of pancreatic carcinogenesis. Clin Cancer Res. 2006;12:5411-5416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Suh H, Pillai K, Morris DL. Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am J Cancer Res. 2017;7:1372-1383. [PubMed] |
| 38. | Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Ohuchida K, Mizumoto K, Yamada D, Fujii K, Ishikawa N, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. Quantitative analysis of MUC1 and MUC5AC mRNA in pancreatic juice for preoperative diagnosis of pancreatic cancer. Int J Cancer. 2006;118:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 632] [Article Influence: 90.3] [Reference Citation Analysis (6)] |
| 42. | Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, Liu L, Huang D, Jiang J, Cui GS, Yang Y, Wang W, Guo D, Dai M, Guo J, Zhang T, Liao Q, Liu Y, Zhao YL, Han DL, Zhao Y, Yang YG, Wu W. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29:725-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 903] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 43. | Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168-1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1041] [Cited by in RCA: 1605] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 44. | Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, Lengrand J, Deshpande V, Selig MK, Ferrone CR, Settleman J, Stephanopoulos G, Dyson NJ, Zoncu R, Ramaswamy S, Haas W, Bardeesy N. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 669] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 45. | Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, Tomar G, Papke B, Hobbs GA, Yan L, Hayes TK, Diehl JN, Goode GD, Chaika NV, Wang Y, Zhang GF, Witkiewicz AK, Knudsen ES, Petricoin EF 3rd, Singh PK, Macdonald JM, Tran NL, Lyssiotis CA, Ying H, Kimmelman AC, Cox AD, Der CJ. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25:628-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 545] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 46. | Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap JT, Burrell LD, Lum DH, Whisenant JR, Gilcrease GW 3rd, Cavalieri CC, Rehbein KM, Cutler SL, Affolter KE, Welm AL, Welm BE, Scaife CL, Snyder EL, McMahon M. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 507] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 47. | Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, Illies AL, Gebregiworgis T, Dai B, Augustine JJ, Murthy D, Attri KS, Mashadova O, Grandgenett PM, Powers R, Ly QP, Lazenby AJ, Grem JL, Yu F, Matés JM, Asara JM, Kim JW, Hankins JH, Weekes C, Hollingsworth MA, Serkova NJ, Sasson AR, Fleming JB, Oliveto JM, Lyssiotis CA, Cantley LC, Berim L, Singh PK. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell 2017; 32: 71-87. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 48. | Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O, Pinault M, Guimaraes C, Nigri J, Loncle C, Lavaut MN, Garcia S, Tailleux A, Staels B, Calvo E, Tomasini R, Iovanna JL, Vasseur S. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112:2473-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 49. | Tadros S, Shukla SK, King RJ, Gunda V, Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, Grem J, Sasson AR, Singh PK. De Novo Lipid Synthesis Facilitates Gemcitabine Resistance through Endoplasmic Reticulum Stress in Pancreatic Cancer. Cancer Res. 2017;77:5503-5517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 50. | Kuzmickiene I, Everatt R, Virviciute D, Tamosiunas A, Radisauskas R, Reklaitiene R, Milinaviciene E. Smoking and other risk factors for pancreatic cancer: a cohort study in men in Lithuania. Cancer Epidemiol. 2013;37:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Pelucchi C, Galeone C, Polesel J, Manzari M, Zucchetto A, Talamini R, Franceschi S, Negri E, La Vecchia C. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Rahman F, Cotterchio M, Cleary SP, Gallinger S. Association between alcohol consumption and pancreatic cancer risk: a case-control study. PLoS One. 2015;10:e0124489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003;89:519-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 244] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 54. | Lightsey D; National Council Against Health Fraud and Quackwatch. Comment on 'Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies'. Br J Cancer. 2012;107:754-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Paluszkiewicz P, Smolińska K, Dębińska I, Turski WA. Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case-control and cohort studies. Cancer Epidemiol. 2012;36:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 57. | Greer JB, Whitcomb DC, Brand RE. Genetic predisposition to pancreatic cancer: a brief review. Am J Gastroenterol. 2007;102:2564-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Petersen G, Zheng W, Agalliu I, Allen NE, Amundadottir L, Boutron-Ruault MC, Buring JE, Canzian F, Clipp S, Dorronsoro M, Gaziano JM, Giovannucci EL, Hankinson SE, Hartge P, Hoover RN, Hunter DJ, Jacobs KB, Jenab M, Kraft P, Kooperberg C, Lynch SM, Sund M, Mendelsohn JB, Mouw T, Newton CC, Overvad K, Palli D, Peeters PH, Rajkovic A, Shu XO, Thomas G, Tobias GS, Trichopoulos D, Virtamo J, Wactawski-Wende J, Wolpin BM, Yu K, Zeleniuch-Jacquotte A. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer. 2010;127:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology 2010; 139: 1076-1080, 1080.e1-1080. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 60. | Raphael KL, Willingham FF. Hereditary pancreatitis: current perspectives. Clin Exp Gastroenterol. 2016;9:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 790] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 62. | Bosetti C, Rosato V, Li D, Silverman D, Petersen GM, Bracci PM, Neale RE, Muscat J, Anderson K, Gallinger S, Olson SH, Miller AB, Bas Bueno-de-Mesquita H, Scelo G, Janout V, Holcatova I, Lagiou P, Serraino D, Lucenteforte E, Fabianova E, Baghurst PA, Zatonski W, Foretova L, Fontham E, Bamlet WR, Holly EA, Negri E, Hassan M, Prizment A, Cotterchio M, Cleary S, Kurtz RC, Maisonneuve P, Trichopoulos D, Polesel J, Duell EJ, Boffetta P, La Vecchia C, Ghadirian P. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2014;25:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 63. | Risch HA, Lu L, Kidd MS, Wang J, Zhang W, Ni Q, Gao YT, Yu H. Helicobacter pylori seropositivities and risk of pancreatic carcinoma. Cancer Epidemiol Biomarkers Prev. 2014;23:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011;128:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Zhang BL, He N, Huang YB, Song FJ, Chen KX. ABO blood groups and risk of cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:4643-4650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am. 2012;41:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 67. | Canto MI. Strategies for screening for pancreatic adenocarcinoma in high-risk patients. Semin Oncol. 2007;34:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Scarà S, Bottoni P, Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 69. | Goggins M. Identifying molecular markers for the early detection of pancreatic neoplasia. Semin Oncol. 2007;34:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Bünger S, Laubert T, Roblick UJ, Habermann JK. Serum biomarkers for improved diagnostic of pancreatic cancer: a current overview. J Cancer Res Clin Oncol. 2011;137:375-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Zubarik R, Gordon SR, Lidofsky SD, Anderson SR, Pipas JM, Badger G, Ganguly E, Vecchio J. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Meng Q, Shi S, Liang C, Liang D, Xu W, Ji S, Zhang B, Ni Q, Xu J, Yu X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:4591-4598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 73. | Gold DV, Modrak DE, Ying Z, Cardillo TM, Sharkey RM, Goldenberg DM. New MUC1 serum immunoassay differentiates pancreatic cancer from pancreatitis. J Clin Oncol. 2006;24:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Gold DV, Goggins M, Modrak DE, Newsome G, Liu M, Shi C, Hruban RH, Goldenberg DM. Detection of early-stage pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2786-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380-7387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17:867-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (7)] |
| 77. | Simeone DM, Ji B, Banerjee M, Arumugam T, Li D, Anderson MA, Bamberger AM, Greenson J, Brand RE, Ramachandran V, Logsdon CD. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas. 2007;34:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Rückert F, Pilarsky C, Grützmann R. Serum tumor markers in pancreatic cancer-recent discoveries. Cancers (Basel). 2010;2:1107-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Bouvet M, Nardin SR, Burton DW, Lee NC, Yang M, Wang X, Baranov E, Behling C, Moossa AR, Hoffman RM, Deftos LJ. Parathyroid hormone-related protein as a novel tumor marker in pancreatic adenocarcinoma. Pancreas. 2002;24:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Louhimo J, Alfthan H, Stenman UH, Haglund C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Kumar Y, Gurusamy K, Pamecha V, Davidson BR. Tumor M2-pyruvate kinase as tumor marker in exocrine pancreatic cancer a meta-analysis. Pancreas. 2007;35:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Frena A. SPan-1 and exocrine pancreatic carcinoma. The clinical role of a new tumor marker. Int J Biol Markers. 2001;16:189-197. [PubMed] |
| 83. | Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X, Wallace MB, Killary AM, Frazier ML, Woodward TA, Wang J, Sen S. Circulating microRNAs in Pancreatic Juice as Candidate Biomarkers of Pancreatic Cancer. J Cancer. 2014;5:696-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Tada M, Komatsu Y, Kawabe T, Sasahira N, Isayama H, Toda N, Shiratori Y, Omata M. Quantitative analysis of K-ras gene mutation in pancreatic tissue obtained by endoscopic ultrasonography-guided fine needle aspiration: clinical utility for diagnosis of pancreatic tumor. Am J Gastroenterol. 2002;97:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Eshleman JR, Norris AL, Sadakari Y, Debeljak M, Borges M, Harrington C, Lin E, Brant A, Barkley T, Almario JA, Topazian M, Farrell J, Syngal S, Lee JH, Yu J, Hruban RH, Kanda M, Canto MI, Goggins M. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol 2015; 13: 963-9. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Matsubayashi H, Canto M, Sato N, Klein A, Abe T, Yamashita K, Yeo CJ, Kalloo A, Hruban R, Goggins M. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Maitra A, Cohen Y, Gillespie SE, Mambo E, Fukushima N, Hoque MO, Shah N, Goggins M, Califano J, Sidransky D, Chakravarti A. The Human MitoChip: a high-throughput sequencing microarray for mitochondrial mutation detection. Genome Res. 2004;14:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 88. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7422] [Article Influence: 353.4] [Reference Citation Analysis (5)] |
| 89. | Zakharova OP, Karmazanovsky GG, Egorov VI. Pancreatic adenocarcinoma: Outstanding problems. World J Gastrointest Surg. 2012;4:104-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Canto MI. Screening and surveillance approaches in familial pancreatic cancer. Gastrointest Endosc Clin N Am. 2008;18:535-553, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Zhao WY, Luo M, Sun YW, Xu Q, Chen W, Zhao G, Wu ZY. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2009;8:457-464. [PubMed] |
| 92. | Buchs NC, Chilcott M, Poletti PA, Buhler LH, Morel P. Vascular invasion in pancreatic cancer: Imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol. 2010;16:818-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 93. | Lami G, Biagini MR, Galli A. Endoscopic ultrasonography for surveillance of individuals at high risk for pancreatic cancer. World J Gastrointest Endosc. 2014;6:272-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Steinberg WM, Barkin JS, Bradley EL 3rd, DiMagno E, Layer P, Canto MI, Levy MJ. Should patients with a strong family history of pancreatic cancer be screened on a periodic basis for cancer of the pancreas? Pancreas. 2009;38:e137-e150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA, Bergman W, Hes FJ, Hommes DW, Offerhaus GJ, Morreau H, Bonsing BA, de Vos tot Nederveen Cappel WH. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology. 2011;140:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (4)] |
| 96. | Mitra A, D'Souza A, Goel M, Shrikhande SV. Surgery for Pancreatic and Periampullary Carcinoma. Indian J Surg. 2015;77:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 98. | Tang S, Huang G, Liu J, Liu T, Treven L, Song S, Zhang C, Pan L, Zhang T. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol. 2011;78:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 99. | Wu LM, Hu JN, Hua J, Liu MJ, Chen J, Xu JR. Diagnostic value of diffusion-weighted magnetic resonance imaging compared with fluorodeoxyglucose positron emission tomography/computed tomography for pancreatic malignancy: a meta-analysis using a hierarchical regression model. J Gastroenterol Hepatol. 2012;27:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Wang XY, Yang F, Jin C, Fu DL. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol. 2014;20:15580-15589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 101. | Wakabayashi H, Nishiyama Y, Otani T, Sano T, Yachida S, Okano K, Izuishi K, Suzuki Y. Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol. 2008;14:64-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, Rinta-Kiikka I, Alanen K, Borra RJ, Puolakkainen PA, Nuutila P, Ovaska JT. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 103. | Strobel K, Heinrich S, Bhure U, Soyka J, Veit-Haibach P, Pestalozzi BC, Clavien PA, Hany TF. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. 2008;49:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 104. | Wang C, Xu Y, Lu X. Should preoperative biliary drainage be routinely performed for obstructive jaundice with resectable tumor? Hepatobiliary Surg Nutr. 2013;2:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 105. | Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 281] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 106. | Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008;CD005444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X, Wang C. Meta-analysis of randomized clinical trials on safety and efficacy of biliary drainage before surgery for obstructive jaundice. Br J Surg. 2013;100:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 108. | Eshuis WJ, van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, Kuipers EJ, Coene PP, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 109. | Crippa S, Cirocchi R, Partelli S, Petrone MC, Muffatti F, Renzi C, Falconi M, Arcidiacono PG. Systematic review and meta-analysis of metal versus plastic stents for preoperative biliary drainage in resectable periampullary or pancreatic head tumors. Eur J Surg Oncol. 2016;42:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 110. | Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 111. | Jin T, Altaf K, Xiong JJ, Huang W, Javed MA, Mai G, Liu XB, Hu WM, Xia Q. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB (Oxford). 2012;14:711-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 112. | Nickel F, Haney CM, Kowalewski KF, Probst P, Limen EF, Kalkum E, Diener MK, Strobel O, Müller-Stich BP, Hackert T. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg. 2020;271:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 113. | Yoo D, Song KB, Lee JW, Hwang K, Hong S, Shin D, Hwang DW, Lee JH, Lee W, Kwon J, Park Y, Jun E, Kim SC. A Comparative Study of Laparoscopic versus Open Pancreaticoduodenectomy for Ampulla of Vater Carcinoma. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Chen K, Zhou Y, Jin W, Zhu Q, Lu C, Niu N, Wang Y, Mou Y, Chen Z. Laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic outcomes and long-term survival. Surg Endosc. 2020;34:1948-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Zhou W, Jin W, Wang D, Lu C, Xu X, Zhang R, Kuang T, Zhou Y, Wu W, Jin D, Mou Y, Lou W. Laparoscopic versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a propensity score matching analysis. Cancer Commun (Lond). 2019;39:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 116. | Chen XM, Sun DL, Zhang Y. Laparoscopic versus open pancreaticoduodenectomy combined with uncinated process approach: A comparative study evaluating perioperative outcomes (Retrospective cohort study). Int J Surg. 2018;51:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Dang C, Wang M, Zhu F, Qin T, Qin R. Comparison of laparoscopic and open pancreaticoduodenectomy for the treatment of nonpancreatic periampullary adenocarcinomas: a propensity score matching analysis. Am J Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Palanivelu C, Senthilnathan P, Sabnis SC, Babu NS, Srivatsan Gurumurthy S, Anand Vijai N, Nalankilli VP, Praveen Raj P, Parthasarathy R, Rajapandian S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017;104:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 119. | Nassour I, Wang SC, Porembka MR, Yopp AC, Choti MA, Augustine MM, Polanco PM, Mansour JC, Minter RM. Robotic Versus Laparoscopic Pancreaticoduodenectomy: a NSQIP Analysis. J Gastrointest Surg. 2017;21:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 120. | Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, White SA. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 121. | Wong JC, Raman S. Surgical resectability of pancreatic adenocarcinoma: CTA. Abdom Imaging. 2010;35:471-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA. 'Artery-first' approaches to pancreatoduodenectomy. Br J Surg. 2012;99:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 123. | Yu XZ, Li J, Fu DL, Di Y, Yang F, Hao SJ, Jin C. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: a meta-analysis. Eur J Surg Oncol. 2014;40:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 124. | Bell R, Ao BT, Ironside N, Bartlett A, Windsor JA, Pandanaboyana S. Meta-analysis and cost effective analysis of portal-superior mesenteric vein resection during pancreatoduodenectomy: Impact on margin status and survival. Surg Oncol. 2017;26:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, Weitz J. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2011;254:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 126. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 3199] [Article Influence: 355.4] [Reference Citation Analysis (35)] |
| 127. | Zhou Y, Yu J, Wu L, Li B. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy on occurrences of postoperative pancreatic fistula after pancreaticoduodenectomy. Asian J Surg. 2015;38:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 128. | Liu FB, Chen JM, Geng W, Xie SX, Zhao YJ, Yu LQ, Geng XP. Pancreaticogastrostomy is associated with significantly less pancreatic fistula than pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: a meta-analysis of seven randomized controlled trials. HPB (Oxford). 2015;17:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Que W, Fang H, Yan B, Li J, Guo W, Zhai W, Zhang S. Pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Am J Surg. 2015;209:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 130. | Kawaida H, Kono H, Hosomura N, Amemiya H, Itakura J, Fujii H, Ichikawa D. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol. 2019;25:3722-3737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (4)] |
| 131. | Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg. 2009;96:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 132. | Kawakatsu S, Inoue Y, Mise Y, Ishizawa T, Ito H, Takahashi Y, Saiura A. Comparison of pancreatojejunostomy techniques in patients with a soft pancreas: Kakita anastomosis and Blumgart anastomosis. BMC Surg. 2018;18:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 133. | Hirono S, Kawai M, Okada KI, Miyazawa M, Kitahata Y, Hayami S, Ueno M, Yamaue H. Modified Blumgart Mattress Suture Versus Conventional Interrupted Suture in Pancreaticojejunostomy During Pancreaticoduodenectomy: Randomized Controlled Trial. Ann Surg. 2019;269:243-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 134. | Chen Y, Ke N, Tan C, Zhang H, Wang X, Mai G, Liu X. Continuous versus interrupted suture techniques of pancreaticojejunostomy after pancreaticoduodenectomy. J Surg Res. 2015;193:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 135. | Ji W, Shao Z, Zheng K, Wang J, Song B, Ma H, Tang L, Shi L, Wang Y, Li X, Zhang Y, Jin G. Pancreaticojejunostomy with double-layer continuous suturing is associated with a lower risk of pancreatic fistula after pancreaticoduodenectomy: a comparative study. Int J Surg. 2015;13:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Peng SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, Li JT. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;245:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 137. | Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, Hyslop T, Schmidt CM, Rosato EL, Lavu H, Nakeeb A, Pitt HA, Lillemoe KD, Yeo CJ. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? J Am Coll Surg. 2009;208:738-47; discussion 747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 138. | Bai X, Zhang Q, Gao S, Lou J, Li G, Zhang Y, Ma T, Xu Y, Liang T. Duct-to-Mucosa vs Invagination for Pancreaticojejunostomy after Pancreaticoduodenectomy: A Prospective, Randomized Controlled Trial from a Single Surgeon. J Am Coll Surg. 2016;222:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 139. | Kojima T, Niguma T, Watanabe N, Sakata T, Mimura T. Modified Blumgart anastomosis with the "complete packing method" reduces the incidence of pancreatic fistula and complications after resection of the head of the pancreas. Am J Surg. 2018;216:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 140. | Bin X, Lian B, Jianping G, Bin T. Comparison of patient outcomes with and without stenting tube in pancreaticoduodenectomy. J Int Med Res. 2018;46:403-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 141. | Suzuki S, Kaji S, Koike N, Harada N, Tanaka S, Hayashi T, Suzuki M, Hanyu F. Pancreaticojejunostomy of duct to mucosa anastomosis can be performed more safely without than with a stenting tube. Am J Surg. 2009;198:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 142. | Sachs TE, Pratt WB, Kent TS, Callery MP, Vollmer CM Jr. The pancreaticojejunal anastomotic stent: friend or foe? Surgery. 2013;153:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Motoi F, Egawa S, Rikiyama T, Katayose Y, Unno M. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg. 2012;99:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 144. | Patel K, Teta A, Sukharamwala P, Thoens J, Szuchmacher M, DeVito P. External pancreatic duct stent reduces pancreatic fistula: a meta-analysis and systematic review. Int J Surg. 2014;12:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Hong S, Wang H, Yang S, Yang K. External stent versus no stent for pancreaticojejunostomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg. 2013;17:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 146. | Zhang GQ, Li XH, Ye XJ, Chen HB, Fu NT, Wu AT, Li Y. Internal Versus External Drainage With a Pancreatic Duct Stent For Pancreaticojejunostomy During Pancreaticoduodenectomy for Patients at High Risk for Pancreatic Fistula: A Comparative Study. J Surg Res. 2018;232:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 147. | Zhou W, Lv R, Wang X, Mou Y, Cai X, Herr I. Stapler vs suture closure of pancreatic remnant after distal pancreatectomy: a meta-analysis. Am J Surg. 2010;200:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 148. | Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, Tomazic A, Bruns CJ, Busch OR, Farkas S, Belyaev O, Neoptolemos JP, Halloran C, Keck T, Niedergethmann M, Gellert K, Witzigmann H, Kollmar O, Langer P, Steger U, Neudecker J, Berrevoet F, Ganzera S, Heiss MM, Luntz SP, Bruckner T, Kieser M, Büchler MW. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377:1514-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 412] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 149. | Jensen EH, Portschy PR, Chowaniec J, Teng M. Meta-analysis of bioabsorbable staple line reinforcement and risk of fistula following pancreatic resection. J Gastrointest Surg. 2013;17:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 150. | Klein F, Glanemann M, Faber W, Gül S, Neuhaus P, Bahra M. Pancreatoenteral anastomosis or direct closure of the pancreatic remnant after a distal pancreatectomy: a single-centre experience. HPB (Oxford). 2012;14:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Meniconi RL, Caronna R, Borreca D, Schiratti M, Chirletti P. Pancreato-jejunostomy versus hand-sewn closure of the pancreatic stump to prevent pancreatic fistula after distal pancreatectomy: a retrospective analysis. BMC Surg. 2013;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 152. | Martin I, Au K. Does fibrin glue sealant decrease the rate of anastomotic leak after a pancreaticoduodenectomy? HPB (Oxford). 2013;15:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 153. | Gong J, He S, Cheng Y, Cheng N, Gong J, Zeng Z. Fibrin sealants for the prevention of postoperative pancreatic fistula following pancreatic surgery. Cochrane Database Syst Rev. 2018;6:CD009621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 154. | Tian Y, Ma H, Peng Y, Li G, Yang H. Preventive effect of omental flap in pancreaticoduodenectomy against postoperative complications: a meta-analysis. Hepatogastroenterology. 2015;62:187-189. [PubMed] |
| 155. | Hassenpflug M, Hinz U, Strobel O, Volpert J, Knebel P, Diener MK, Doerr-Harim C, Werner J, Hackert T, Büchler MW. Teres Ligament Patch Reduces Relevant Morbidity After Distal Pancreatectomy (the DISCOVER Randomized Controlled Trial). Ann Surg. 2016;264:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 156. | Behrman SW, Zarzaur BL, Parmar A, Riall TS, Hall BL, Pitt HA. Routine drainage of the operative bed following elective distal pancreatectomy does not reduce the occurrence of complications. J Gastrointest Surg. 2015;19:72-9; discussion 79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 157. | Van Buren G 2nd, Bloomston M, Schmidt CR, Behrman SW, Zyromski NJ, Ball CG, Morgan KA, Hughes SJ, Karanicolas PJ, Allendorf JD, Vollmer CM Jr, Ly Q, Brown KM, Velanovich V, Winter JM, McElhany AL, Muscarella P 2nd, Schmidt CM, House MG, Dixon E, Dillhoff ME, Trevino JG, Hallet J, Coburn NSG, Nakeeb A, Behrns KE, Sasson AR, Ceppa EP, Abdel-Misih SRZ, Riall TS, Silberfein EJ, Ellison EC, Adams DB, Hsu C, Tran Cao HS, Mohammed S, Villafañe-Ferriol N, Barakat O, Massarweh NN, Chai C, Mendez-Reyes JE, Fang A, Jo E, Mo Q, Fisher WE. A Prospective Randomized Multicenter Trial of Distal Pancreatectomy With and Without Routine Intraperitoneal Drainage. Ann Surg. 2017;266:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 158. | Witzigmann H, Diener MK, Kienkötter S, Rossion I, Bruckner T, Bärbel Werner, Pridöhl O, Radulova-Mauersberger O, Lauer H, Knebel P, Ulrich A, Strobel O, Hackert T, Büchler MW. No Need for Routine Drainage After Pancreatic Head Resection: The Dual-Center, Randomized, Controlled PANDRA Trial (ISRCTN04937707). Ann Surg. 2016;264:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 159. | Van Buren G 2nd, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Vollmer C, Velanovich V, Riall T, Muscarella P, Trevino J, Nakeeb A, Schmidt CM, Behrns K, Ellison EC, Barakat O, Perry KA, Drebin J, House M, Abdel-Misih S, Silberfein EJ, Goldin S, Brown K, Mohammed S, Hodges SE, McElhany A, Issazadeh M, Jo E, Mo Q, Fisher WE. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 160. | Wang YC, Szatmary P, Zhu JQ, Xiong JJ, Huang W, Gomatos I, Nunes QM, Sutton R, Liu XB. Prophylactic intra-peritoneal drain placement following pancreaticoduodenectomy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:2510-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 161. | Nagakawa Y, Matsudo T, Hijikata Y, Kikuchi S, Bunso K, Suzuki Y, Kasuya K, Tsuchida A. Bacterial contamination in ascitic fluid is associated with the development of clinically relevant pancreatic fistula after pancreatoduodenectomy. Pancreas. 2013;42:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 162. | Kurumboor P, Palaniswami KN, Pramil K, George D, Ponnambathayil S, Varma D, Aikot S. Octreotide Does Not Prevent Pancreatic Fistula Following Pancreatoduodenectomy in Patients with Soft Pancreas and Non-dilated Duct: A Prospective Randomized Controlled Trial. J Gastrointest Surg. 2015;19:2038-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 163. | Fernández-Cruz L, Jiménez Chavarría E, Taurà P, Closa D, Boado MA, Ferrer J. Prospective randomized trial of the effect of octreotide on pancreatic juice output after pancreaticoduodenectomy in relation to histological diagnosis, duct size and leakage. HPB (Oxford). 2013;15:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 164. | Sarr MG; Pancreatic Surgery Group. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg. 2003;196:556-64; discussion 564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 165. | Allen PJ, Gönen M, Brennan MF, Bucknor AA, Robinson LM, Pappas MM, Carlucci KE, D'Angelica MI, DeMatteo RP, Kingham TP, Fong Y, Jarnagin WR. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;370:2014-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 166. | Young S, Sung ML, Lee JA, DiFronzo LA, O'Connor VV. Pasireotide is not effective in reducing the development of postoperative pancreatic fistula. HPB (Oxford). 2018;20:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 167. | Elliott IA, Dann AM, Ghukasyan R, Damato L, Girgis MD, King JC, Hines OJ, Reber HA, Donahue TR. Pasireotide does not prevent postoperative pancreatic fistula: a prospective study. HPB (Oxford). 2018;20:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 168. | Furuse J, Shibahara J, Sugiyama M. Development of chemotherapy and significance of conversion surgery after chemotherapy in unresectable pancreatic cancer. J Hepatobiliary Pancreat Sci. 2018;25:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 169. | Kunzmann V, Algül H, Goekkurt E, Siegler GM, Martens UM, Waldschmidt D, Network NCC. Pancreatic Adenocarcinoma (Version 1.2021) 2021. [cited 12 December 2020]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. |
| 170. | Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jäger D, Ulrich A, Büchler MW. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016;264:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 396] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 171. | Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, Hawkins WG, Williams G, Maduekwe U, Kim HJ, Ahmad SA, Patel SH, Abbott DE, Schwartz P, Weber SM, Scoggins CR, Martin RCG, Dudeja V, Franceschi D, Livingstone AS, Merchant NB. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg. 2019;270:400-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 172. | Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, Brawley CD, Calvert J, Chowdhury S, Cook A, Cross W, Dearnaley DP, Douis H, Gilbert D, Gillessen S, Jones RJ, Langley RE, MacNair A, Malik Z, Mason MD, Matheson D, Millman R, Parker CC, Ritchie AWS, Rush H, Russell JM, Brown J, Beesley S, Birtle A, Capaldi L, Gale J, Gibbs S, Lydon A, Nikapota A, Omlin A, O'Sullivan JM, Parikh O, Protheroe A, Rudman S, Srihari NN, Simms M, Tanguay JS, Tolan S, Wagstaff J, Wallace J, Wylie J, Zarkar A, Sydes MR, Parmar MKB, James ND; STAMPEDE investigators. Corrigendum to Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial: Ann Oncol 2019; 30: 1992-2003. Ann Oncol. 2020;31:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 173. | Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, Wo JY, Ryan DP, Allen JN, Blaszkowsky LS, Clark JW, Murphy JE, Nipp RD, Parikh A, Qadan M, Warshaw AL, Hong TS, Lillemoe KD, Ferrone CR. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann Surg. 2019;269:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 174. | Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, Segersvärd R, Arnelo U, Del Chiaro M. Surgery Improves Survival After Neoadjuvant Therapy for Borderline and Locally Advanced Pancreatic Cancer: A Single Institution Experience. Ann Surg. 2021;273:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 175. | Yoo C, Shin SH, Kim KP, Jeong JH, Chang HM, Kang JH, Lee SS, Park DH, Song TJ, Seo DW, Lee SK, Kim MH, Park JH, Hwang DW, Song KB, Lee JH, Ryoo BY, Kim SC. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 176. | Satoi S, Yamamoto T, Yamaki S, Sakaguchi T, Sekimoto M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020;4:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 177. | Tajima H, Ohta T, Kitagawa H, Okamoto K, Sakai S, Makino I, Kinoshita J, Furukawa H, Nakamura K, Hayashi H, Oyama K, Inokuchi M, Nakagawara H, Fujita H, Takamura H, Ninomiya I, Fushida S, Tani T, Fujimura T, Ikeda H, Kitamura S. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. 2012;3:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 178. | O’Reilly EM, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M, Tang LH, LaValle J, Winston C, DeMatteo RP, DʼAngelica M, Kurtz RC, Abou-Alfa GK, Klimstra DS, Lowery MA, Brennan MF, Coit DG, Reidy DL, Kingham TP, Allen PJ. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 179. | Motoi F, Ishida K, Fujishima F, Ottomo S, Oikawa M, Okada T, Shimamura H, Takemura S, Ono F, Akada M, Nakagawa K, Katayose Y, Egawa S, Unno M. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreatic ductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol. 2013;20:3794-3801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 180. | Scott E, Mamawala M, Epstein JI, Landis P, Wolf S, Trock, Carter HB. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. Tosoian JJ, Mamawala M, Epstein JI, Landis P, Wolf S, Trock BJ, Carter HB.J Clin Oncol. 2015 Oct 20;33(30):3379-85. [Epub 2015 Aug 31]. doi: 10.1200/JCO.2015.62.5764. Urol Oncol. 2017;35:121-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 181. | Labori KJ, Lassen K, Hoem D, Grønbech JE, Søreide JA, Mortensen K, Smaaland R, Sorbye H, Verbeke C, Dueland S. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1)) - study protocol for a national multicentre randomized controlled trial. BMC Surg. 2017;17:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 182. | Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR, Raoul JL, Bachellier P, Dufour P, Moehler M, Weber A, Lang H, Rogiers X, Clavien PA. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer. 2011;11:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 183. | Sohal D, McDonough SL, Ahmad SA, Gandhi N, Hochster HS. SWOG S1505: A randomized phase II study of perioperative mFOLFIRINOX vs. gemcitabine/nab-paclitaxel as therapy for resectable pancreatic adenocarcinom. J Clin Oncol. 2017;35:TPS4152-TPS4152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 184. | Turrini O, Viret F, Moureau-Zabotto L, Guiramand J, Moutardier V, Lelong B, de Chaisemartin C, Giovannini M, Delpero JR. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology. 2009;76:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 185. | Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, Jungnickel H, Schreiber S, Grabenbauer GG, Meyer T, Merkel S, Fietkau R, Hohenberger W. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 186. | Okano K, Suto H, Oshima M, Maeda E, Yamamoto N, Kakinoki K, Kamada H, Masaki T, Takahashi S, Shibata T, Suzuki Y. A Prospective Phase II Trial of Neoadjuvant S-1 with Concurrent Hypofractionated Radiotherapy in Patients with Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2017;24:2777-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 187. | Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640-3650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 188. | Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, van Cutsem E, van Dekken H, Klinkenbijl JH, Jeekel J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 189. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 759] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 190. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1791] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 191. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1025] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 192. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1459] [Article Influence: 162.1] [Reference Citation Analysis (0)] |
| 193. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 2098] [Article Influence: 262.3] [Reference Citation Analysis (0)] |
| 194. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 195. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 196. | Sinn M, Liersch T, Riess H, Gellert K, Stübs P, Waldschmidt D, Lammert F, Maschmeyer G, Bechstein W, Bitzer M, Denzlinger C, Hofheinz R, Lindig U, Ghadimi M, Hinke A, Striefler JK, Pelzer U, Bischoff S, Bahra M, Oettle H. CONKO-006: A randomised double-blinded phase IIb-study of additive therapy with gemcitabine + sorafenib/placebo in patients with R1 resection of pancreatic cancer - Final results. Eur J Cancer. 2020;138:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 197. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5891] [Article Influence: 392.7] [Reference Citation Analysis (23)] |
| 198. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 5135] [Article Influence: 395.0] [Reference Citation Analysis (12)] |
| 199. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 880] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 200. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 201. | Roth MT, Cardin DB, Berlin JD. Recent advances in the treatment of pancreatic cancer. F1000Res. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 202. | Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 203. | Upadhrasta S, Zheng L. Strategies in Developing Immunotherapy for Pancreatic Cancer: Recognizing and Correcting Multiple Immune "Defects" in the Tumor Microenvironment. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 204. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2199] [Article Influence: 366.5] [Reference Citation Analysis (1)] |
| 205. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 2067] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 206. | Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, Doebele R, Giannetta L, Cerea G, Marrapese G, Schirru M, Amatu A, Bencardino K, Palmeri L, Sartore-Bianchi A, Vanzulli A, Cresta S, Damian S, Duca M, Ardini E, Li G, Christiansen J, Kowalski K, Johnson AD, Patel R, Luo D, Chow-Maneval E, Hornby Z, Multani PS, Shaw AT, De Braud FG. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 633] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 207. | Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 208. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1762] [Article Influence: 251.7] [Reference Citation Analysis (0)] |
| 209. | Rouanet M, Lebrin M, Gross F, Bournet B, Cordelier P, Buscail L. Gene Therapy for Pancreatic Cancer: Specificity, Issues and Hopes. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (4)] |
| 210. | Chen S, Xu M, Zhao J, Shen J, Li J, Liu Y, Cao G, Ma J, He W, Chen X, Shan T. MicroRNA-4516 suppresses pancreatic cancer development via negatively regulating orthodenticle homeobox 1. Int J Biol Sci. 2020;16:2159-2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 211. | Roth S, Springfeld C, Diener MK, Tjaden C, Knebel P, Klaiber U, Michalski CW, Mieth M, Jäger D, Büchler MW, Hackert T. Protocol of a prospective, monocentric phase I/II feasibility study investigating the safety of multimodality treatment with a combination of intraoperative chemotherapy and surgical resection in locally confined or borderline resectable pancreatic cancer: the combiCaRe study. BMJ Open. 2019;9:e028696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin Q, Petrusel L, Qin R S-Editor: Fan JR L-Editor: A P-Editor: Wang LL