Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1580
Peer-review started: December 13, 2019
First decision: January 16, 2020
Revised: January 22, 2020
Accepted: March 14, 2020
Article in press: March 14, 2020
Published online: April 14, 2020
Processing time: 123 Days and 2.7 Hours
The role of cancer stem cells in gastrointestinal cancer-associated death has been widely recognized. Gastrointestinal cancer stem cells (GCSCs) are considered to be responsible for tumor initiation, growth, resistance to cytotoxic therapies, recurrence and metastasis due to their unique properties. These properties make the current therapeutic trials against GCSCs ineffective. Moreover, recent studies have shown that targeting stem cell surface markers or stemness associated pathways might have an additional off-target effect on the immune system. Recent advances in oncology and precision medicine have opened alternative therapeutic strategies in the form of cancer immunotherapy. This approach differs from classical anti-cancer therapy through its mechanism of action involving the activation and use of a functional immune system against tumor cells, instead of aiming physically destruction of cancer cells through radio- or chemotherapy. New immunological approaches for GCSCs targeting involve the use of different immune cells and various immune mechanisms like targeting specific surface antigens, using innate immune cells like the natural killer and T cells, T-cell chimeric antigen receptor technology, dendritic cell vaccine, or immune checkpoint inhibitors. In this respect, better understandings of immune regulatory mechanisms that govern anti-tumor response bring new hope in obtaining long-term remission for cancer therapy.
Core tip: Cancer immunotherapy has emerged in recent years as an alternative to classical anti-tumor therapy. It involves the activation of the host immune system in the fight against tumor cells, including cancer stem cells, which are responsible for tumor maintenance, relapse, and metastasis. Here we discuss the different forms of immunotherapy for gastrointestinal cancer stem cells targeting such as using monoclonal antibodies against surface antigens, generation of effector natural killer cells and T cells genetic engineered to target tumor antigens, dendritic vaccines, and immune checkpoints inhibitors.
- Citation: Chivu-Economescu M, Necula LG, Matei L, Dragu DL, Neagu AI, Alexiu I, Bleotu C, Diaconu CC. Gastrointestinal cancer stem cells as targets for innovative immunotherapy. World J Gastroenterol 2020; 26(14): 1580-1593
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1580
Gastrointestinal cancers include several malignancies of the gastrointestinal tract and accessory organs such as stomach, liver and intrahepatic bile duct, gallbladder and pancreas. All of them have epithelial cell origin, and combined accounts for 4974672 estimated new cases, representing 28% of all cancer incidence in 2018. According to GLOBOCAN 2018, all together gastrointestinal cancer are responsible for over 3.5 million deaths which correspond to 37% deaths of total human cancers[1]. Thus the need to understand the molecular background of gastrointestinal cancer, as well as mechanisms involved in occurrences and tumor maintenance, are of tremendous importance.
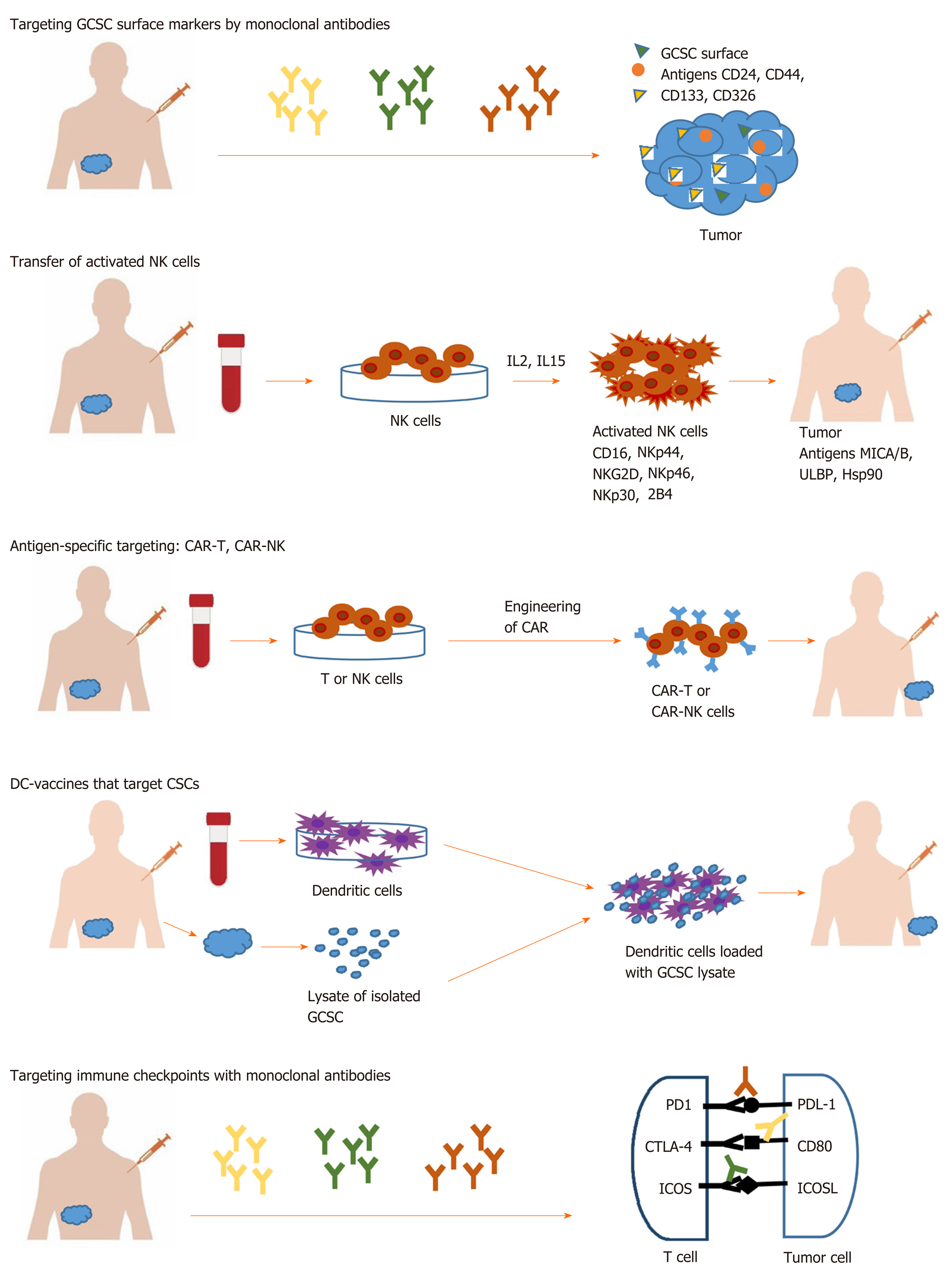
In the last years, the hypothesis that cancer appears from a population of stem cells has gained widespread support. Cancer stem cells (CSCs) are a distinct subpopulation within the tumor with unique properties. There are two theories about how tumors appear. Stochastic theory, involving the occurrence of unpredictable genetic and epigenetic changes during tumor growth[2,3] and hierarchical theory, which supports the idea of a subpopulation of cells, that have an intrinsic ability to initiate self-regeneration and tumor growth[4-6]. CSCs have been discovered in a wide field of tumors, including gastrointestinal cancer. These cells are at the origin of tumorigenesis and also, are responsible for tumor maintenance due to resistance towards standard oncology treatments, relapse, and metastasis[7]. More and more evidence is constantly accumulating that mechanisms of resistance of gastrointestinal cancer stem cells (GCSC) to conventional therapy are epithelial mesenchymal transition (EMT), drug efflux proteins, and upregulation of aldehyde dehydrogenase (ALDH) activity. Therefore, CSC raised an important challenge regarding the efficacy of current cancer treatment due to these special properties. In this way, more effective therapies that target the GCSCs subpopulation are needed, instead of addressing the entire tumor population. New immunological approaches involve the use of various immune mechanisms like targeting specific surface antigens or immune checkpoints on GCSC surface or using innate immune cells like the natural killer (NK) and T cells, T cell chimeric antigen receptor technology and dendritic cell vaccine (Figure 1).
To target GCSCs markers and track the effect of anti-tumor therapies, it is necessary to identify them. Commonly, the surface antigens such as CD24, CD44, EpCAM, CD133, alone or in combinations, are among the most used markers for the identification of GCSC[8]. Combination of CD44, CD90, CD133 and ALDH1 was commonly used for esophageal tumor type[9,10], CD44 and ALDH1 for gastric tumor type[8,11], CD24, CD44, CD133, EpCAM and ALDH for pancreatic tumor type[12-14], CD44, CD90, CD133 and EpCAM for liver tumor type[15-18], CD24, CD44, CD49f, CD133, EpCAM and ALDH1 for colorectal tumor type[15,19,20] (Table 1). Several other molecules, such as CD90, Musashi-1, LINGO2, oval cell marker OV6[8,21] have been reported as potential surface markers for GCSCs. Identification of specific antigens on the CSC surface may provide more targets for immunotherapy (Table 1).
These markers are relatively conserved across to the broad spectrum of solid cancers and also are common with normal stem cells[22]. Monoclonal antibodies, chimeric, humanized or fully human antibodies that are able to target specific markers were developed for the treatment of major malignant diseases, including gastrointestinal cancers (Table 2).
| Targeting approach | Cancer model | Effects | Ref. |
| RG7576 mAb against CD44 | Solid tumors | Inhibited tumor growth and induced activation of macrophages | [23-26] |
| SWA11 against CD24 | Colorectal cancer | Reduced tumor cell proliferation and angiogenesis | [27,28] |
| G7 mAbs against CD24 | Liver cancer | Suppressed tumor growth | [29-31] |
| Catumaxomab (Removab®) mAb targeting EpCAM (CD326) | Gastric, colon cancers, pancreas | Activated immune cells (NK cells, macrophages, and T cells); prolonged survival period | [32-35] |
CD44 is a transmembrane receptor commonly expressed on solid tumor CSC surface. Targeting CD44 with RG7356, a recombinant anti-CD44 monoclonal specific antibody, blocks the binding of CD44 to hyaluronic acid (HA) and inhibits cell adhesion, leading to tumor growth inhibition, and activates macrophages in preclinical models[23-25]. However, only a modest clinical efficacy was observed in patients with metastatic or locally advanced CD44-expressing solid malignancies (including breast cancer, melanoma, renal and lung cancer) treated with RG7356 (phase I clinical trial)[26].
CD24 is a highly glycosylated protein localized in the membrane of many type of CSCs. Some CD24 monoclonal antibodies (mAb), such as SWA11and G7 mAbs, were developed for therapeutic purpose, demonstrating a good efficiency in human cancer xenograft models. A potent anti-tumor activity of SWA11 mAb[27] has been demonstrated in human colorectal cancer models, reducing tumor cell proliferation and angiogenesis[28]. Other anti-CD24 monoclonal antibody, G7 mAb was used in liver cancer xenograft models[29,30] demonstrating specificity for tumor tissue and efficacy in suppressing tumor growth, as single-agent treatment or in combination with doxorubicin[31], or cetuximab, a chimerical monoclonal anti-EGFR antibody[31].
CD326 (EpCAM) is a transmembrane glycoprotein mediating intercellular cell-adhesion in epithelial tissues being involved in cell signaling, proliferation, differentiation, invasion, metastasis, and chemo-/radioresistance. EpCAM is a target for immunotherapeutic strategies in epithelial-derived neoplasms of colon, stomach, pancreas etc.[32,33]. The antibody Catumaxomab (Removab®) targeting EpCAM was used in clinical trials on patients with ovarian, gastric, colon, breast cancers and malignant ascites (NCT00836654) delaying deterioration in quality of life for a prolonged survival period[34]. Moreover, one phase III clinical trial (NCT00822809) demonstrated that intraperitoneal infusion of catumaxomab activates immune cells such as NK cells, macrophages and T cells in ascites, and favors CD8+T cell accumulation into the peritoneal cavity showing a clinical benefits in treatment of malignant ascites associated with EpCAM positive carcinomas[33,35].
However, precise targeting with monoclonal antibodies seems difficult, since most GCSCs are identified based on a combination of surface markers, often unspecific[36]. Since none of these markers is unique for GCSCs, additionally features of stem cells are such as ALDH activity, side population phenotype, the expression of pluripotency genes (OCT4, SOX2, and NANOG), and aberrant expression of ABC transporters[37] are analyzed in order to permit a more reliable identification and targeting of the cancer cells with stem properties[38,39].
Another widely used strategy against GCSC involved targeting of key signaling pathways like Notch, Hedgehog, Wnt, and IL6. However, accumulating evidence pointed out that the inhibitors used to target self-renewal pathways might have off-target effects on immune cells, impairing T cell proliferation, function, and cytokine production[22,40-43].
At this point, scientists consider that cancer immunotherapy represents a new approach, different from the conventional one that uses chemo and radiotherapy.
Cancer immunotherapy differs from classical anti-cancer therapy through its mechanism of action involving the activation and use of a functional immune system against tumor cells, instead of aiming physically destruction of cancer cells through radio- or chemotherapy[44]. The immune system is an active part of the tumor microenvironment (TME). Here GCSCs co-exist with other cellular components like stromal cells, endothelial cells, immune cells such as dendritic cells (DC), NK cells, T cells, tumor-associated macrophages, regulatory T cells, tumor-infiltrating lymphocytes, and myeloid derived suppressor cells. There are several mechanisms used by GCSC in their interaction with TME to escape from tumor killer cells like NK and T cells. One of these refers to having a low expression of MHC class I surface molecules. Another one is represented by the crosstalk between GCSC and the other components of TME, these interactions being mediated by cytokines and chemokines that eventually suppress antitumor immunity. Moreover, recently, co-inhibitory molecules and immune checkpoint ligands such as programmed death-ligand (PD-L) 1 and PD-L2 were identified to be overexpressed on GCSC surface. Due to PD1/PD-L1 (L2) axis, GCSC can easily escape from immune cell action. Understanding the TME and the dynamic cross talk between GCSCs and the TME is equally important to initiate an efficient anti-tumor therapy without impairing the anti-tumor immune response.
Immunological approaches for GCSC targeting involve different immune cells and various immune mechanisms like using innate immune cells such as NK and T cells, using antigen-specific targeting by T cell chimeric antigen receptor (CAR) technology, DC vaccine, or immune checkpoints therapy[45,46]. The targeting strategies against GCSC are listed in Table 3.
| Targeting approach | Cancer model | Ref. |
| CIK cells via NKG2D ligands expressed on CSC | Hepatocellular carcinoma | [56] |
| NK cells via NKG2D ligands expressed on CSC | Pancreatic cancer | [57] |
| CAR-T for CSC antigen ASB4 | Colon cancer | [59] |
| CAR-T for EGFR and CAR-T for CSC antigen CD133 | Cholangiocarcinoma | [60] |
| CAR-T for CSC antigen CD24 | Pancreatic adenocarcinoma | [61] |
| DC loaded with Panc-1 CSC lysate | Pancreatic cancer | [62] |
| DC loaded with total mRNA from gastric CSC | Gastric cancer | [63] |
NK cells, the third largest population of immune cells after B and T lymphocytes, serve the innate immunity, usually defending the human organism against infections. NK are good candidates for immunotherapy since they trigger special attacks on cancer cells that express ligands that couples activating receptors on NK cells. This action is mediated through a group of activating receptors containing CD16, NKG2D, NKp30, NKp44, NKp46, 2B4 and DNAM-1 with PVR and NECTIN-2[47-50]. The major activating ligands for NK cells are MICA/B, ULBP and Hsp90 usualy overexpressed on tumor cells[51]. For tumor eradication is necessary total destruction of CSCs. Different studies showed that there are CSCs that express ligands that can be recognized by NK cells and, consequently can be killed[52-54], and certain CSCs which do not show detectable ligands for NK and escape cytotoxicity[55]. An in vitro study conducted by Rong et al[56] showed that cytokine-induced killer cells, which are NK lymphocytes characterized by the co-expression of CD3 and CD56 surface antigens, killed CSCs in hepatocellular carcinoma via interaction of their membrane receptor NKG2D with stress-inducible molecules, MIC A/B and ULBPs, on target cells. In vivo, cytokine-induced killer infusion significantly delayed tumor growth. Similarly, Ames et al[57] demonstrated that activated NK cells are capable of preferentially killing tumor cells with a CSC phenotype identified by multiple markers (CD24+/CD44+, CD133+, and aldehyde dehydrogenasebright) from a wide variety of human cancer cell lines, including pancreatic cell lines like PANC-1 and BXPC3. The mechanism of action implicated an NKG2D-dependent NK activation via MICA/B, Fas, and DR5 ligands expressed on GCSCs. Also, Yin et al[58] showed that cells with stem cell phenotype can be more easily killed by NK cells activated by IL2 and IL15. Taken together, these preliminary studies provide evidence that activated NK cells can have translational potential as NK immunotherapy against GCSC phenotype, and other CSC from solid malignancies.
The next step in cancer therapy resided in the generation of effector T cells and NK cells genetic engineered to produce an artificial T cell receptor, named CAR that gives T cells both the ability to target a specific protein/tumor antigen and to be consequently activated. CAR-T cells against GCSC antigens have been developed and evaluated in several gastrointestinal cancer models (Table 3).
Miyamoto et al[59] used CAR-T-based immunotherapies against colorectal CSCs based on the ASB4 gene that was identified as being expressed on colorectal CSC, but not on non-CSC cells or normal cells/tissue. ASB4 as tumor-associated antigen was used to generate specific cytotoxic T lymphocytes (CTL) in vitro, that were able to infiltrate implanted colorectal tumors in a mouse model, preventing tumor growth. Another clinical trial was developed by Feng et al[60] that used CAR-T cells targeting epidermal growth factor receptor (EGFR) and GCSC surface antigen CD133, respectively. The patient received successive infusions of CAR-T cells for the treatment of unresectable/metastatic cholangiocarcinoma. The results showed a partial response of 8.5 mo from CAR-T for EGFR and 4.5-mo-lasting from the CAR-T for CD133 treatment, however, both therapies induced acute toxicities. Maliar et al[61] developed CAR-T for CSC antigen CD24 and evaluated it in mice with pancreatic adenocarcinoma xenografts. The results showed that more than 50% of the animals remained tumor-free after 16 wk.
As concluding remarks, treatment of solid tumors using CAR-T cells induced less favorable results then hematological malignancies, mainly due to short efficacy and off-target toxicity. Davenport et al[62] reported in 2018 that responses triggered by CAR-T cytotoxic cells are fast but short, being followed by a rapid loss after 20 h in cytotoxic activity against tumor. The main concern remains on the severe toxicity associated with CAR-T therapy like cytokine release syndrome, which can trigger organ damage and death, neurologic toxicity, insertional oncogenesis, graft versus-host disease, off-target antigen recognition[63]. Two trails using CAR-T cells engineered against ERBB2 or CEACAM5 used for the treatment of gastrointestinal cancers reported poor efficacy and caused acute pulmonary toxicity due to antigen expression on lung epithelium. This resulted in the death of one patient within 5 d post-transfer of the cellular product due to multiple organ failure[64,65].
In order to avoid CAR-T therapy accompanied toxicity is essential to choose accurate target antigens and improve tumor discrimination[66,67]. Also, there are some studies on introducing a suicidal gene that can induce apoptosis of T cells to prevent over activation and critical off-tumor cytotoxicity. Such genes are the thymidine kinase gene of herpes simplex virus and inducible caspase 9. If the latter seems successful, the first strategy, related to the thymidine kinase gene of herpes simplex virus seems to raise immunogenicity problems and it will not be used in the clinic[67-69].
Recently, the knowledge of producing CAR-T has been transferred to CAR-NK cells. CAR-NK use seems to be safer in the clinic, as NK cells do not initiate similar toxicity[70]. Between the advantages: CAR-NK cells are able to retain the expression of their activating receptors, showing longer efficacy, and appear safer in terms of cytokine release syndrome and neurotoxicity due to a different pattern of cytokines/chemokines released after activation[71,72]. However, there are also major drawbacks like the poor ability of NK to reach tumor tissue due to TME and some changes that may intervene in the expression of activating receptors/ligands. For example, the level of NKG2D ligand is increased in the early stages of colorectal cancer, but it decreases during tumor progression[73].
There are not ongoing clinical trials for GCSC or other solid CSC. The only clinical trial for solid cancers is a phase I study that use anti-GD2 CAR-T for sarcoma and neuroblastoma patients (NCT02107963), without published results. There are however 30 clinical trials that are recruiting patients with solid cancers including liver, stomach and colorectal for CAR-T therapies using PD-1, CTLA4, EGFR or NKG2D-ligand. Also, there are three trials for solid cancers that are recruiting for CAR-NK therapies using antigens like MUC3, ROBO1, and NKG2D-ligand. The creation of NKG2D/NKG2DL-based multi-functional fusion proteins is becoming one of the most promising strategies in immunotherapy for cancer. Activated CAR-NKG2D receptor on the T or NK cell surface can bind to its respective NKG2D ligand expressed in tumor cells, enabling immune cells to kill tumor cells.
Dendritic cells are crucial players in immune responses, and their ability to control the activation, proliferation and differentiation of specific T cell subsets make them strategic tools for cancer vaccines that target CSCs. A great advantage of DC-vaccines might be the potential capacity to induce immunological memory, eliminating existing CSCs and, at the same time, offering long-term protection against new arising CSCs[74].
Most clinical trials using DC-vaccines are based on DC loaded with lysates of isolated CSCs. Yin et al[75] used such DC loaded with pancreatic Panc-1 CSC lysate and observed that modified DC induced proliferation of T cell lymphocytes during co-culture. This approach has at least a few disadvantages like the lack of reliable surface makers that may be used for CSCs isolation, unknowing which neoantigens in the lysate elicit an immune response, and the variability of the number of immunogenic neoantigens on different types of tumors[76,77].
ALDH, a marker frequently used for CSC identification was used in obtaining DC vaccines that significantly inhibited tumor growth, reduced development of pulmonary metastases and prolonged survival. Direct targeting of CSCs was confirmed by the specific binding of IgG produced by ALDHhigh CSC-DC vaccine primed B cells to ALDHhigh CSCs, lysing these target CSCs in the presence of complement[78]. This promising approach was reported so far for squamous cell cancer and metastatic melanoma, however, ALDH is a highly expressed marker on various GCSC (Table 1), so we can hypothesize similar positive results in gastrointestinal cancers.
In spite of the promising results, DC-based vaccination strategies need to be improved[78]. A more efficient strategy to eradicate CSCs might be to load autologous DCs with peptides, proteins or even mRNA rather than tumor lysate, this way controlling more accurately the generated immune response. In this regard, Bagheri V et al[79] loaded DC with total mRNA from gastric CSC expressing CD44, CD54, and EpCAM markers. These DC were able to induce IFN-γ gene expression in T-lymphocytes after a 12-d co-culture.
Recent studies proposed loading DCs with transcription factors as NANOG, OKT4a, SOX2, c-MYC, and KLF4, which also transform somatic cells into stem cells (iPS). Targeting CSCs unique proteins might be one of the best ways to destroy CSCs. Since the expression of NANOG is low or absent in normal cells and CSCs re-express it, makes NANOG an ideal therapeutic target. DCs loaded with NANOG peptides will be able to generate immunological memory after vaccination and would help the immune system to manage CSC plasticity[22].
In order to maximize the response rates to vaccines, if NANOG peptides cannot be presented by a patient HLA class I molecules, peptides against other stem cell transcription factor OKT4 or SOX2 may be used[76]. Combining DC vaccination against CSCs with other therapies, as immune checkpoint inhibitors, for example, might overcome immunosuppressive mechanisms in cancer and avoid bone marrow damaging by the chemotherapeutics.
It is considered that CSCs escape immune surveillance due to their immune suppressive profiles based on the expression of co-inhibitory molecules, immune checkpoints ligands and cytokines, drug-resistance and ability to activate an EMT programme[80]. Based on these properties, CSC not only escapes immune surveillance but also directly inhibits T and NK cells anti-tumor activity via modulating immune checkpoints.
Several immune checkpoints have been stated during last years with either co-stimulatory activity on immune cells such as CD28/CD80 (CD86), ICOS (CD278)/ICOSL, CD27/CD70, GITR/GITRL, or co-inhibitory like PD-1/PDL-1 (PD-L2), BTLA/HVEM, CTLA4/CD80 (CD86), B7H3, B7H4, B7H5/HVEM, LAG3/MHC II, TIM3/GAL9, TIGIT/Nectin-2, or IDO. Many of them are highly expressed on various CSCs, but the type of molecule seems to vary with tumor type and localization.
From these, PD-L1 (also known as CD274 or B7H1) and B7H3 have been identified as promoters of CSC-like phenotype, EMT, tumor cell proliferation, metastasis and resistance to therapy[81-83].
PD-L1 is one of the most studied immune checkpoints. The interaction between PD-L1/PD-L2 and PD-1 aids CSCs in escaping from the killing through inhibiting tumor-reactive T cells by binding to its PD-1 receptor. Moreover, PD-L1 is also expressed by tumor-associated myeloid-derived suppressor cells, contributing to T cells blocking and immune deficiency in TME[84]. Hsu et al[85] established that PD-L1 high expression in CSCs is due to EMT and to EMT/β-catenin/STT3/PD-L1 signaling axis. Moreover, PD-L1 expression could be enhanced via PI3K/AKT and RAS/MAPK pathways. All these major pathways could be activated by OCT4 and SOX2, key regulatory genes involved in CSC self-renewal and function[86]. The final effect of PD-L1 overexpression on CSC will be an increase in cancer invasion and proliferation via EMT.
This hypothesis was sustained by several experiments on GCSC. Yang et al[87] detected PD-L1 overexpression on gastric CSCs, defined as Lgr5+/CD326+/CD45−, were enhanced in vitro tumor-promoting capacity of GCSCs by colony-forming assay, and induces their proliferation. In reverse, knockdown of PD-L1 expression in gastric cancer cells significantly suppressed proliferation and invasion in vitro[88], and tumor growth in nude mice[89].
An increased level of PD-L1 was observed in esophageal and colorectal CD133+ GCSCs with EMT phenotype. The authors showed by manipulating PD-L1 expression, that higher PD-L1 expression promoted cell proliferation, migration and EMT phenotype. The EMT mechanism could help GCSC escape immune attack during metastasis[90].
The assessment of PD-L1 level on biopsies could bring useful information for establishing therapies regimen. The dynamic change of PD-L1 expression may indicate the response to therapy and have predictive significance on progression free survival. This could be monitored with the help of circulating tumor cells, which may act as substitute for tissue biopsies, and have great utility in real-time cancer management[91].
The expression of these molecules with an immunosuppressive effect on the GCSC surface may be a major problem as cytotoxic T lymphocytes therapies become less effective. However, is an indicator that GCSC resistant to classical anti-tumor therapy could be targets for immune checkpoints inhibitors.
Targeting immune checkpoints with monoclonal antibodies has become a custom treatment since early studies have shown their ability to improve tumor infiltration of CD8+ and CD4+ T cells and inhibit tumorigenesis.
Antibodies blocking PD-L1, PD-1 or CTLA-4 were developed and tested in clinical trials for their cancer therapeutic potential. 2014 was the year of nivolumab and pembrolizumab approval, both being monoclonal antibodies targeting PD-1. They were authorized for clinical use with benefits in various types of cancer such as refractory malignant melanoma[92], Hodgkin lymphoma[93], NSCLC[94], head and neck squamous cell carcinoma[95], urothelial cancer[96], gastric adenocarcinoma[97], colorectal cancer[98] and advanced hepatocellular carcinoma[99] (Table 4). Moreover, pem-brolizumab has received general approval for the treatment of all solid tumors with high microsatellite instability and deficiency in the mismatch repair genes. Next, a combination of ipilimumab (anti-CTLA-4) with nivolumab (anti-PD-1) inhibitors was shown to significantly enhance antitumor efficacy and the response rates in patients with colorectal cancer expressing high microsatellite instability phenotype.
| Target | Drug | Commercial name | Indication |
| PD-1 | Nivolumab | Opdivo | Hepatocellular carcinoma, colorectal cancer with MSI-H |
| PD-1 | Pembrolizumab | Keytruda | Gastric cancer, hepatocellular carcinoma, colorectal cancer, solid tumors with MSI-H |
| CTLA-4 and PD-1 | Ipilimumab plus nivolumab | Yervoy plus Opdivo | Colorectal cancer with MSI-H |
In addition to targeting PD-1 and CTLA-4 receptors, PD-L1 has been also confirmed to be useful for immunotherapy. It was demonstrated that increased expression of PD-L1, decreased T cell infiltration and activation, protecting tumor and GCSCs against immune response. In 2016 atezolizumab, an anti-PD-L1 monoclonal antibody received approval for the treatment of several solid cancers, but not yet for gastrointestinal cancers.
The clinical benefits of immunotherapy cannot be questioned. The development of new inhibitors for immune checkpoints or their ligands continues, addressing newly identified regulators Lag-3, Tim-3, TIGIT, V-domain Ig suppressor of T cell activation, etc. And this is only the beginning, as many clinical trials are under way to assess the effectiveness of combining these inhibitors either with or without classical chemotherapy in treating gastrointestinal cancers[100].
To target GCSC and completely eradicate them, it might be necessary to combine these immunotherapy approaches.
Several clinical trials are now proposing interesting strategies for combining immune checkpoints therapy with DC-vaccines or CAR-T technology. Most of these clinical trials are either in the phase of patient recruitment or in phase I. There are several trials that are testing the combination between anti PD-1 compound (nivolumab) and gene-modified T-cells and dendritic cell vaccine targeting cancer-testis antigen (CTA). CTAs such as New York esophageal squamous cell carcinoma 1 and melanoma-associated antigen A (MAGEA) are considered excellent candidates for cancer immunotherapy since a large majority of them have their espression limited to the embrionic stem cells, testes, ovaries and endometrium in normal tissue, and are re-expressed in metastatic tumours[101]. MAGEA1-3, MAGEA9, LAGE1, and New York esophageal squamous cell carcinoma 1 were found to be highly expressed in hepatocellular carcinoma, oesophageal, gastric and colorectal carcinoma stem/ progenitor cells and associated with poor survival, high risk of tumor recurrence[102,103]. However, there is a small number of CTAs that are expressed on normal tissue as well. Although targeting CTA seems to be a promising strategy, carefull selection of CTA type for immunotherapy is manadatory. Serious neuronal adverse events followed by death were observed during clinical trials using anti-MAGE3 CAR-T cells in patients with solid cancers. Histopathological examination showed that normal neuronal cells also expressed MAGEA proteins that became targets of modified T cell therapy, thus being destroyed[104].
Another approach is targeting of inhibitory immune checkpoints like PD-1 and TIGIT to unblock the NK and T cells activity. Zhang et al[105] found that blockade of TIGIT promoted NK cell mediated immunity in a mouse model with colon cancer, and the response was enhanced by additional anti-PD-L1 immunotherapy. Moreover, it seems that the animal model developed a persistent memory immunity that was functional after tumor cell reinfusion.
Combined immune therapies may be very effective having the advantage of addressing both GCSC and TME simultaneously. Thus, they can target, for example, GCSC surface markers with monoclonal antibodies, DC-vaccines or CAR-T therapy, and at the same time, they can reactivate the immune system by blocking the negative signals induced by immune checkpoints in effector immune cells. However, there are some limitations since most of the known solid tumor-associated antigens are expressed also in normal tissues, resulting in damaging off-target toxicity. Therefore, there is a continue effort to identify tumor-specific antigens that can be addressed using immune therapies. Another limiting factor that can influence the clinical response is the level of inflammatory infiltration and the expression of immune checkpoints. Unlike liquid cancers, where immunotherapy has been a real success, in solid tumors, their efficiency has been diminished by the consistency, content and dynamics of TME that modulates the anti-tumor response through access and phenotype of immune cells.
To improve the efficiency and ensure the safety of the treatment it is imperative to carefully select the target antigens, assuring that they are highly immunogenic and expressed only in targeted the cell population.
Tumor-immune profiling has highlighted the mechanisms of immune evasion of cancer based not only on CSC properties but also on the interaction of these cells with TME. These include features such as antigen presentation and regulation of immune cells activation and functioning through immunosuppressive elements like immune checkpoints. Novel immunotherapeutic approaches addressed to all these features. There are several approaches that involve expanding of NK and T cells for CSC antigen-specific targeting or dendritic cell-based vaccines against CSCs. However, the most exciting approach is related to immune checkpoints discovery. Targeting PD-1, CTLA-4, Lag-3, Tim-3, and TIGIT, or their respective ligands on CSC allows activation of the immune cells like T-lymphocytes, NK, neutrophils, dendritic cells and destruction of CSC. The main purpose of these approaches is to modify the TME so that tumor cells and CSC become more responsive to chemotherapy.
An important limitation may come from gaining resistance to immunotherapy. It may be caused by the absence of tumor antigens, loss or decrease of MHC expression, alteration of signaling pathways affecting immune cell infiltration, or presence of regulatory T cells or myeloid derived suppressor cells in the tumor mi-croenvironment. In order to prevent resistance and extend the clinical benefits of immunotherapy, it is necessary to better understand the anti-tumor response mechanisms of the strategies discussed here in order to combine them, as combinatorial therapy might be the answer for acquiring long-term remission in cancer therapy.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56678] [Article Influence: 7084.8] [Reference Citation Analysis (135)] |
| 2. | Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3291] [Cited by in RCA: 3531] [Article Influence: 271.6] [Reference Citation Analysis (0)] |
| 3. | Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 581] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 4. | Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4428] [Cited by in RCA: 4234] [Article Influence: 84.7] [Reference Citation Analysis (1)] |
| 5. | Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, Gollin SM, Gamblin TC, Geller DA, Lagasse E. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932-6941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6983] [Article Influence: 279.3] [Reference Citation Analysis (0)] |
| 7. | Dragu DL, Necula LG, Bleotu C, Diaconu CC, Chivu-Economescu M. Therapies targeting cancer stem cells: Current trends and future challenges. World J Stem Cells. 2015;7:1185-1201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (2)] |
| 8. | Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, Salto-Tellez M. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105:658-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Qian X, Tan C, Wang F, Yang B, Ge Y, Guan Z, Cai J. Esophageal cancer stem cells and implications for future therapeutics. Onco Targets Ther. 2016;9:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Tang KH, Dai YD, Tong M, Chan YP, Kwan PS, Fu L, Qin YR, Tsao SW, Lung HL, Lung ML, Tong DK, Law S, Chan KW, Ma S, Guan XY. A CD90(+) tumor-initiating cell population with an aggressive signature and metastatic capacity in esophageal cancer. Cancer Res. 2013;73:2322-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J, Chen R, Zhou Y. Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol. 2013;42:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 13. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2163] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 14. | Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3068] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 16. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 937] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 17. | Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 971] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 18. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 934] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 19. | Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlöw B, Nestor M. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One. 2014;9:e94621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Haraguchi N, Ishii H, Mimori K, Ohta K, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Yamamoto H, Doki Y, Mori M. CD49f-positive cell population efficiently enriches colon cancer-initiating cells. Int J Oncol. 2013;43:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Jo JH, Park SB, Park S, Lee HS, Kim C, Jung DE, Song SY. Novel Gastric Cancer Stem Cell-Related Marker LINGO2 Is Associated with Cancer Cell Phenotype and Patient Outcome. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Codd AS, Kanaseki T, Torigo T, Tabi Z. Cancer stem cells as targets for immunotherapy. Immunology. 2018;153:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Birzele F, Voss E, Nopora A, Honold K, Heil F, Lohmann S, Verheul H, Le Tourneau C, Delord JP, van Herpen C, Mahalingam D, Coveler AL, Meresse V, Weigand S, Runza V, Cannarile M. CD44 Isoform Status Predicts Response to Treatment with Anti-CD44 Antibody in Cancer Patients. Clin Cancer Res. 2015;21:2753-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Weigand S, Herting F, Maisel D, Nopora A, Voss E, Schaab C, Klammer M, Tebbe A. Global quantitative phosphoproteome analysis of human tumor xenografts treated with a CD44 antagonist. Cancer Res. 2012;72:4329-4339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Maisel D, Birzele F, Voss E, Nopora A, Bader S, Friess T, Goller B, Laifenfeld D, Weigand S, Runza V. Targeting Tumor Cells with Anti-CD44 Antibody Triggers Macrophage-Mediated Immune Modulatory Effects in a Cancer Xenograft Model. PLoS One. 2016;11:e0159716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Menke-van der Houven van Oordt CW, Gomez-Roca C, van Herpen C, Coveler AL, Mahalingam D, Verheul HM, van der Graaf WT, Christen R, Rüttinger D, Weigand S, Cannarile MA, Heil F, Brewster M, Walz AC, Nayak TK, Guarin E, Meresse V, Le Tourneau C. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget. 2016;7:80046-80058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Salnikov AV, Bretz NP, Perne C, Hazin J, Keller S, Fogel M, Herr I, Schlange T, Moldenhauer G, Altevogt P. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br J Cancer. 2013;108:1449-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Shapira S, Shapira A, Starr A, Kazanov D, Kraus S, Benhar I, Arber N. An immunoconjugate of anti-CD24 and Pseudomonas exotoxin selectively kills human colorectal tumors in mice. Gastroenterology. 2011;140:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Chen Z, Wang T, Tu X, Xie W, He H, Wang M, Zhang J. Antibody-based targeting of CD24 enhances antitumor effect of cetuximab via attenuating phosphorylation of Src/STAT3. Biomed Pharmacother. 2017;90:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | He H, Tu X, Zhang J, Acheampong DO, Ding L, Ma Z, Ren X, Luo C, Chen Z, Wang T, Xie W, Wang M. A novel antibody targeting CD24 and hepatocellular carcinoma in vivo by near-infrared fluorescence imaging. Immunobiology. 2015;220:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Ma Z, He H, Sun F, Xu Y, Huang X, Ma Y, Zhao H, Wang Y, Wang M, Zhang J. Selective targeted delivery of doxorubicin via conjugating to anti-CD24 antibody results in enhanced antitumor potency for hepatocellular carcinoma both in vitro and in vivo. J Cancer Res Clin Oncol. 2017;143:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G, Baeuerle PA. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 33. | Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 34. | Wimberger P, Gilet H, Gonschior AK, Heiss MM, Moehler M, Oskay-Oezcelik G, Al-Batran SE, Schmalfeldt B, Schmittel A, Schulze E, Parsons SL. Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol. 2012;23:1979-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Fossati M, Buzzonetti A, Monego G, Catzola V, Scambia G, Fattorossi A, Battaglia A. Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti-EpCAM×anti-CD3). Gynecol Oncol. 2015;138:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Dragu DL, Chivu-Economescu M, Bleotu C, Necula LG, Matei L, Stoian M, Diaconu CC. Establishing a mouse disease model for future studies regarding gastric anti-cancer therapies. Romanian Biotechnological Letters. 2019;24:874-882. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 38. | Liu LL, Fu D, Ma Y, Shen XZ. The power and the promise of liver cancer stem cell markers. Stem Cells Dev. 2011;20:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Greve B, Kelsch R, Spaniol K, Eich HT, Götte M. Flow cytometry in cancer stem cell analysis and separation. Cytometry A. 2012;81:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 40. | Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1040] [Article Influence: 94.5] [Reference Citation Analysis (2)] |
| 41. | Colombo M, Mirandola L, Chiriva-Internati M, Basile A, Locati M, Lesma E, Chiaramonte R, Platonova N. Cancer Cells Exploit Notch Signaling to Redefine a Supportive Cytokine Milieu. Front Immunol. 2018;9:1823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1646] [Cited by in RCA: 1993] [Article Influence: 221.4] [Reference Citation Analysis (0)] |
| 43. | Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 853] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 44. | Cogdill AP, Andrews MC, Wargo JA. Hallmarks of response to immune checkpoint blockade. Br J Cancer. 2017;117:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 45. | Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1228] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 46. | Pan Q, Li Q, Liu S, Ning N, Zhang X, Xu Y, Chang AE, Wicha MS. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells. 2015;33:2085-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 343] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 48. | Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 3000] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 49. | Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 50. | Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 440] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 51. | Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2096] [Cited by in RCA: 2205] [Article Influence: 105.0] [Reference Citation Analysis (1)] |
| 52. | Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F, Moretta L, Moretta A, Corte G, Bottino C. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530-3539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 53. | Tseng HC, Arasteh A, Paranjpe A, Teruel A, Yang W, Behel A, Alva JA, Walter G, Head C, Ishikawa TO, Herschman HR, Cacalano N, Pyle AD, Park NH, Jewett A. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS One. 2010;5:e11590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R, Mandelboim O, Stassi G, Di Fabrizio E, Parmiani G, Moretta A, Dieli F, Kärre K, Carbone E. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 55. | Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, Yang J, Xu SL, Ye XZ, Xu C, Yang L, Qian C, Wang JM, Cui YH, Zhang X, Bian XW. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746-5757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 56. | Rong XX, Wei F, Lin XL, Qin YJ, Chen L, Wang HY, Shen HF, Jia LT, Xie RY, Lin TY, Hao WC, Yang J, Yang S, Cheng YS, Huang WH, Li AM, Sun Y, Luo RC, Xiao D. Recognition and killing of cancer stem-like cell population in hepatocellular carcinoma cells by cytokine-induced killer cells via NKG2d-ligands recognition. Oncoimmunology. 2016;5:e1086060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, Hagino T, Perez-Cunningham J, Sckisel GD, Urayama S, Monjazeb AM, Fragoso RC, Sayers TJ, Murphy WJ. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J Immunol. 2015;195:4010-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 58. | Yin T, Wang G, He S, Liu Q, Sun J, Wang Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell Immunol. 2016;300:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Miyamoto S, Kochin V, Kanaseki T, Hongo A, Tokita S, Kikuchi Y, Takaya A, Hirohashi Y, Tsukahara T, Terui T, Ishitani K, Hata F, Takemasa I, Miyazaki A, Hiratsuka H, Sato N, Torigoe T. The Antigen ASB4 on Cancer Stem Cells Serves as a Target for CTL Immunotherapy of Colorectal Cancer. Cancer Immunol Res. 2018;6:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, Huang JH, Yang QM, Han WD. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 61. | Maliar A, Servais C, Waks T, Chmielewski M, Lavy R, Altevogt P, Abken H, Eshhar Z. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology. 2012;143:1375-1384.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Davenport AJ, Cross RS, Watson KA, Liao Y, Shi W, Prince HM, Beavis PA, Trapani JA, Kershaw MH, Ritchie DS, Darcy PK, Neeson PJ, Jenkins MR. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci USA. 2018;115:E2068-E2076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 63. | Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 677] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 64. | Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2048] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 65. | Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW, Sharma SK, Chester KA, Westwood NB, Halford SER, Nabarro S, Wan S, Austin E, Hawkins RE. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017;66:1425-1436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 315] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 66. | Zhang Q, Zhang Z, Peng M, Fu S, Xue Z, Zhang R. CAR-T cell therapy in gastrointestinal tumors and hepatic carcinoma: From bench to bedside. Oncoimmunology. 2016;5:e1251539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Yu S, Yi M, Qin S, Wu K. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer. 2019;18:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 68. | Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1228] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 69. | Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, Heslop HE, Rooney CM, Brenner MK, Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 70. | Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell. 2018;23:181-192.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 71. | Siegler EL, Zhu Y, Wang P, Yang L. Off-the-Shelf CAR-NK Cells for Cancer Immunotherapy. Cell Stem Cell. 2018;23:160-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 72. | Ingegnere T, Mariotti FR, Pelosi A, Quintarelli C, De Angelis B, Tumino N, Besi F, Cantoni C, Locatelli F, Vacca P, Moretta L. Human CAR NK Cells: A New Non-viral Method Allowing High Efficient Transfection and Strong Tumor Cell Killing. Front Immunol. 2019;10:957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, Trowsdale J, Durrant LG. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res. 2009;15:6993-7002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Wefers C, Schreibelt G, Massuger LFAG, de Vries IJM, Torensma R. Immune Curbing of Cancer Stem Cells by CTLs Directed to NANOG. Front Immunol. 2018;9:1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Yin T, Shi P, Gou S, Shen Q, Wang C. Dendritic cells loaded with pancreatic Cancer Stem Cells (CSCs) lysates induce antitumor immune killing effect in vitro. PLoS One. 2014;9:e114581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 5762] [Article Influence: 443.2] [Reference Citation Analysis (0)] |
| 77. | Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, Egenti M, Owen J, Moyer JS, Prince ME, Huang S, Wicha MS, Xia JC, Li Q. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology. 2015;4:e990767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Mac Keon S, Ruiz MS, Gazzaniga S, Wainstok R. Dendritic cell-based vaccination in cancer: therapeutic implications emerging from murine models. Front Immunol. 2015;6:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Bagheri V, Abbaszadegan MR, Memar B, Motie MR, Asadi M, Mahmoudian RA, Gholamin M. Induction of T cell-mediated immune response by dendritic cells pulsed with mRNA of sphere-forming cells isolated from patients with gastric cancer. Life Sci. 2019;219:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N. Immune responses to human cancer stem-like cells/cancer-initiating cells. Cancer Sci. 2016;107:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. B7H3 As a Promoter of Metastasis and Promising Therapeutic Target. Front Oncol. 2018;8:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 82. | Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion. Front Oncol. 2018;8:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 83. | Jiang B, Zhang T, Liu F, Sun Z, Shi H, Hua D, Yang C. The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget. 2016;7:31755-31771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1367] [Cited by in RCA: 1311] [Article Influence: 145.7] [Reference Citation Analysis (9)] |
| 85. | Hsu JM, Xia W, Hsu YH, Chan LC, Yu WH, Cha JH, Chen CT, Liao HW, Kuo CW, Khoo KH, Hsu JL, Li CW, Lim SO, Chang SS, Chen YC, Ren GX, Hung MC. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. 2018;9:1908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 86. | Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 626] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 87. | Yang Y, Wu KE, Zhao E, Li W, Shi L, Xie G, Jiang B, Wang Y, Li R, Zhang P, Shuai X, Wang G, Tao K. B7-H1 enhances proliferation ability of gastric cancer stem-like cells as a receptor. Oncol Lett. 2015;9:1833-1838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Chen L, Xiong Y, Li J, Zheng X, Zhou Q, Turner A, Wu C, Lu B, Jiang J. PD-L1 Expression Promotes Epithelial to Mesenchymal Transition in Human Esophageal Cancer. Cell Physiol Biochem. 2017;42:2267-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 89. | Li Y, Yang X, Wu Y, Zhao K, Ye Z, Zhu J, Xu X, Zhao X, Xing C. B7-H3 promotes gastric cancer cell migration and invasion. Oncotarget. 2017;8:71725-71735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 90. | Zhi Y, Mou Z, Chen J, He Y, Dong H, Fu X, Wu Y. B7H1 Expression and Epithelial-To-Mesenchymal Transition Phenotypes on Colorectal Cancer Stem-Like Cells. PLoS One. 2015;10:e0135528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 307] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (3)] |
| 92. | Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, Cranmer LD, Blank CU, O'Day SJ, Ascierto PA, Salama AK, Margolin KA, Loquai C, Eigentler TK, Gangadhar TC, Carlino MS, Agarwala SS, Moschos SJ, Sosman JA, Goldinger SM, Shapira-Frommer R, Gonzalez R, Kirkwood JM, Wolchok JD, Eggermont A, Li XN, Zhou W, Zernhelt AM, Lis J, Ebbinghaus S, Kang SP, Daud A. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1288] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 93. | Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2598] [Cited by in RCA: 2866] [Article Influence: 260.5] [Reference Citation Analysis (0)] |
| 94. | Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 5181] [Article Influence: 518.1] [Reference Citation Analysis (2)] |
| 95. | Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, Keam B, Kang H, Muro K, Weiss J, Geva R, Lin CC, Chung HC, Meister A, Dolled-Filhart M, Pathiraja K, Cheng JD, Seiwert TY. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol. 2016;34:3838-3845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 664] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 96. | Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF; KEYNOTE-045 Investigators. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2051] [Cited by in RCA: 2722] [Article Influence: 302.4] [Reference Citation Analysis (0)] |
| 97. | Li YP, Wang YM. [Pressor mechanism of tussilagone]. Zhongguo Yao Li Xue Bao. 1986;7:333-336. [PubMed] |
| 98. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2188] [Article Influence: 243.1] [Reference Citation Analysis (9)] |
| 99. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3454] [Article Influence: 383.8] [Reference Citation Analysis (2)] |
| 100. | Chivu-Economescu M, Matei L, Necula LG, Dragu DL, Bleotu C, Diaconu CC. New therapeutic options opened by the molecular classification of gastric cancer. World J Gastroenterol. 2018;24:1942-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Gordeeva O. Cancer-testis antigens: Unique cancer stem cell biomarkers and targets for cancer therapy. Semin Cancer Biol. 2018;53:75-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 102. | Yamada R, Takahashi A, Torigoe T, Morita R, Tamura Y, Tsukahara T, Kanaseki T, Kubo T, Watarai K, Kondo T, Hirohashi Y, Sato N. Preferential expression of cancer/testis genes in cancer stem-like cells: proposal of a novel sub-category, cancer/testis/stem gene. Tissue Antigens. 2013;81:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Wei Y, Wang Y, Gong J, Rao L, Wu Z, Nie T, Shi D, Zhang L. High expression of MAGE-A9 contributes to stemness and malignancy of human hepatocellular carcinoma. Int J Oncol. 2018;52:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 898] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 105. | Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R, Tian Z. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 849] [Article Influence: 106.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Vetvicka V, Shimizu Y S-Editor: Zhou JJ L-Editor: A E-Editor: Zhang YL