Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1594
Peer-review started: December 24, 2019
First decision: January 12, 2020
Revised: January 13, 2020
Accepted: March 22, 2020
Article in press: March 22, 2020
Published online: April 14, 2020
Processing time: 111 Days and 22.7 Hours
Many studies investigating postoperative pancreatic fistula (POPF) after gastrectomy, including studies measuring drain amylase content (D-AMY) as a predictive factor have been reported. This article reviews previous studies and looks to the future of measuring D-AMY in patients after gastrectomy. The causes of pancreatic fluid leakage are; the parenchymal and/or thermal injury to the pancreas, and blunt injury to the pancreas by compression and retraction. Measurement of D-AMY to predict POPF has become common in clinical practice after pancreatic surgery and was later extended to the gastric surgery. Several studies have reported associations between D-AMY and POPF after gastrectomy, and the high value of D-AMY on postoperative day (POD) 1 was an independent risk factor. To improve both sensitivity and specificity, attempts have been made to enhance the predictive accuracy of factors on POD 1 as well as on POD 3 as combined markers. Although several studies have shown a high predictive ability of POPF, it has not necessarily been exploited in clinical practice. Many problems remain unresolved; ideal timing for measurement, optimal cut-off value, and means of intervention after prediction. Prospective clinical trial could be imperative in order to develop D-AMY measurement in common clinical practice for gastric surgery.
Core tip: Many studies investigating postoperative pancreatic fistula after gastrectomy, including measurement of drain amylase content (D-AMY) as a predictive factor. This article reviews previous studies and looks to the future of measuring D-AMY in patients after gastrectomy. Several studies have reported that the high D-AMY on postoperative day 1 or day 3 was an independent risk factor for postoperative pancreatic fistula. However, issues for clinical use remain unresolved, including the ideal timing of measurement, optimal cut-off value and intervention after prediction. Prospective clinical trials might be indispensable for D-AMY to become a common marker in clinical practice.
- Citation: Nakanishi K, Kanda M, Sakamoto J, Kodera Y. Is the measurement of drain amylase content useful for predicting pancreas-related complications after gastrectomy with systematic lymphadenectomy? World J Gastroenterol 2020; 26(14): 1594-1600
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1594.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1594
Gastrectomy with radical lymph node dissection, especially peri-pancreatic lymph node dissection, is the mainstay for resectable gastric cancers. Pancreas-related complications, especially postoperative pancreatic fistula (POPF), is one of the most common postoperative complications, and can sometimes lead to serious results, including intra-abdominal abscess, subsequent sepsis, and intraperitoneal bleeding, which usually require prolongation of hospitalization[1,2].
Many studies predicting POPF after gastrectomy have been investigated and age, operation time, body mass index, total gastrectomy, splenectomy, anatomical position of the pancreas, and high value of drain amylase content (D-AMY) have been reported as substantial predictive factors[2-13]. Among those, D-AMY, which is the measurement of the amylase content in drained abdominal fluids taken through an indwelling intra-abdominal drain, is promising because it can be measured objectively regardless of the patient’s preoperative condition, type of surgical procedure, and surgeon’s skill[14]. However, several problems remain unsolved precluding the implication in common clinical practice.
This article reviews previous reports and looks to the future of measuring D-AMY to predict POPF in gastric cancer surgery.
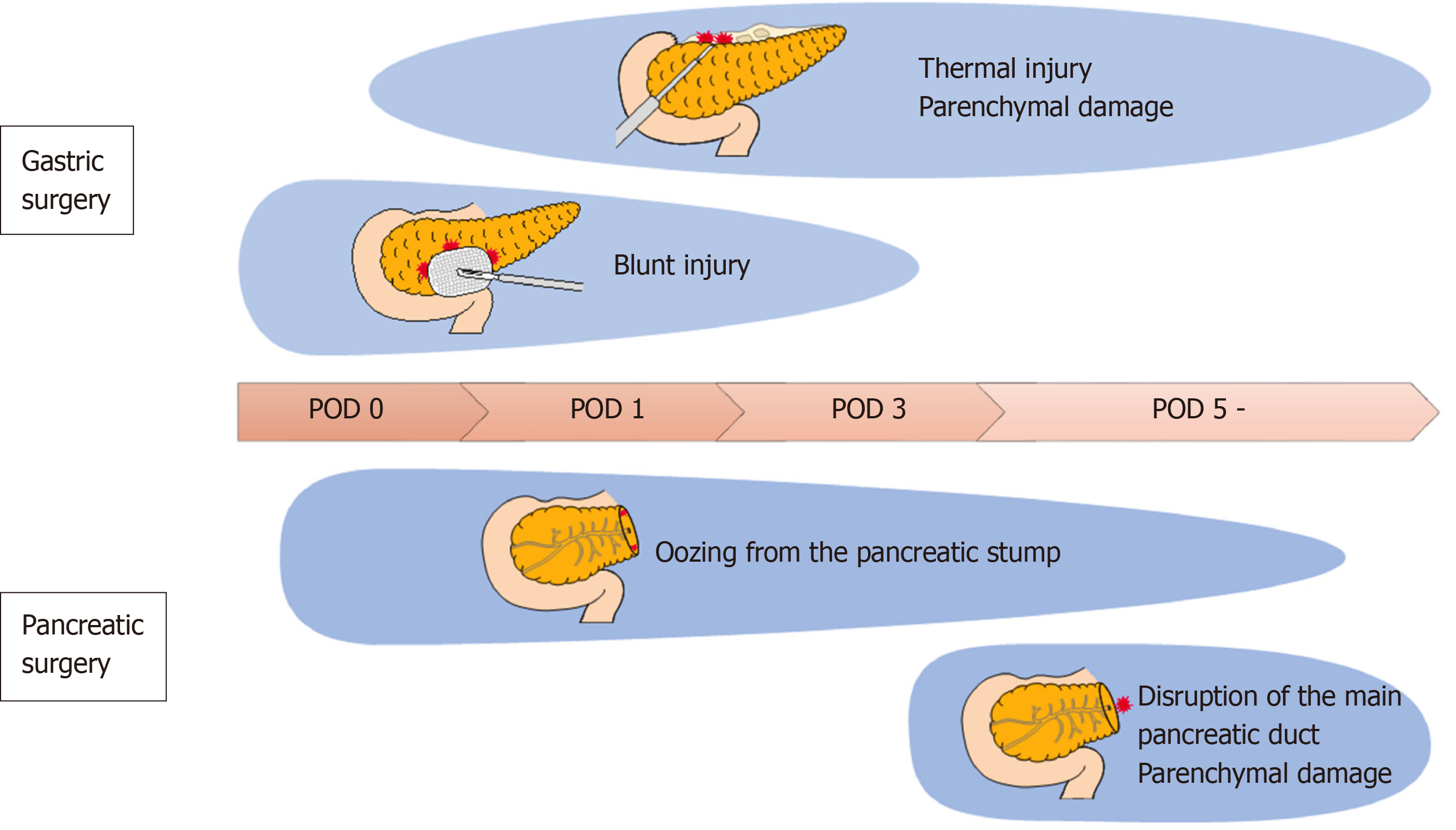
In gastric cancer surgery, the pancreatic duct is not usually transected, and the mechanism of pancreatic fluid leakage after surgery is different from that of pancreatectomy. Mainly, three mechanisms of pancreatic fluid leakage in gastric cancer surgery are reported, presumably caused by the operator and assistant surgeons. The operator can injure the surface of the pancreas by parenchymal and/or thermal injury during the dissection of the suprapancreatic lymph nodes[15]. Second, the assistant could compress and retract the pancreas to achieve a good view of the suprapancreatic area during suprapancreatic lymph node dissection, so called “blunt injury”[16]. Ida et al[16] conducted animal experiments using pigs and reported that blunt injury causes pancreatic necrosis and inflammatory cell infiltration, and the value of amylase content around the pancreas increases 2-4 h after the procedure.
Another mechanism is that pancreatic tail mobilization during combined splenectomy or splenectomy with distal pancreatectomy can damage the pancreatic parenchyma, resulting in pancreatic fluid leakage[3]. However, indications for such extended lymphadenectomy for gastric cancer has recently become limited[17].
In this regard, pancreatic fluid leakage involving gastric cancer surgery could mostly be minor leakage, that tends to be subsided spontaneously without clinically relevant pancreas-related postoperative complications.
In pancreatic surgery, the measurement of D-AMY is also used in the diagnostic criteria of POPF[18]. In general, pancreatectomy is recognized as a highly invasive surgery and is associated with a mortality of approximately 5% and a morbidity of 30%-60%[19]. Approximately 16% of patients develop POPF, making it one of the common complications of pancreatectomy[20]. The clinical stratification of POPFs was established by the International Study Group on Pancreatic Fistula (ISGPF) definition in 2005[18]. The presence of POPFs can be determined on postoperative day (POD) 3 by the amylase content in the drained fluid; therefore, the measurement of D-AMY has become common in clinical practice in the field of pancreatic surgery. After pancreatectomy, pancreatic fluid leakage is caused by a disruption of the main pancreatic duct, and D-AMY directly reflects pancreatic fluid leakage[21,22]; therefore, the measurement of D-AMY is a reasonable prediction tool for pancreas-related complications. This concept was later extended to gastric cancer surgery; however, the mechanisms responsible for pancreatic fluid leakage are supposed to differ between pancreatectomy in which the main pancreatic duct is transected, and gastrectomy, which causes some problems. The source of pancreatic fluid leakage after gastrectomy is the seepage of pancreatic juice from the parenchymal damage of the pancreas and blunt damage by compression or retraction[15,16]. Even if the value of D-AMY is high on POD 3, this minor pancreatic leakage seems to subside spontaneously without proceeding to clinical fistula formation[3]. Thus, the establishment of a gastric cancer surgery-specific definition and prediction tool for POPF is desirable.
Despite clinical importance, POPF had not been uniformly defined until 2005 when the ISGPF established the definition based on the clinical impact of POPF-related complications[18], and it has been well accepted in the pancreatic surgery community. The stratifications are as follows: Grade A, pancreatic fistulas with no clinical impact, although D-AMY on or after POD 3 is three times more than the upper normal serum amylase level; grade B requires a change in management or adjustment in the clinical pathway; and grade C requires a major change in clinical management and aggressive clinical intervention. This ISGPF classification is sometimes applied in the gastric cancer surgery community. However, validation of applying this definition to POPF following gastrectomy still remains unclear. As described in the previous section, the mechanisms of pancreatic fluid leakage in gastric cancer surgery are different from those in pancreatic surgery, and are shown in Figure 1; POPF of ISGPF grade A is not necessarily clinically significant; in other words, POPF with no clinical impact need not be defined in gastric cancer surgery. In addition, almost all POPFs in gastric cancer surgery are classified as ISGPF grade B, and ISGPF grade C is very rare.
As another definition of POPF, the Clavien-Dindo classification has been adopted, which is a comprehensive evaluation of postoperative complications and has gained widespread acceptance[23,24]. This classification system regards grade II or higher as clinically relevant and grade III or higher as severe complications. However, this definition is sometimes inconsistent with clinical severity, although objective and simple. For example, replacement of drainage tubes under fluoroscopy is classified as Clavien-Dindo grade IIIa, despite minor changes in clinical management and regarded severe complications.
An establishment of a new grading system of POPF after gastrectomy may be necessary. However, it is less frequently used and may not be familiar because the prevalence of POPF after gastrectomy is not as high compared with that after pancreatomy; it occurs in approximately 1.6% of patients who underwent distal gastrectomy[25] and in 2.6% of patients who underwent total gastrectomy[26]. At present, the ISGPF and Clavien-Dindo classification systems have each of their advantages and disadvantages, and it is desirable to use both appropriately.
Several studies have reported associations between D-AMY and POPF after gastrectomy, and Table 1 shows these studies in the gastric cancer surgical field. In 1997, Sano et al[3] reported for the first time that the measurement of D-AMY was useful for POPF after gastrectomy. However, a definition of POPF had not been established at that time, and their study defined it as a condition in which the D-AMY level was more than three times the upper normal serum amylase level for more than 7 d after operation. In their study, the prevalence of POPF in patients with D-AMY ≥ 4000 IU/L on POD 1 was significantly higher compared with that in patients with D-AMY < 4000 IU/L on POD 1, indicating the high level of D-AMY on POD 1 was retained to POD 7, regardless of clinically relevant complications.
| Ref. | Sample size | Surgical procedure | POD 1 | POD 3 | Definition of POPF | ||
| Data available | Cut-off | Data available | Cut-off | ||||
| Sano et al[3], 1997 | 102 | OTG, D1 - ≥ D2 | Yes | 4000 IU/L | No | NG | D-AMY > 3 times more than S-AMY for ≥ 7 days |
| Iwata et al[2], 2010 | 372 | Gastrectomy, D1 - ≥ D2 | Yes | 1000 IU/L | No | NG | ISGPF definition (grade A/B/C) |
| Tomimaru et al[4], 2011 | 172 | TG, D1 plus - D2 | Yes | 5000 IU/L | No | NG | ISGPF definition (grade B/C) |
| Miki et al[6], 2011 | 104 | TG, D2 | Yes | 3398 IU/L | No | NG | ISGPF definition (grade B/C) |
| Kobayashi et al[7], 2015 | 448 | Gastrectomy, D1 - ≥ D2 | Yes | 1949 IU/L | No | NG | C-D classification (grade III or higher) |
| De Sol et al[5], 2015 | 53 | Gastrectomy,D2 | N0 | NG | Yes | D-AMY > 3 times more than S-AMY | ISGPF definition (grade B/C) |
| Kanda et al[8], 2016 | 265 | LDG, D1 plus - D2 | Yes | 904 IU/L | Yes | Retained at ≥ 31.2% of D-AMY on POD 1 | C-D classification (grade II or higher) |
| Taniguchi et al[9], 2017 | 591 | Gastrectomy D1- ≥ D2 | Yes | 2900 IU/L | Yes | 2100 IU/L | ISGPF definition (grade B/C) |
| Kamiya et al[10], 2018 | 801 | Gastrectomy D1 plus - ≥ D2 | Yes | 2218 IU/L | Yes | 555 IU/L | C-D classification (grade III or higher) |
| Wakahara et al[12], 2019 | 327 | Gastrectomy D0-D2 | No | NG | Yes | 761 IU/L | C-D classification (grade II or higher) |
The ISGPF established the definition of POPF in 2005, and Iwata et al[2] adapted this definition and reported associations between D-AMY and POPF after gastrectomy in 2010. They reported that the prevalence of ISGPF grade A or higher was 16.3% and that D-AMY ≥ 1000 U/L on POD 1 along with body mass index were independent risk factors for POPF. However, the study suffered from the inclusion of a broad spectrum of surgical procedures ranging from the laparoscopic approach for early-stage cancer to extended lymphadenectomy accompanied by splenectomy for advanced cancer. In 2011, there were two studies that defined ISGPF grades B or C as POPF. Miki et al[6] reported that the prevalence of POPF after total gastrectomy with D2 lymphadenectomy was 22.1% and that D-AMY ≥ 3398 IU/L on POD 1 was an independent predictor of POPF. Tomimaru et al[4] reported that the prevalence of POPF after total gastrectomy with D1 plus or D2 lymphadenectomy was 9.2% and that D-AMY ≥ 5000 IU/L on POD 1 was a predictor of POPF. The above three studies were validated by applying the ISGPF classification and the predictive ability of D-AMY on POD 1, but the surgical procedures were different among studies, which caused the prevalence of POPF and the cut-off values to be inconsistent. In 2012, Kobayashi et al[7] adapted the Clavien-Dindo classification and reported that D-AMY ≥ 2000 IU/L on POD 1 and C-reactive protein ≥ 20 mg/dL on POD 3 were predictive of Clavien-Dindo classification grade III or higher POPF. The Clavien-Dindo classification grade III or higher POPF is the same as the ISGPF grade B or higher (grade C) excluding antibiotic treatment. So far, we summarized the studies to evaluate the predictive value of D-AMY on POD 1.
Two time point measurements of D-AMY have been developed to enhance the predictive value of POPF. In 2016, Kanda et al[8] reported that D-AMY on POD 1 served as a predictive factor for POPF. In addition, patients whose D-AMY level on POD 3 was retained at ≥ 31.2% of that on POD 1 were more likely to develop POPF after laparoscopic distal gastrectomy[8]. After that, two studies reported that the combined use of D-AMY on POD 1 and POD 3 had a higher predictive performance for POPF compared with each alone[9,10]. The combined use had high sensitivity; however, it did not serve as an early prediction.
A few studies have considered drainage volume[5,27] and concluded that drainage volume was not significant in gastric cancer surgery.
As described in the previous section, the definition of POPF has not been established; in addition, several problems have remained for clinical use, such as timing of measuring D-AMY, optimal cut-off value, and means of intervention after early prediction.
The timing of measuring D-AMY has not been determined. There is a dilemma between early prediction and diagnostic accuracy. With a single predictive marker, there is a limitation to increasing both sensitivity and specificity, and attempts have been made to enhance the predictive accuracy of factors on POD 1 as well as on POD 3 as combined markers[8-10]. However, it has limited clinical use for early prediction and early intervention. In other words, the timing of measurement that has both high diagnostic accuracy and early detection has not been determined.
Second, the cut-off values were different among studies. Differences in the definition of POPF, differences in the surgical procedure, and small-scale retrospective studies prevent the establishment of the optimal cut-off value. From the viewpoint of the mechanism of POPF, the differences in surgical procedures do not affect fistula formation. A large-scale prospective trial is warranted to establish an optimal cut-off value that applies to any surgical procedure.
Third, it is unclear whether early intervention will improve outcomes even if early prediction is successful. Prophylactic antibiotics in gastric cancer surgery are usually administered until the next morning after surgery. Additional prophylactic antibiotic administration may be beneficial for preventing deterioration in particular POPF high-risk patients who underwent pancreaticoduodenectomy[28]; although so far, the benefit is unclear in gastric cancer surgery[29]. Currently, a prospective exploratory randomized trial to evaluate prolonged prophylactic antibacterial drug treatment for patients with high levels of D-AMY on POD 1 after gastrectomy is in progress (UMIN000012152). In addition, the benefit of the measurement of D-AMY content as an indicator of early drainage tube removal is unknown. The prophylactic drain is helpful for the detection of not only POPF but also other serious complications, including anastomotic leakage, intraoperative bleeding, and injury of the intestine. It is possible that the drainage of intra-abdominal fluids, including pancreatic juice, may prevent subsequent POPF[2]. From the viewpoint of the enhanced recovery after surgery program, the drainage tube should be removed as soon as it is deemed unnecessary in order to reduce drain-related complications and shorten the hospital stay after gastrectomy[30-32]. Additionally, unnecessary drain placement is harmful in terms of intra-abdominal fluid loss. At present, the measurement of D-AMY is not used as an indicator of early drainage tube removal, but if the level of D-AMY is low, the drainage tube can be removed with little concern for later pancreas-related complications.
The measurement of D-AMY is promising because of its high predictive ability of POPF, even in the gastric cancer surgical field. However, many problems remain unresolved, i.e., definition of POPF, ideal timing for measurement, optimal cut-off value, and means of intervention after prediction. Prospective clinical trial could be imperative in order to develop D-AMY measurement in common clinical practice for gastric surgery.
We are grateful to the nonprofit organization Epidemiological and Clinical Research Information Network (ECRIN) for providing their valuable support with this work.
| 1. | Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 737] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 2. | Iwata N, Kodera Y, Eguchi T, Ohashi N, Nakayama G, Koike M, Fujiwara M, Nakao A. Amylase concentration of the drainage fluid as a risk factor for intra-abdominal abscess following gastrectomy for gastric cancer. World J Surg. 2010;34:1534-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 3. | Sano T, Sasako M, Katai H, Maruyama K. Amylase concentration of drainage fluid after total gastrectomy. Br J Surg. 1997;84:1310-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 4. | Tomimaru Y, Miyashiro I, Kishi K, Motoori M, Yano M, Shingai T, Noura S, Ohue M, Ohigashi H, Ishikawa O. Is routine measurement of amylase concentration in drainage fluid necessary after total gastrectomy for gastric cancer? J Surg Oncol. 2011;104:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 5. | De Sol A, Cirocchi R, Di Patrizi MS, Boccolini A, Barillaro I, Cacurri A, Grassi V, Corsi A, Renzi C, Giuliani D, Coccetta M, Avenia N. The measurement of amylase in drain fluid for the detection of pancreatic fistula after gastric cancer surgery: an interim analysis. World J Surg Oncol. 2015;13:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 6. | Miki Y, Tokunaga M, Bando E, Tanizawa Y, Kawamura T, Terashima M. Evaluation of postoperative pancreatic fistula after total gastrectomy with D2 lymphadenectomy by ISGPF classification. J Gastrointest Surg. 2011;15:1969-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Kobayashi D, Iwata N, Tanaka C, Kanda M, Yamada S, Nakayama G, Fujii T, Koike M, Fujiwara M, Kodera Y. Factors related to occurrence and aggravation of pancreatic fistula after radical gastrectomy for gastric cancer. J Surg Oncol. 2015;112:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Kanda M, Fujiwara M, Tanaka C, Kobayashi D, Iwata N, Mizuno A, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Kodera Y. Predictive value of drain amylase content for peripancreatic inflammatory fluid collections after laparoscopic (assisted) distal gastrectomy. Surg Endosc. 2016;30:4353-4362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 9. | Taniguchi Y, Kurokawa Y, Mikami J, Tanaka K, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. Amylase concentration in drainage fluid as a predictive factor for severe postoperative pancreatic fistula in patients with gastric cancer. Surg Today. 2017;47:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Kamiya S, Hiki N, Kumagai K, Honda M, Nunobe S, Ohashi M, Sano T, Yamaguchi T. Two-point measurement of amylase in drainage fluid predicts severe postoperative pancreatic fistula after gastric cancer surgery. Gastric Cancer. 2018;21:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Kumagai K, Hiki N, Nunobe S, Kamiya S, Tsujiura M, Ida S, Ohashi M, Yamaguchi T, Sano T. Impact of anatomical position of the pancreas on postoperative complications and drain amylase concentrations after laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2018;32:3846-3854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Wakahara T, Kanemitsu K, Asari S, Tsuchida S, Ueno N, Toyokawa A, Sasako M. The Combined Use of Drainage Amylase Concentration and Serum C-reactive Protein as Predictors of Pancreas-Related Complications after Elective Gastrectomy. Oncology. 2020;98:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kodera Y, Sasako M, Yamamoto S, Sano T, Nashimoto A, Kurita A; Gastric Cancer Surgery Study Group of Japan Clinical Oncology Group. Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg. 2005;92:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Aranha GV, Aaron JM, Shoup M, Pickleman J. Current management of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2006;140:561-568; discussion 568-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Irino T, Hiki N, Ohashi M, Nunobe S, Sano T, Yamaguchi T. The Hit and Away technique: optimal usage of the ultrasonic scalpel in laparoscopic gastrectomy. Surg Endosc. 2016;30:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 16. | Ida S, Hiki N, Ishizawa T, Kuriki Y, Kamiya M, Urano Y, Nakamura T, Tsuda Y, Kano Y, Kumagai K, Nunobe S, Ohashi M, Sano T. Pancreatic Compression during Lymph Node Dissection in Laparoscopic Gastrectomy: Possible Cause of Pancreatic Leakage. J Gastric Cancer. 2018;18:134-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 17. | Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2017;265:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 18. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3557] [Article Influence: 169.4] [Reference Citation Analysis (35)] |
| 19. | Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2013;CD008370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Davidson TB, Yaghoobi M, Davidson BR, Gurusamy KS. Amylase in drain fluid for the diagnosis of pancreatic leak in post-pancreatic resection. Cochrane Database Syst Rev. 2017;4:CD012009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, Kodera Y. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014;18:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Kanda M, Fujii T, Takami H, Suenaga M, Inokawa Y, Yamada S, Kobayashi D, Tanaka C, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Kodera Y. Novel diagnostics for aggravating pancreatic fistulas at the acute phase after pancreatectomy. World J Gastroenterol. 2014;20:8535-8544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26151] [Article Influence: 1188.7] [Reference Citation Analysis (2)] |
| 24. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9228] [Article Influence: 542.8] [Reference Citation Analysis (1)] |
| 25. | Kurita N, Miyata H, Gotoh M, Shimada M, Imura S, Kimura W, Tomita N, Baba H, Kitagawa Y, Sugihara K, Mori M. Risk Model for Distal Gastrectomy When Treating Gastric Cancer on the Basis of Data From 33,917 Japanese Patients Collected Using a Nationwide Web-based Data Entry System. Ann Surg. 2015;262:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Watanabe M, Miyata H, Gotoh M, Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Kitagawa Y, Sugihara K, Mori M. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg. 2014;260:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Seo KW, Yoon KY, Lee SH, Shin YM, Choi KH, Hwang HY. Amylase, lipase, and volume of drainage fluid in gastrectomy for the early detection of complications caused by pancreatic leakage. J Korean Surg Soc. 2011;81:402-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Jin K, Zhou H, Zhang J, Wang W, Sun Y, Ruan C, Hu Z, Wang Y. Systematic review and meta-analysis of somatostatin analogues in the prevention of postoperative complication after pancreaticoduodenectomy. Dig Surg. 2015;32:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Hirao M, Tsujinaka T, Imamura H, Kurokawa Y, Inoue K, Kimura Y, Shimokawa T, Furukawa H; Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG). Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013;16:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Yamagata Y, Yoshikawa T, Yura M, Otsuki S, Morita S, Katai H, Nishida T. Current status of the "enhanced recovery after surgery" program in gastric cancer surgery. Ann Gastroenterol Surg. 2019;3:231-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Wee IJY, Syn NL, Shabbir A, Kim G, So JBY. Enhanced recovery versus conventional care in gastric cancer surgery: a meta-analysis of randomized and non-randomized controlled trials. Gastric Cancer. 2019;22:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Tanaka R, Lee SW, Kawai M, Tashiro K, Kawashima S, Kagota S, Honda K, Uchiyama K. Protocol for enhanced recovery after surgery improves short-term outcomes for patients with gastric cancer: a randomized clinical trial. Gastric Cancer. 2017;20:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aurello P, Chen S, Ju SQ S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ