Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1564
Peer-review started: December 17, 2019
First decision: January 16, 2020
Revised: March 4, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: April 14, 2020
Processing time: 118 Days and 23 Hours
A number of digestive and extra-digestive disorders, including inflammatory bowel diseases, irritable bowel syndrome, intestinal infections, metabolic syndrome and neuropsychiatric disorders, share a set of clinical features at gastrointestinal level, such as infrequent bowel movements, abdominal distension, constipation and secretory dysfunctions. Several lines of evidence indicate that morphological and molecular changes in intestinal epithelial barrier and enteric neuromuscular compartment contribute to alterations of both bowel motor and secretory functions in digestive and extra-digestive diseases. The present review has been conceived to provide a comprehensive and critical overview of the available knowledge on the morphological and molecular changes occurring in intestinal epithelial barrier and enteric neuromuscular compartment in both digestive and extra-digestive diseases. In addition, our intent was to highlight whether these morphological and molecular alterations could represent a common path (or share some common features) driving the pathophysiology of bowel motor dysfunctions and related symptoms associated with digestive and extra-digestive disorders. This assessment might help to identify novel targets of potential usefulness to develop original pharmacological approaches for the therapeutic management of such disturbances.
Core tip: Current evidence suggests that impairments of intestinal epithelial barrier and enteric neuromuscular compartment might represent a common condition underlying the onset/progression of bowel functional disturbances in both digestive and extra-digestive diseases. In this review, we summarize the impact of morphological and molecular alterations occurring in intestinal epithelial barrier and enteric neuromuscular compartment on bowel motor and secretory functions in digestive and extra-digestive diseases. This assessment, beyond to provide insight on the pathophysiology of bowel motor dysfunctions, could pave the way to the identification of novel therapeutic targets for the management of bowel dysfunctions associated with digestive and extra-digestive disorders.
- Citation: D’Antongiovanni V, Pellegrini C, Fornai M, Colucci R, Blandizzi C, Antonioli L, Bernardini N. Intestinal epithelial barrier and neuromuscular compartment in health and disease. World J Gastroenterol 2020; 26(14): 1564-1579
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1564.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1564
A number of digestive and extra-digestive disorders, such as inflammatory bowel diseases (IBDs), irritable bowel syndrome (IBS), intestinal infections, metabolic syndrome and neuropsychiatric disorders, share a set of clinical features at gastrointestinal (GI) level. Digestive functional disturbances, such as infrequent bowel movements, abdominal distension, constipation and secretory dysfunctions, are often complained by patients affected by the above diseases, undermining their quality of life and contributing relevantly to morbidity[1-4].
Several lines of evidence indicate that morphological and molecular changes in intestinal epithelial barrier (IEB) and enteric neuromuscular compartment can be associated with both digestive and extra-digestive diseases. For instance, both IBD and obese patients are characterized by an impairment of IEB and remodeling of enteric neuromuscular compartment, which appear to contribute to alterations of both intestinal motor and secretory functions[5,6]. In parallel, the same or similar morphofunctional GI alterations characterize different neuropsychiatric disorders, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), autism spectrum disorder (ASD) and depression[7-9].
Based on this background, the present review has been conceived to provide a comprehensive and critical overview of available knowledge on the morphological and molecular changes occurring in IEB and enteric neuromuscular compartment in both digestive and extra-digestive diseases. In addition, our intent was to highlight whether these alterations could represent a common path (or share some common features) driving the pathophysiology of bowel motor dysfunctions and related symptoms associated with digestive and extra-digestive disorders. This assessment might help to identify novel targets of potential usefulness to develop novel pharmacological approaches for the therapeutic management of such disturbances.
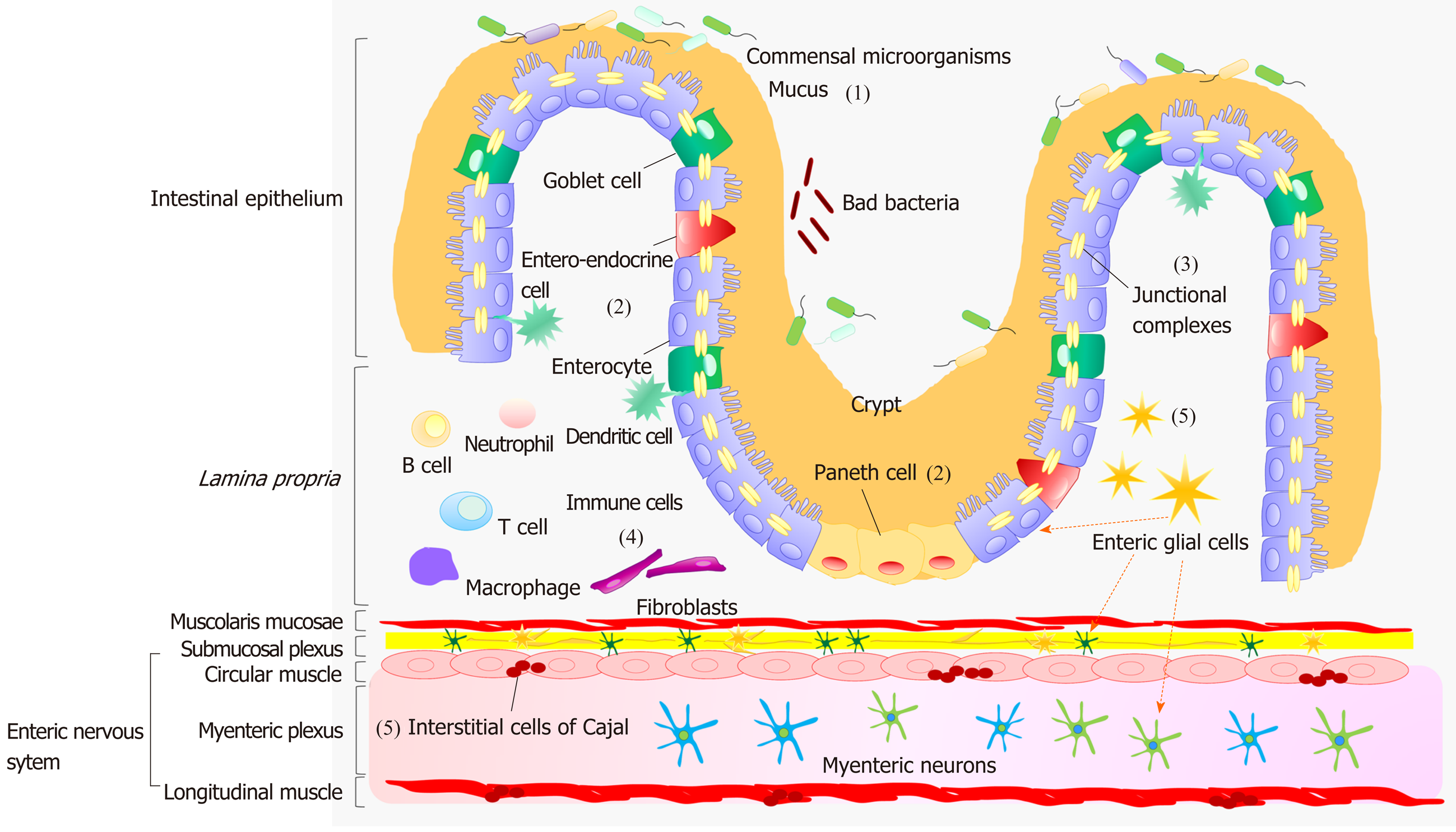
A dynamic interplay, occurring between IEB, enteric immune system and neuromuscular compartment, contributes relevantly to the maintenance of gut homeostasis[10]. The IEB represents the main physical barrier between the lumen and tissue compartments[11]. The luminal surface of intestinal mucosa is covered by a hydrated gel, consisting mainly of mucins secreted by goblet cells[11]. The outer mucus layer provides a habitat for commensal microorganisms, while the inner mucus layer acts as a physical barrier preventing the penetration of microorganisms and other noxious agents into bowel tissues[11] (Figure 1). Under physiological conditions, there is an equilibrium between the mucus secretion rate and its erosion, due to the movement of luminal contents, ensuring a stable thicknesses of the mucus layer.
Below the mucus layer, the IEB, an epithelial cell monolayer arranged into finger-like protrusions (villi) and invaginations (crypts), forms a selective physical barrier[11]. The villi provide an efficient surface for nutrient absorption, while stem cells, located at the basis of crypts, give rise to several types of epithelial cells: Enterocytes, goblet cells, entero-endocrine cells and Paneth cells[11] (Figure 1). Enterocytes are the major cell type in intestinal epithelium. Beyond their critical role as selective physical barrier, they tightly regulates the nutrient absorption (e.g., ions, water, sugar, peptides, and lipids) as well as the secretion of immunoglobulins. In parallel, the entero-endocrine cells release intestinal hormones or peptides into bloodstream upon stimulation, to activate nervous responses. Finally, Paneth cells, located at the base of small intestinal crypts, regulate microbial populations and protect neighboring stem cells, through the secretion of antimicrobial peptides[11].
The IEB holds three fundamental functions: (1) It acts as a physical barrier, preventing the passage of harmful intraluminal entities; (2) It operates as a selective filter, allowing the passage of nutrients and water; and (3) It has secretory functions, such as the release of mucus and immunoglobulins[11].
The efficiency of IEB depends on the maintenance of its integrity, ensured by three junctional complexes that join adjacent epithelial cells and include tight junctions (TJs), adherent junctions and desmosomes[11] (Figure 1). TJs, the most apical intercellular junctions, consist of trans-membrane proteins, such as claudins, occludin and tri-cellulin, which are anchored to the actin cytoskeleton via a cytoplasmic plaque including the zona occludens (ZO-1, ZO-2 and ZO-3)[11]. Adherent junctions, located just beneath TJs, share a common structural organization with the junctional complex mentioned above. Desmosomes are located along the lateral membranes beneath adherent junctions. The main tasks of such junctional complexes are to confer mechanical strength to the IEB and regulate paracellular permeability[11].
With regard for the enteric immune system, several review articles have provided a thorough overviews about the intricate networks occurring among the immune cells, resident both in the lamina propria and Peyer’s patches, and the mucosal and neuromuscular compartment[10] (Figure 1).
The enteric nervous system (ENS) holds a pivotal role in shaping the majority of GI functions[12]. This nervous network is arranged into two plexuses: The submucosal plexus (or Meissner’s plexus), located in the submucosa, and the myenteric plexus (or Auerbach’s plexus), located between the circular and longitudinal muscle layer[12] (Figure 1). The neurons of submucosal plexus, besides contributing to the motor control of smooth muscles, regulate secretive and absorptive functions, whereas those of the myenteric plexus are involved mainly in the initiation and control of gut motor activity[12]. The ENS, beyond the regulation of GI motor functions, contributes to the control of key functions involved in the maintenance of IEB homeostasis, including paracellular or transcellular permeability, epithelial cell proliferation and TJ expression; it regulates also several mucosal functions, independently of cerebral inputs[13].
Among the cellular components of ENS, there is increasing evidence highlighting a pivotal involvement of enteric glial cells (EGCs), interstitial cells of Cajal (ICC) and smooth muscle cells in the regulation of gut homeostasis. EGCs are associated with both submucosal and myenteric neurons and are located also in proximity to epithelial cells[12]. They coordinate signal propagation from and to myenteric neurons and epithelial cells, thus taking a significant part to the control of bowel motility as well as the secretory and absorptive functions of the enteric epithelium[14,15] (Figure 1). A crucial role in the control of the motor functions of enteric smooth myocytes is played by the ICC, located in the tunica muscularis[12]. These cells generate spontaneous and rhythmic electrical activity, on the basis of which they are considered as pacemakers for gut motility[12] (Figure 1). The muscular compartment consists of two layers of smooth muscle cells: The circular one, where fibers are oriented along the transversal axis and generate forward transit with relatively little mixing, and the longitudinal muscle layer, equipped with fibers oriented along the longitudinal axis, that, beyond the maintenance of intestinal muscle tone, contributes to shorten the lumen and support the propulsion[12] (Figure 1). The outer surface of the muscular layer is covered by the adventitia, which secretes lubricating fluids to reduce friction generated by muscle movements[12].
Overall, the structural and functional integrity of IEB and neuromuscular compartment are essential to ensure an adequate implementation of digestive motor and secretory functions. In particular, a proper interplay between IEB and ENS gives rise to a dynamic network aimed at coordinating the GI physiology and preserving the integrity of gut microenvironment.
IBDs, comprising mainly ulcerative colitis (UC) and Crohn’s disease (CD), are chronic intestinal inflammatory disorders, characterized clinically by abdominal pain, diarrhea or constipation, and weight loss[1]. Anatomically, UC is restricted to the rectum, colon and caecum, while CD can affect the entire GI tract, although it commonly affects the terminal ileum and colon[1]. Currently, the etiology of IBDs has not been completely elucidated. Intensive research efforts have been focused on the characterization of the role of IEB and enteric neuromuscular compartment in the onset of IBDs and related digestive disturbances.
Several studies have documented a defective mucus layer in IBD patients. In particular, the histological analysis of UC colonic biopsies has shown a depletion of goblet cells, a reduced mucin glycosylation, and a decrease in mucin (MUC)-2 biosynthesis and secretion[16-19]. By contrast, CD patients display an abnormal glycosylation and mucin hyperproduction accompanied by goblet cell hyperplasia[17] (Table 1). Such alterations can increase the epithelial permeability to luminal bacteria and microbial products, which, upon interaction with immune cells, trigger and maintain the inflammatory response[18-20].
| Digestive disorder | Morphofunctional changes in intestinal epithelial barrier | Morphofunctional changes in enteric neuromuscular compartment | Notes | Ref. |
| Human investigations | ||||
| IBD | Altered composition of mucus layer | ↓ Myenteric neurons (b) | (a) UC ↓ claudin-1 and -4; CD ↓ claudin-3, -5 and -8 | [5,16-19,23-26,29-36] |
| Abnormal glycosylation of mucins | ↑ SP release (c) | (b) Another study reported an increment of the enteric neuron number | ||
| ↑ Paracellular and transcellular permeability | ↑ NK-1 and NK-2 receptors | |||
| ↑ Claudin-2 and claudin-18 (a) | Altered morphology of ICC | (c) Other authors reported a significant reduction of both AChE activity and ACh release in IBD patients suffering from moderate-severe disease, as compared with healthy controls or IBD patients with low disease severity | ||
| ↓ Occludin and ZO-1 | Functional alterations of EGCs | |||
| IBS | ↑ Mucus secretion | ↓ Thickness of muscle layer | (d) Positive correlation between increased intestinal permeability and visceral pain | [51,54-63] |
| ↑ Paracellular permeability (d) | ↑ Entero-endocrine cell activity | |||
| ↓ Occludin and ZO-1 | ↑ SP release (f) | |||
| Altered expression of claudins (e) | Altered circulating levels of 5-HT | (e) IBS-D: ↓ claudin-1 and claudin-4, resulting in diarrhea; IBS-C: ↑ claudin-1, claudin-3 and claudin-4, resulting in constipation | ||
| Altered number and morphology of ICC | (f) Positive correlation between increased SP release and pain scores | |||
| ↑ EGC density | ||||
| Intestinal infections | Altered composition of mucus layer | ↓ Circulating levels of 5-HT | [72,74,75,76,78,79] | |
| ↓ Goblet cell number | ↑ SP release | |||
| ↑ Paracellular permeability altered TJs | ||||
| ↑ Epithelial apoptosis | ||||
| Diverticulosis and diverticulitis | ↑ Mucosal folds | Altered smooth muscle cells | (g) A more recent study did not observe alterations of ENS | [77,80-83] |
| Mucosal ulcerations | Altered serotonergic system | |||
| Crypt distortion | ↑ Tachykinergic contractile activity | |||
| ↓ Cholinergic pathway activity | ||||
| ↓ ICC number | ||||
| ↓ EGC density (g) | ||||
| Experimental models | ||||
| IBD | Altered composition of mucus layer | ↓ Myenteric neurons | [37-50] | |
| ↓ Goblet cell number | Altered morphology of ICC | |||
| ↑ Paracellular and transcellular permeability | ↓ EGC density | |||
| ↑ Claudin-1 and claudin-2 | ||||
| ↓ Occludin and ZO-1 | ||||
| IBS | ↑ Mucus secretion | ↓ Thickness of muscle layer | (h) Positive correlation between increased intestinal permeability and visceral pain | [63,65-68,70] |
| ↑ Paracellular permeability (h) | Altered number of ICC | |||
| ↓ Occludin and ZO-1 | ↑ SP release | |||
| ↓ Circulating levels of 5-HT | ||||
| ↑ EGC density | ||||
| Intestinal infections | ↑ MUC1 expression | ↑ SP release | [84-87] | |
| ↓ MUC2 expression | ||||
| ↑ Paracellular permeability | ||||
| Altered TJs | ||||
A common feature of IBD patients is the increase in paracellular permeability due to TJ abnormalities that, besides altering the transport of solutes and water and causing leak flux diarrhea, allow the tissue penetration of large molecules and luminal pathogens, triggering innate immune responses[5,21,22]. In this regard, IBD patients have been found to display an increased expression of claudin-2 and claudin-18 as well as a decreased expression and tissue redistribution of occludin, along with an increased serum ZO-1 concentration[5,23-26] (Table 1).
IBD patients are commonly affected by GI motility disorders[27,28]. Indeed, changes in small bowel transit have been reported in both UC and CD patients[27]. Consistent with these clinical findings, several lines of evidence indicate the occurrence of neuroplastic changes in the neuromuscular compartment and suggest that these are critical steps in contributing to the alterations of digestive motility in the presence of IBDs. In particular, several studies have described a reduction of myenteric neurons[29], mainly in UC than CD tissues[30], likely resulting from increased apoptotic processes, not restricted to specific neural populations[31]. IBD patients display also subtle changes in the expression of enteric neurotransmitters or their receptors. For instance, high levels of substance P (SP) and upregulation of NK-1 and NK-2 receptors have been observed in the colon and rectum of IBD patients[32-34]. Other human studies reported morphological abnormalities of ICC and EGCs, that could participate to the initiation/maintenance of IBDs and their associated symptoms[28,29,35]. In support of this view, histological examinations of UC and CD bowel biopsies pointed out an increase in glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B), and glial cell line-derived neurotrophic factor (GDNF) in the inflamed area, suggesting that EGCs were activated during the inflammatory processes[36] (Table 1).
The mechanisms underlying pathological interplays among immune/inflammatory processes, IEB, neuromuscular compartment and bowel motor dysfunctions in IBDs remain to be elucidated. In this respect, interesting evidence comes from studies on IBD animal models. Il10-/- mice (lacking the expression of IL-10 and developing colitis spontaneously), as well as colitis induced by dextran sodium sulfate (DSS) or dinitrobenzene sulfonic acid (DNBS) display a significant loss of goblet cells and alterations of mucus layer composition, implying a dysfunction in the mucus barrier permeability[18,37-39]. In addition, mouse with DSS colitis showed a reduced expression of occludin and ZO-1 as well as an increase of claudin-1 and claudin-2, along with a marked increase in apoptotic death of epithelial cells[40,41] (Table 1). Of note, the reduction of ZO-1 expression was found to precede the onset of intestinal inflammation, suggesting that the ZO-1 alteration was not a consequence of the inflammatory process, but rather an early event, prodromal to the onset of colitis[40]. In support to this view, studies conducted in Il10-/- mice, beyond showing alterations of villus and crypt architecture, displayed an increment of intestinal permeability, that occurred as a primary defect, before the onset of mucosal inflammation, suggesting a disruption of IEB[42,43].
The occurrence of ENS abnormalities, including axonal hypertrophy, a decrease in the number of enteric neurons and morphological alterations of ICC, has been described also in animal models of IBD[44-48]. Brown et al[49] reported that the activation of EGCs in the context of neuroinflammation induce enteric neuronal death in DNBS-treated mice, suggesting that glial response to inflammatory mediators might contribute to the development of bowel motor abnormalities. Currently, only one pre-clinical study, conducted in rats with 2,4,6-trinitrobenzene sulfonic acid (TNBS) colitis, reported a loss of EGCs following bowel inflammation, demonstrating that colitis can affect differently the EGCs in the submucosal and myenteric plexus[50] (Table 1). Of note, at present studies on histological alterations of EGC markers such as GFAP, S100B and GDNF in animal tissues of IBDs are lacking. Therefore, further investigations should be implemented to help better clarifying putative correlations among the morphofunctional alterations of EGCs, bowel inflammation and motor dysfunctions in IBDs.
IBS is a frequent disorder affecting up to 15%-25% of the adult population[2]. IBS patients are classified into subtypes by predominant stool pattern: IBS with diarrhea (IBS-D); constipation (IBS-C); mixed (IBS-M); and unsubtyped IBS (IBS-U)[2]. Among the patients complaining of constipation, 11% have functional slow transit constipation (STC); such patients differ from IBS-C due to the absence of abdominal pain. Emerging evidence suggests that, beyond psychosocial factors and low-grade intestinal inflammation, alterations of IEB and enteric neuromuscular compartment could contribute to IBS onset, development and related symptoms.
Human studies have reported a status of exuberant mucin secretion by goblet cells along with an increased paracellular permeability due to TJ abnormalities in IBS patients[51]. The increment of IEB permeability is thought to represent an important step in the sequence of events leading to the onset of low-grade intestinal inflammation and disturbed bowel functions[52,53]. The integrity of IEB in IBS patients has been investigated by evaluating the urinary excretion of oral probes, such as 13C mannitol[54]. This approach has allowed to document an increase in the intestinal permeability of IBS patients, likely reflecting alterations of TJs occurring during the acute phase of the disorder[54]. Histological examinations of colonic biopsies showed an abnormal cellular distribution of claudins as well as a reduced expression of ZO-1 and occludin in all IBS subtypes as compared to healthy controls[51,55,56] (Table 1). Currently there is no evidence regarding changes in IEB in STC patients.
As far as the neuromuscular compartment is concerned, several alterations have been described in patients, suggesting their contribution to the pathophysiology of IBS symptoms, such as bowel dysmotility. However, no predominant patterns of motor activity have emerged as markers for IBS. In this context, translational evidence highlighted a hypertrophy of the muscle layer, mainly in IBS-D patients, and alterations of the number and size of ICC both in IBS and STC patients[57-60]. Cheng et al[51] reported an abnormal density of entero-endocrine cells in rectal biopsies of IBS patients, along with a strong secretory status, suggesting that the endocrine system may play an important role in the pathophysiology of IBS. Other studies observed an increase in circulating serotonin levels in IBS-D patients, contrary to IBS-C, characterized by reduced levels of circulating serotonin[61,62]. These findings suggest that serotonin, beyond regulating gut motility, plays an important role in immune activation and inflammation, thus contributing to the pathophysiology of IBS. Currently, only few studies have taken into consideration the morphology of EGCs in IBS. For instance, Wang et al[63] observed an increment of EGCs in the colonic mucosa of IBS patients (Table 1). By contrast, STC patients displayed a significant decrease in EGCs in both the myenteric and submucosal plexus[64]. At present, there is no evidence to explain the relationship between the altered number of EGCs and bowel motor dysfunctions in IBS and STC patients. Therefore further studies are needed.
Consistently with human findings, an increment of mucus secretion and hyperplasia of goblet cells has been observed in IBS animal models[65]. In addition, in an IBS-D rat model induced by acetic acid, a significant reduction of ZO-1 and occludin expression has been shown[66]. These findings suggest that morphological alterations of mucus layer and TJ proteins could contribute to the increased sensitivity to visceral pain and other aspects of IBS symptoms[65,67] (Table 1).
The occurrence of ENS abnormalities has been described also in IBS animal models. Indeed, similarly to patients, murine models of IBS showed a significant reduction of the total thickness of muscle layer and alterations of ICC[65,68]. Likewise, Wang et al[69] showed a significant reduction of ICC number in a rat model of STC. Thus, current data from human and pre-clinical studies indicate that changes in ICC numbers are closely associated with alterations of intestinal motor patterns in both IBS and STC[57,68,70]. Of interest, similarly to IBS patients, Wang et al[63] reported an increase in the number of EGCs, observing a positive correlation between changes in EGCs and abdominal pain (Table 1).
For a variety of digestive disorders, such as intestinal infections and diverticular disease (including diverticulosis and diverticulitis), the pathogenesis remains unclear and several hypotheses have been formulated. Nevertheless, alterations of IEB and enteric neuromuscular compartment have been described as common features likely involved in the pathogenesis and progression of these diseases.
In intestinal infections, the presence of pathogens in the intestine can induce pathological alterations of the mucus layer and IEB, resulting in the onset of inflammatory responses within the gut wall[71]. Indeed, infectious agents may damage the intestinal mucosa by a direct interaction with mucins or the release of toxins[72,73]. In this regard, human studies have documented a depletion of goblet cells and an altered composition of mucus, resulting in an enhanced interaction between harmful intraluminal entities and enteric epithelium, exacerbating intestinal inflammation[72,74]. On the other hand, infectious agents have developed mechanisms that target the host's TJs. Clinical data from norovirus-infected patients showed a flattening of epithelium and a severe loss of villi as well as a reduction of TJ expression and an increment of epithelial apoptosis[75,76] (Table 1).
When considering the morphofunctional alterations of the mucus layer and IEB occurring in diverticular disease, a limited number of clinical data are currently available. For instance, a recent study showed a prominent mucosal folding with crypt distortion, mucosal ulcerations and infiltration of inflammatory cells in patients with diverticulitis[77] (Table 1).
With regard for the neuromuscular compartment, structural and functional abnormalities have been observed, either in patients with intestinal infections and subjects affected by diverticular disease. A common feature in such disorders is the alteration of enteric neurotransmitters. Clinical evidence in Giardia duodenalis-infected patients showed a reduction of circulating serotonin and a decreased number of serotonin-containing enterochromaffin cells in the duodenal mucosa[78]. Other authors reported an increment of SP levels in the gut of patients infected with Cryptosporidium[79] (Table 1). Similarly to intestinal infections, patients with diverticular disease displayed alterations of the serotonergic system[80] and an increment of tachykinergic motor activity as well as a reduction of cholinergic motility[81]. Other authors reported an altered expression patterns of important molecular factors involved in the regulation of smooth muscle cells contractility at level of the tunica muscularis[82]. In addition, Wedel et al[83] observed a thickening of muscle layers, along with a reduced number of EGCs and ICC (Table 1).
Consistently with human findings, pre-clinical studies in mice infected with Citrobacter rodentium or Campylobacter jejuni, beyond showing a depletion of MUC2, displayed an increment of MUC1 secretion[84]. Such an increase, observed both in human and pre-clinical studies, highlights a mechanism of host defense aimed at trapping parasites in the mucus, thereby favoring their expulsion. On the other hand, Elmi et al[85] reported an increment of IEB permeability due to TJ alterations in mice infected with Campylobacter jejuni, Escherichia coli and Citrobacter rodentium, that contributed to promote bacterial invasion into host cells and the development of inflammatory process (Table 1).
When considering the morphofunctional alterations of neuromuscular compartment in animal models of intestinal infections, some authors reported a significant increase in SP levels in Cryptosporidium-infected macaque or rats infected with Trichinella spiralis, suggesting a relationship between the SP content and inflammation associated with pathogen invasion as well as a positive correlation between SP levels and the severity of diarrhea[86,87] (Table 1). Current animal models of diverticular disease, based on low-fiber diets, have generated very inconsistent results and/or a significant impairment of the systemic health status[88]. Thus, at present, pre-clinical studies on the histological alterations of IEB and ENS in models of diverticular disease are strongly needed.
Patients with metabolic disorders, including obesity and type 2 diabetes mellitus, often experience GI dysfunctions, such as impaired gastric emptying, infrequent bowel movements and constipation[3]. In this setting, several lines of evidence support the contention that a chronic low-grade systemic inflammatory condition, besides interfering with the metabolic processes, could contribute to alterations of IEB and enteric neuromuscular compartment, which, in turn, could lead to the onset of bowel motor abnormalities.
A recent study showed that obese patients display an increase in IEB permeability, along with a decreased expression of occludin and tri-cellulin as well as an increase in circulating lipopolysaccharide (LPS), an indirect index of intestinal permeability, and ZO-1 levels[6] (Table 2). However, despite these interesting observations, human studies, showing a correlation between altered IEB, changes in the enteric neuromuscular compartment and intestinal motor dysfunctions, are currently lacking. In this respect, pioneering evidence, supporting the relevance of IEB alterations in the pathophysiology of bowel dysmotility in metabolic disorders, comes from pre-clinical studies. For instance, mice with high fat diet (HFD)-induced obesity displayed a decrease in ZO-1, occludin and claudin expression, as well as an increase in circulating LPS levels[89-91]. Likewise, leptin-deficient mice (genetic model of obesity) showed an increased IEB permeability along with morphological changes in villi/crypt length and decreased expression of TJ- and mucus-related genes, that could contribute to the alterations of intestinal motility[92] (Table 2).
| Metabolic disorder | Morphofunctional changes in intestinal epithelial barrier | Morphofunctional changes in enteric neuromuscular compartment | Ref. |
| Human investigations | |||
| Obesity | ↑ Circulating LPS | NA | [6] |
| ↓ Occludin and tri-cellulin immunopositivity | |||
| ↑ ZO-1 | |||
| Diabetes | ↑ Intestinal permeability (urinary excretion of lactulose) | NA | [6] |
| Experimental models | |||
| HFD-induced obese mice | ↓ ZO-1, occludin and claudins | ↓ Nitrergic and VIPergic neurons Altered smooth muscle cell excitability | [89-91,93,94,96,97] |
| ↑ Circulating LPS | ↓ Enteric inhibitory neurotransmission | ||
| ↑ Enteric excitatory tachykininergic neurotransmission | |||
| ↑ SP immunopositivity | |||
| ↑ A2B adenosine receptor expression | |||
| Lep ob/ob mice | ↑ Intestinal permeability | NA | [92] |
| Alterations of villi/crypt length | |||
| ↓ TJs and mucus-related genes | |||
| Ob/ob mice | ↑ Paracellular permeability | ↓ Intestinal motor activity | [95] |
| Altered TJs | ↓ ACh receptors | ||
| Delayed intestinal transit rate | |||
Of note, pre-clinical studies have shown that obese mice are characterized by a remarkable morphofunctional rearrangement of the ENS, such as a decrease in the density of nitrergic and VIPergic neurons and an altered intestinal smooth muscle cell excitability, with consequent impairment of enteric inhibitory neurotransmission[93,94]. In addition, Schacht et al[95] showed that ob/ob mice (a genetic model of diabetes) displayed a decrease in the intestinal transit rate, likely resulting from a loss of acetylcholine receptors in muscle layers and an impaired intestinal motor activity (Table 2). These findings support the view that alterations of the enteric neuromuscular compartment could contribute to bowel dysmotility in metabolic disorders. Consistently with this hypothesis, a recent study showed that HFD mice displayed a marked enhancement of enteric excitatory tachykininergic neurotransmission along with an increase in SP immunoreactivity that contributes to colonic dysmotility[96]. In addition, these authors demonstrated that an increase in colonic adenosine A2B receptor expression modulated the activity of excitatory tachykininergic nerves, participating to the enteric dysmotility associated with obesity[97] (Table 2).
Patients with neuropsychiatric diseases, including PD, AD, ALS, MS, ASD and depression, are often characterized by functional digestive disturbances, including infrequent bowel movements, abdominal distension and constipation[4]. Several lines of evidence suggest that changes in gut microbiota composition, impairments of IEB, intestinal inflammation and rearrangements of the enteric neuromuscular compartment contribute to these bowel motor dysfunctions[4]. In this section, we summarize the most prominent data about the morphofunctional changes in IEB and neuromuscular compartment in the most common central nervous system (CNS) disorders.
Patients with early PD display an increase in IEB permeability, which correlates with staining of intestinal mucosa for Escherichia coli, tissue oxidative stress and enteric α-synuclein accumulation[98]. Clairembault et al[99] reported an alteration of occludin expression in colonic biopsies from PD patients, although the paracellular and transcellular permeability did not differ among PD patients and controls. Others observed an increase in IEB permeability and decreased colonic ZO-1 expression in PD patients with severe intestinal symptoms, thus supporting the view that morphofunctional alterations of IEB could contribute to bowel motor dysfunctions in PD[7]. Of note, changes in intestinal permeability have been documented also in patients with MS and ASD, and in all these settings the respective patterns appear to correlate with the disability status[8,9] (Table 3). Nevertheless, current evidence doesn’t allow to establish a clear casual link between IEB alterations and bowel motor dysfunctions in CNS disorders.
| Central nervous system disorder | Morphofunctional changes in intestinal epithelial barrier | Morphofunctional changes in enteric neuromuscular compartment | Ref. |
| Human investigations | |||
| PD | ↑ Intestinal permeability | ↑ EGC density | [7,98-100] |
| ↓ Occludin and ZO-1 expression | α-syn accumulation in myenteric neurons | ||
| AD | NA | ↑ Aβ, AβPP and p-Tau immunoreactivity in colonic myenteric and submucosal neurons | [102] |
| MS | ↑ Intestinal permeability (urinary mannitol concentration) | ENS fiber disgregation | [8,101] |
| EGC activation | |||
| ASD | Altered intestinal permeability | NA | [9] |
| Experimental models | |||
| Rotenone-induced central dopaminergic neurodegeneration | ↑ Intestinal permeability | α-syn accumulation in myenteric neurons | [7,104] |
| Delayed bowel transit | |||
| LPS-induced central dopaminergic neurodegeneration | ↑ intestinal permeability (lactulose/mannitol ratio and sucralose levels) | α-syn accumulation in myenteric neurons | [7,104] |
| Delayed bowel transit | |||
| 6-OHDA-induced nigrostriatal neurodegeneration | NA | Impairment of colonic cholinergic and tachykininergic motor activity | [105-106] |
| Tg A53T mice (genetic model of PD) | NA | Impairment of colonic cholinergic motor activity | [107] |
| α-syn accumulation in myenteric and submucosal neurons | |||
| APP/PS1 mouse (genetic model of AD) | NA | ↑ Aβ protein precursor, Aβ | [4] |
| Protein and p-Tau | |||
| ↓ nNOS and ChAT | |||
| EGC activation | |||
| Tg CRND8 mice (genetic models of AD) | NA | ↑ Aβ protein precursor in myenteric neurons | [4] |
| Enteric glial activation (GFAP, nestin) | |||
| Enteric neuronal loss | |||
| Smooth muscle cell atrophy | |||
| EAE (animal model of MS) | Abnormal intestinal permeability (plasma Na-F and FITC levels) | Crypt depth and thickness of submucosal and muscular layers | [4] |
| ↓ ZO-1 expression | Enteric glial activation | ||
| Neuronal loss | |||
| Abnormal GI motility | |||
| G93A mice (genetic model of ALS) | ↑ Circulating LPS | NA | [4,103] |
| ↓ ZO-1 and E-cadherin expression | |||
| ↑ Paneth cells number | |||
Besides IEB alterations, several evidence suggest that patients with CNS diseases display alterations of enteric neuromuscular compartment, that could contribute to bowel dysmotility. A recent study has reported an increment of EGCs in colonic biopsies from PD patients[100]. Wunsch et al[101] described the presence of ENS nerve fiber disintegration and EGC activation in MS patients. Others reported an increased α-synuclein as well as β-amyloid (Aβ) protein, β-amyloid protein precursor (AβPP) and phosphorylated Tau (p-Tau) immunoreactivity in colonic myenteric and submucosal neurons from PD and AD patients, respectively, suggesting that morphological changes in ENS and protein accumulation in enteric neurons could contribute to bowel motor dysfunctions in CNS diseases[98,102] (Table 3).
However, current human studies don’t allow to establish a clear casual link among changes in IEB, alterations of neuromuscular compartment and bowel motor dysfunctions in CNS disorders. In this regard, research efforts have been made in pre-clinical models of neurological disorders. Wu et al[103] showed an increase in circulating LPS levels, a decrease in ZO-1 and E-cadherin expression, and an abnormal increase in the number of Paneth cells in ALS mice. Other studies observed the concomitance of abnormal intestinal permeability, enteric α-synuclein accumulation and delayed bowel transit in mice with PD induced by LPS and rotenone[7,104]. Recent pioneering studies in different animal models of PD highlighted relevant rearrangements in the chemical coding of both enteric inhibitory and excitatory neurons, along with impairments of ileum and colonic motor activity, which likely contribute to the decrease in small intestinal and colonic transit rate as well as the efficiency of peristaltic reflex[105-107]. Of note, alterations of enteric neurochemical coding, characterized by a decrease in neuronal nitric oxide synthase (nNOS) and choline acetyltransferase (ChAT), age-related loss of myenteric neurons, EGC activation, intestinal smooth muscle cell atrophy and altered bowel motility have been observed in several animal models of CNS diseases, including AD, MS and ALS[4] (Table 3).
Current data from human and pre-clinical studies suggest that impairments of IEB and enteric neuromuscular compartment might represent a common condition underlying the onset/progression of bowel functional disturbances in both digestive and extra-digestive diseases. Indeed, even though each disease displays different clinical and neuropathological features, patients with IBD, IBS, intestinal infections, diverticular disease as well as metabolic and CNS disorders are characterized by significant molecular and morphofunctional alterations of IEB, ENS and intestinal muscular layers. In particular, changes in TJ protein expression and distribution as well as morphofunctional alterations of EGCs represent a common feature of such disorders, that could contribute to the pathophysiology of bowel motor disturbances. However, the molecular mechanisms underlying the interplays between IEB and enteric neuromuscular compartment as well as their role in the pathophysiology of bowel dysmotility in digestive and extra-digestive disorders remain to be elucidated.
Another important aspect of the current evidence from the literature is that changes in gut microbiota composition could also promote the development of functional bowel disorders[108,109]. Indeed, a number of exhaustive review articles have widely described changes of intestinal microbiota in patients with digestive and neuropsychiatric disorders[110-113]. However, human studies do not allow to establish a causal role between gut dysbiosis and bowel functional disturbances in digestive and extra-digestive diseases. Therefore, an integrated overview about the relationship between alterations in gut microbiota composition and bowel functional disturbances associated with digestive and extra-digestive diseases is missing and requires investigations.
In conclusion, based on current knowledge, some important issues remain to be addressed: (1) What is the role of IEB in bowel motor dysfunctions associated with digestive and extra-digestive diseases? (2) What are the molecular mechanisms underlying the interplay between IEB and enteric neuromuscular compartment in the onset of bowel motor abnormalities associated with digestive and extra-digestive diseases? (3) Can diet influence the alterations of IEB and enteric neuromuscular compartment in digestive and extra-digestive diseases? And (4) What is the impact of gut dysbiosis in bowel motor dysfunctions associated with digestive and extra-digestive diseases?
To address these points, research efforts should be made to characterize simultaneously the alterations of IEB and neuromuscular compartment, regarded as an integrated network, in animal models and patients. Understanding these aspects could pave the way to the identification of novel therapeutic targets and the development of novel pharmacological entities for the management of bowel dysfunctions associated with digestive and extra-digestive disorders. Indeed, at present, there is a lack of therapeutic interventions able to restore IEB integrity and dysfunctions of the enteric neuromuscular compartment. A limited number of clinical studies have reported some benefits in terms of improvement of IEB integrity and restoration of ENS functions, following the administration of probiotics and prebiotics. However, clinical results remain patchy due to heterogenitcity of study protocols, related mainly to the selection of study population, sample size, dosage, formulation and bacterial strains used, as well as the duration of therapy and outcome measures. Therefore, intensive research efforts are needed to deepen the beneficial effects of probiotics and prebiotics observed in clinical studies. Moreover, further research in this area is necessary to identify novel therapeutic targets suitable for strengthening IEB and to treat or prevent GI disorders.
| 1. | Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care. 2017;44:673-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (13)] |
| 2. | Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 1504] [Article Influence: 150.4] [Reference Citation Analysis (1)] |
| 3. | Le Pluart D, Sabaté JM, Bouchoucha M, Hercberg S, Benamouzig R, Julia C. Functional gastrointestinal disorders in 35,447 adults and their association with body mass index. Aliment Pharmacol Ther. 2015;41:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 4. | Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018;136:345-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 5. | Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, Filippi J, Saint-Paul MC, Tulic MK, Verhasselt V, Hébuterne X, Piche T. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 6. | Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, Salem JE, Vaillant JC, Oppert JM, Laugerette F, Michalski MC, Wind P, Rousset M, Brot-Laroche E, Leturque A, Clément K, Thenet S, Poitou C. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol. 2018;246:217-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (4)] |
| 7. | Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ, Kordower JH, Shannon KM, Garssen J, Kraneveld AD, Keshavarzian A. Role of TLR4 in the gut-brain axis in Parkinson's disease: a translational study from men to mice. Gut. 2019;68:829-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (3)] |
| 8. | Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, Mechelli R, Romano S, Fornasiero A, Mattei G, Piras E, Angelini DF, Battistini L, Simmaco M, Umeton R, Salvetti M, Ristori G. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler. 2017;23:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 9. | Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 344] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Veiga-Fernandes H, Mucida D. Neuro-Immune Interactions at Barrier Surfaces. Cell. 2016;165:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (4)] |
| 11. | Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (2)] |
| 12. | Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 561] [Article Influence: 46.8] [Reference Citation Analysis (1)] |
| 13. | Puzan M, Hosic S, Ghio C, Koppes A. Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci Rep. 2018;8:6313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 334] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L, Gulbransen BD. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 2018;315:G473-G483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 16. | Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, Griger J, Fritz P, Fellermann K, Schwab M, Wehkamp J, Stange EF. Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation. 2009;77:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn's disease. Gastroenterol Res Pract. 2013;2013:431231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 18. | Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 791] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 19. | van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H, Johansson MEV, Hansson GC. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68:2142-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 20. | Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dörffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 21. | Xu CM, Li XM, Qin BZ, Liu B. Effect of tight junction protein of intestinal epithelium and permeability of colonic mucosa in pathogenesis of injured colonic barrier during chronic recovery stage of rats with inflammatory bowel disease. Asian Pac J Trop Med. 2016;9:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 22. | Michielan A, D'Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 517] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 23. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 972] [Article Influence: 51.2] [Reference Citation Analysis (1)] |
| 24. | Zwiers A, Fuss IJ, Leijen S, Mulder CJ, Kraal G, Bouma G. Increased expression of the tight junction molecule claudin-18 A1 in both experimental colitis and ulcerative colitis. Inflamm Bowel Dis. 2008;14:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Yamamoto-Furusho JK, Mendivil EJ, Fonseca-Camarillo G. Differential expression of occludin in patients with ulcerative colitis and healthy controls. Inflamm Bowel Dis. 2012;18:E1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Caviglia GP, Dughera F, Ribaldone DG, Rosso C, Abate ML, Pellicano R, Bresso F, Smedile A, Saracco GM, Astegiano M. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Med. 2019;110:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Fischer M, Siva S, Wo JM, Fadda HM. Assessment of Small Intestinal Transit Times in Ulcerative Colitis and Crohn's Disease Patients with Different Disease Activity Using Video Capsule Endoscopy. AAPS PharmSciTech. 2017;18:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 28. | Bassotti G, Villanacci V, Cathomas G, Maurer CA, Fisogni S, Cadei M, Baron L, Morelli A, Valloncini E, Salerni B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum Pathol. 2006;37:1252-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Bernardini N, Segnani C, Ippolito C, De Giorgio R, Colucci R, Faussone-Pellegrini MS, Chiarugi M, Campani D, Castagna M, Mattii L, Blandizzi C, Dolfi A. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J Cell Mol Med. 2012;16:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Ganguli SC, Kamath MV, Redmond K, Chen Y, Irvine EJ, Collins SM, Tougas G. A comparison of autonomic function in patients with inflammatory bowel disease and in healthy controls. Neurogastroenterol Motil. 2007;19:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Bassotti G, Villanacci V, Nascimbeni R, Cadei M, Fisogni S, Antonelli E, Corazzi N, Salerni B. Enteric neuroglial apoptosis in inflammatory bowel diseases. J Crohns Colitis. 2009;3:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 32. | Mazumdar S, Das KM. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol. 1992;87:176-181. [PubMed] |
| 33. | Goode T, O'Connell J, Anton P, Wong H, Reeve J, O'Sullivan GC, Collins JK, Shanahan F. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut. 2000;47:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Menzies JR, McKee R, Corbett AD. Differential alterations in tachykinin NK2 receptors in isolated colonic circular smooth muscle in inflammatory bowel disease and idiopathic chronic constipation. Regul Pept. 2001;99:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Rumessen JJ, Vanderwinden JM, Horn T. Ulcerative colitis: ultrastructure of interstitial cells in myenteric plexus. Ultrastruct Pathol. 2010;34:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | von Boyen GB, Schulte N, Pflüger C, Spaniol U, Hartmann C, Steinkamp M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Morampudi V, Bhinder G, Wu X, Dai C, Sham HP, Vallance BA, Jacobson K. DNBS/TNBS colitis models: providing insights into inflammatory bowel disease and effects of dietary fat. J Vis Exp. 2014;e51297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Schwerbrock NM, Makkink MK, van der Sluis M, Büller HA, Einerhand AW, Sartor RB, Dekker J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Dharmani P, Leung P, Chadee K. Tumor necrosis factor-α and Muc2 mucin play major roles in disease onset and progression in dextran sodium sulphate-induced colitis. PLoS One. 2011;6:e25058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (2)] |
| 41. | Yuan B, Zhou S, Lu Y, Liu J, Jin X, Wan H, Wang F. Changes in the Expression and Distribution of Claudins, Increased Epithelial Apoptosis, and a Mannan-Binding Lectin-Associated Immune Response Lead to Barrier Dysfunction in Dextran Sodium Sulfate-Induced Rat Colitis. Gut Liver. 2015;9:734-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Gomes-Santos AC, Moreira TG, Castro-Junior AB, Horta BC, Lemos L, Cruz DN, Guimarães MA, Cara DC, McCafferty DM, Faria AM. New insights into the immunological changes in IL-10-deficient mice during the course of spontaneous inflammation in the gut mucosa. Clin Dev Immunol. 2012;2012:560817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil. 2005;17:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Park JH, Kwon JG, Kim SJ, Song DK, Lee SG, Kim ES, Cho KB, Jang BI, Kim DH, Sin JI, Kim TW, Song IH, Park KS. Alterations of colonic contractility in an interleukin-10 knockout mouse model of inflammatory bowel disease. J Neurogastroenterol Motil. 2015;21:51-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Kiriukhin SO, Makarova OV. [Morphological changes in the colonic muscular layer and interstitial cells of Cajal in experimental acute ulcerative colitis]. Arkh Patol. 2016;78:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Wang LJ, Tang ZP. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol. 2017;23:4724-4734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Ippolito C, Segnani C, Errede M, Virgintino D, Colucci R, Fornai M, Antonioli L, Blandizzi C, Dolfi A, Bernardini N. An integrated assessment of histopathological changes of the enteric neuromuscular compartment in experimental colitis. J Cell Mol Med. 2015;19:485-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol. 2016;2:77-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 50. | da Silva MV, Marosti AR, Mendes CE, Palombit K, Castelucci P. Submucosal neurons and enteric glial cells expressing the P2X7 receptor in rat experimental colitis. Acta Histochem. 2017;119:481-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Cheng P, Yao J, Wang C, Zhang L, Kong W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol Med Rep. 2015;12:3257-3264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (5)] |
| 52. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 53. | Martínez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F, Santos J, Vicario M. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 54. | Grover M, Camilleri M, Hines J, Burton D, Ryks M, Wadhwa A, Sundt W, Dyer R, Singh RJ. (13) C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil. 2016;28:1114-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 55. | Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 56. | Kong WM, Gong J, Dong L, Xu JR. [Changes of tight junction claudin-1,-3,-4 protein expression in the intestinal mucosa in patients with irritable bowel syndrome]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1345-1347. [PubMed] |
| 57. | Ohgo H, Imaeda H, Yamaoka M, Yoneno K, Hosoe N, Mizukami T, Nakamoto H. Irritable bowel syndrome evaluation using computed tomography colonography. World J Gastroenterol. 2016;22:9394-9399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 58. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 59. | Tong WD, Liu BH, Zhang LY, Zhang SB, Lei Y. Decreased interstitial cells of Cajal in the sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2004;19:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 60. | Ohlsson B, Gustafsson R, Swahn F, Toth E, Veress B, Thorlacius H. Endoscopic full-thickness biopsy, a novel method in the work up of complicated abdominal symptoms. Therap Adv Gastroenterol. 2018;11:1756283X17730747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 62. | Fu R, Chen M, Chen Y, Mao G, Liu S. Expression and clinical significance of 5-HT and 5-HT3R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exp Ther Med. 2019;17:3077-3082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 63. | Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, Akhtar S, Zuo XL, Tan XD, Li YQ. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:20320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (3)] |
| 64. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 65. | O'Malley D, Julio-Pieper M, Gibney SM, Dinan TG, Cryan JF. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress-induced anxiety and depression-like behaviour. Stress. 2010;13:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z, Liu F. MiR-144 Increases Intestinal Permeability in IBS-D Rats by Targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44:2256-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, Mancuso A, Mercade-Loubière M, Salvador-Cartier C, Gillet M, Ferrier L, Loubière P, Dague E, Theodorou V, Mercier-Bonin M. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G420-G429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 68. | Yang B, Zhou XC, Lan C. Impact of the alterations in the interstitial cells of Cajal on intestinal motility in post-infection irritable bowel syndrome. Mol Med Rep. 2015;11:2735-2740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 69. | Wang YB, Ling J, Zhang WZ, Li G, Qiu W, Zheng JH, Zhao XH. Effect of bisacodyl on rats with slow transit constipation. Braz J Med Biol Res. 2018;51:e7372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Lin MJ, Yu BP. Colonic Hypermotility in a Rat Model of Irritable Bowel Syndrome Is Associated with Upregulation of TMEM16A in Myenteric Plexus. Dig Dis Sci. 2018;63:3329-3338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Khan WI, Collins SM. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 2004;26:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 72. | Engevik MA, Yacyshyn MB, Engevik KA, Wang J, Darien B, Hassett DJ, Yacyshyn BR, Worrell RT. Human Clostridium difficile infection: altered mucus production and composition. Am J Physiol Gastrointest Liver Physiol. 2015;308:G510-G524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 73. | Vesterlund S, Karp M, Salminen S, Ouwehand AC. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology. 2006;152:1819-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Bergstrom KS, Guttman JA, Rumi M, Ma C, Bouzari S, Khan MA, Gibson DL, Vogl AW, Vallance BA. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect Immun. 2008;76:796-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Troeger H, Loddenkemper C, Schneider T, Schreier E, Epple HJ, Zeitz M, Fromm M, Schulzke JD. Structural and functional changes of the duodenum in human norovirus infection. Gut. 2009;58:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Karandikar UC, Crawford SE, Ajami NJ, Murakami K, Kou B, Ettayebi K, Papanicolaou GA, Jongwutiwes U, Perales MA, Shia J, Mercer D, Finegold MJ, Vinjé J, Atmar RL, Estes MK. Detection of human norovirus in intestinal biopsies from immunocompromised transplant patients. J Gen Virol. 2016;97:2291-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 77. | Ambrosio MR, Rocca BJ, Ginori A, Barone A, Onorati M, Lazzi S. Long pedunculated colonic polyp with diverticulosis: case report and review of the literature. Pathologica. 2011;103:8-10. [PubMed] |
| 78. | Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Robinson P, Okhuysen PC, Chappell CL, Weinstock JV, Lewis DE, Actor JK, White AC. Substance P expression correlates with severity of diarrhea in cryptosporidiosis. J Infect Dis. 2003;188:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Böttner M, Barrenschee M, Hellwig I, Harde J, Egberts JH, Becker T, Zorenkov D, Wedel T. The enteric serotonergic system is altered in patients with diverticular disease. Gut. 2013;62:1753-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Guagnini F, Valenti M, Mukenge S, Matias I, Bianchetti A, Di Palo S, Ferla G, Di Marzo V, Croci T. Neural contractions in colonic strips from patients with diverticular disease: role of endocannabinoids and substance P. Gut. 2006;55:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Mattii L, Ippolito C, Segnani C, Battolla B, Colucci R, Dolfi A, Bassotti G, Blandizzi C, Bernardini N. Altered expression pattern of molecular factors involved in colonic smooth muscle functions: an immunohistochemical study in patients with diverticular disease. PLoS One. 2013;8:e57023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 83. | Wedel T, Büsing V, Heinrichs G, Nohroudi K, Bruch HP, Roblick UJ, Böttner M. Diverticular disease is associated with an enteric neuropathy as revealed by morphometric analysis. Neurogastroenterol Motil. 2010;22:407-414, e93-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Lindén SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS One. 2008;3:e3952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 85. | Elmi A, Nasher F, Jagatia H, Gundogdu O, Bajaj-Elliott M, Wren B, Dorrell N. Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell E-cadherin and occludin. Cell Microbiol. 2016;18:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 86. | Swain MG, Agro A, Blennerhassett P, Stanisz A, Collins SM. Increased levels of substance P in the myenteric plexus of Trichinella-infected rats. Gastroenterology. 1992;102:1913-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Hernandez J, Lackner A, Aye P, Mukherjee K, Tweardy DJ, Mastrangelo MA, Weinstock J, Griffiths J, D'Souza M, Dixit S, Robinson P. Substance P is responsible for physiological alterations such as increased chloride ion secretion and glucose malabsorption in cryptosporidiosis. Infect Immun. 2007;75:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Patel B, Guo X, Noblet J, Chambers S, Kassab GS. Animal Models of Diverticulosis: Review and Recommendations. Dig Dis Sci. 2018;63:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Nakadate K, Hirakawa T, Tanaka-Nakadate S. Small intestine barrier function failure induces systemic inflammation in monosodium glutamate-induced chronically obese mice. Appl Physiol Nutr Metab. 2019;44:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Ahmad R, Rah B, Bastola D, Dhawan P, Singh AB. Obesity-induces Organ and Tissue Specific Tight Junction Restructuring and Barrier Deregulation by Claudin Switching. Sci Rep. 2017;7:5125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Antonioli L, D'Antongiovanni V, Pellegrini C, Fornai M, Benvenuti L, di Carlo A, van den Wijngaard R, Caputi V, Cerantola S, Giron MC, Németh ZH, Haskó G, Blandizzi C, Colucci R. Colonic dysmotility associated with high-fat diet-induced obesity: Role of enteric glia. FASEB J. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 92. | Nagpal R, Newman TM, Wang S, Jain S, Lovato JF, Yadav H. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. J Diabetes Res. 2018;2018:3462092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 93. | Soares A, Beraldi EJ, Ferreira PE, Bazotte RB, Buttow NC. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol. 2015;15:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Bhattarai Y, Fried D, Gulbransen B, Kadrofske M, Fernandes R, Xu H, Galligan J. High-fat diet-induced obesity alters nitric oxide-mediated neuromuscular transmission and smooth muscle excitability in the mouse distal colon. Am J Physiol Gastrointest Liver Physiol. 2016;311:G210-G220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Schacht S, Masood F, Catmull S, Dolan R, Altabtabaee R, Grow W, Al-Nakkash L. Dietary Genistein Influences Number of Acetylcholine Receptors in Female Diabetic Jejunum. J Diabetes Res. 2017;2017:3568146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Antonioli L, Caputi V, Fornai M, Pellegrini C, Gentile D, Giron MC, Orso G, Bernardini N, Segnani C, Ippolito C, Csóka B, Haskó G, Németh ZH, Scarpignato C, Blandizzi C, Colucci R. Interplay between colonic inflammation and tachykininergic pathways in the onset of colonic dysmotility in a mouse model of diet-induced obesity. Int J Obes (Lond). 2019;43:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Antonioli L, Pellegrini C, Fornai M, Tirotta E, Gentile D, Benvenuti L, Giron MC, Caputi V, Marsilio I, Orso G, Bernardini N, Segnani C, Ippolito C, Csóka B, Németh ZH, Haskó G, Scarpignato C, Blandizzi C, Colucci R. Colonic motor dysfunctions in a mouse model of high-fat diet-induced obesity: an involvement of A2B adenosine receptors. Purinergic Signal. 2017;13:497-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6:e28032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 694] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 99. | Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann MF, Neunlist M, Derkinderen P. Structural alterations of the intestinal epithelial barrier in Parkinson's disease. Acta Neuropathol Commun. 2015;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 100. | Clairembault T, Kamphuis W, Leclair-Visonneau L, Rolli-Derkinderen M, Coron E, Neunlist M, Hol EM, Derkinderen P. Enteric GFAP expression and phosphorylation in Parkinson's disease. J Neurochem. 2014;130:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 101. | Wunsch M, Jabari S, Voussen B, Enders M, Srinivasan S, Cossais F, Wedel T, Boettner M, Schwarz A, Weyer L, Göcer O, Schroeter M, Maeurer M, Woenckhaus M, Pollok K, Radbruch H, Klotz L, Scholz CJ, Nickel J, Friebe A, Addicks K, Ergün S, Lehmann PV, Kuerten S. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol. 2017;134:281-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Puig KL, Lutz BM, Urquhart SA, Rebel AA, Zhou X, Manocha GD, Sens M, Tuteja AK, Foster NL, Combs CK. Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system. J Alzheimers Dis. 2015;44:1263-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Wu S, Yi J, Zhang YG, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. 2015;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 104. | Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. Mov Disord. 2014;29:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 105. | Fornai M, Pellegrini C, Antonioli L, Segnani C, Ippolito C, Barocelli E, Ballabeni V, Vegezzi G, Al Harraq Z, Blandini F, Levandis G, Cerri S, Blandizzi C, Bernardini N, Colucci R. Enteric Dysfunctions in Experimental Parkinson's Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats. J Pharmacol Exp Ther. 2016;356:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 106. | Pellegrini C, Fornai M, Colucci R, Tirotta E, Blandini F, Levandis G, Cerri S, Segnani C, Ippolito C, Bernardini N, Cseri K, Blandizzi C, Haskó G, Antonioli L. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflammation. 2016;13:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 107. | Rota L, Pellegrini C, Benvenuti L, Antonioli L, Fornai M, Blandizzi C, Cattaneo A, Colla E. Constipation, deficit in colon contractions and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy. Transl Neurodegener. 2019;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Enck P, Mazurak N. Dysbiosis in Functional Bowel Disorders. Ann Nutr Metab. 2018;72:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 109. | Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016;14:127-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (2)] |
| 110. | Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 876] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 111. | Nouvenne A, Ticinesi A, Tana C, Prati B, Catania P, Miraglia C, De' Angelis GL, Di Mario F, Meschi T. Digestive disorders and Intestinal microbiota. Acta Biomed. 2018;89:47-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 112. | Grochowska M, Laskus T, Radkowski M. Gut Microbiota in Neurological Disorders. Arch Immunol Ther Exp (Warsz). 2019;67:375-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Passos MDCF, Moraes-Filho JP. INTESTINAL MICROBIOTA IN DIGESTIVE DISEASES. Arq Gastroenterol. 2017;54:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Tang ST S-Editor: Wang J L-Editor: A E-Editor: Zhang YL