Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.511
Peer-review started: October 17, 2017
First decision: November 8, 2017
Revised: December 2, 2017
Accepted: December 5, 2017
Article in press: December 4, 2017
Published online: January 28, 2018
Processing time: 101 Days and 11.2 Hours
The single nucleotide polymorphism (SNP) c.415C>T in exon 3 of NUDT15 affects thiopurine-induced leukopenia in Asian patients with Crohn’s disease. Meanwhile, three additional genetic variants of NUDT15 were reported in patients with acute lymphoblastic leukemia. We evaluated the effects of these additional genetic variants of NUDT15 in patients with inflammatory bowel disease (IBD) treated with thiopurines.
Ninety-six Japanese patients with IBD were enrolled. Genotyping for the NUDT15 and TPMT genes was performed using Custom TaqMan SNP genotyping assays or Sanger sequencing. The changes in white blood cell (WBC) count, mean corpuscular volume (MCV), platelet count, hemoglobin, CRP, amylase, albumin, AST, ALT, and ESR were evaluated.
Genetic variants of exon 1 and exon 3 of NUDT15 were identified in 24 of 96 patients (25.0%). C.52G > A and c.36_37insGGAGTC in exon 1 were found in three patients each. All three patients with c.36_37insGGAGTC in exon 1 were heterozygotes of p.Arg139Cys in exon 3. Eighteen patients had p.Arg139Cys in exon 3 alone. The WBC count gradually decreased after initiation of thiopurine treatment in the mutated cases (n = 24), and was significantly lower at 6, 8, 10, and 16 wk (P = 0.0271, 0.0037, 0.0051, and 0.0185, respectively). The WBC counts were also evaluated in patients with and without prednisolone treatment. In the patients with prednisolone treatment, the WBC count tended to show a greater decrease in the mutated cases, with significant differences at 8 and 10 wk (P = 0.012 and 0.029, respectively). In the patients without prednisolone treatment, the WBC count was significantly lower at 2, 4, 8, and 14 wk in mutated cases (P = 0.0196, 0.0182, 0.0237 and 0.0241, respectively). MCV increased after starting thiopurine treatment in the mutated cases, and was significantly higher at 10 wk (P = 0.0085). Platelet count, hemoglobin, CRP, amylase, albumin, AST, ALT and ESR did not differ significantly between the wild-type and mutated cases. TPMT mutations were not found in any of the patients.
Mutations in exon 1 of NUDT15 also affect thiopurine-induced leukopenia in patients with IBD. To discuss thiopurine-induced leukopenia in more detail, investigation of SNPs in both exon 1 and exon 3 of NUDT15 is needed.
Core tip: Single nucleotide polymorphism (SNP) in NUDT15 c.415C>T in exon 3 affects thiopurine-induced leukopenia in Asian Crohn’s disease patients. Meanwhile, there is a report of additional three genetic variants of NUDT15 in patients with acute lymphoblastic leukemia. We evaluated the effect of these additional genetic variants of NUDT15 on inflammatory bowel disease (IBD) treated with thiopurines. The increase rate of mean corpuscular volume was higher in the variants than the wild, Mutations of NUDT15 in exon 1 also affects thiopurine-induced leukopenia in patients with IBD. To discuss thiopurine-induced leukopenia, investigating SNPs both exons 1 and exon 3 of NUDT15 is needed.
- Citation: Kojima Y, Hirotsu Y, Omata W, Sugimori M, Takaoka S, Ashizawa H, Nakagomi K, Yoshimura D, Hosoda K, Suzuki Y, Mochizuki H, Omata M. Influence of NUDT15 variants on hematological pictures of patients with inflammatory bowel disease treated with thiopurines. World J Gastroenterol 2018; 24(4): 511-518
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.511
The number of patients with inflammatory bowel disease (IBD) are increasing worldwide. As the treatment for ulcerative colitis (UC) and Crohn’s disease (CD), thiopurine drugs are widely used[1]. For UC, thiopurines are used for both steroid-dependent and steroid-resistant cases. For CD, thiopurines are recommended to be used with infliximab for better efficacy and prevention of events such as infusion reaction[2].
The reported major adverse events associated with thiopurines include leukopenia, pancreatitis, hair loss, and liver dysfunction[3,4]. In European descent, this leukopenia is mainly associated with genetic variations of TPMT which encodes thiopurine S-methyltransferase[5]. Meanwhile, in Asian patients, a single nucleotide polymorphism (SNP) in exon 3 of NUDT15 c.415C>T (encoding p.Arg139Cys), was shown to play an important role in thiopurine-induced leukopenia[6-11]. When p.Arg139Cys occurred, the odds ratio of myelosuppression caused by thiopurines was 35.6 (P = 4.88 × 10-94) in Korean patients with CD[6].
Recently, three additional genetic variants of NUDT15 were reported to induce leukopenia in patients with acute lymphoblastic leukemia (ALL)[12]. These three genetic variants were c.36_37insGGAGTC (encoding p.Val18_Val19insGlyVal) and c.52G > A (encoding p.Val18Ile) in exon 1 and c.416G > A (encoding p.Arg139His) in exon 3. However, these three SNPs were not examined in Asian patients with IBD.
In the present study, we investigated the effects of all four SNPs in exon 1 and exon 3 of NUDT15 and their correlations with biochemical parameters. We also analyzed three SNPs in the TPMT gene that are associated with drug responses and commonly performed in Europe.
We enrolled 96 Japanese patients with IBD treated with thiopurines at our hospital between October 2015 and January 2016. These 96 patients comprised 32 females and 64 males with a median age of 28 years at presentation of IBD. Sixty-seven patients had UC and 29 patients had CD (Table 1). The treatment protocols were as follows. All 96 patients were treated with 6-mercaptopurine (6-MP), which was started at a dose of 30 mg daily. Ninety patients received 5-ASA, 55 patients received steroid, and 36 patients received anti-TNF drugs (infliximab, 29; adalimumab, 7) (Table 1). Written informed consent to conduct genetic analysis of NUDT15 and TPMT was obtained from all 96 patients.
| Patient | n |
| Gender (females/males) | 32/64 |
| Median age at presentation | 28 |
| Range | 10-71 |
| UC; CD | 67; 29 |
| Treatment | |
| 6-MP (yes/no) | 96/0 |
| 5-ASA (yes/no) | 90/6 |
| Steroid (yes/no) | 55/41 |
| Anti-TNF drugs (yes/no) | 36/60 |
Adverse events were examined every week for the first month and then every 1-2 mo thereafter. Blood samples were analyzed for white blood cell (WBC) count, hemoglobin, mean corpuscular volume (MCV), platelet count, amylase, lipase, AST, ALT, albumin, CRP, and ESR.
The protocol was approved by the Institutional Review Board of Yamanashi Prefectural Central Hospital.
Peripheral blood samples were obtained from the 96 patients. Buffy coats were isolated by centrifugation of the blood samples at 820 × g at 25 °C for 10 min and stored at -80 °C until required for DNA extraction. Buffy-coat DNA was extracted using a QIAamp® DNA Blood Mini QIAcube Kit (Qiagen, Hilden, Germany) with a QIAcube (Qiagen). The total genomic DNA concentration was determined using a Nano Drop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States) as described previously[13,14].
PCR was performed using genomic DNA as a template and primer pairs flanking the SNP sites in exon 1 (rs869320766, p.Val18_Val19insGlyVal; rs186364861, Val18Ile) and exon 3 (rs116855232, Arg139Cys; rs147390019, Arg139His) of the NUDT15 gene. The PCR products were cleaned up using ExoSAP-IT™ Reagent (Affymetrix, Santa Clara, CA, United States) according to the manufacturer’s instructions. Sequencing was performed with a BigDye Terminator v3.1 (Thermo Fisher Scientific) using forward or reverse primers. The PCR products were purified and subsequently analyzed by a 3500 Genetic Analyzer (Thermo Fisher Scientific)[15,16]. The primer sequences are provided in Table 2.
| Primer | Primer sequence |
| NUDT15 exon1 forward | 5’-CAAAGCACAACTGTAAGCGACT-3’ |
| NUDT15 exon1 reverse | 5’-GAAAGACCCAGCTAGCAAAGAC-3’ |
| NUDT15 exon3 forward | 5’-TTGTATAGCCAAGCAAATGCAAAGC-3’ |
| NUDT15 exon3 reverse | 5’-TCTGTGTCTGGAATACAATTCAATGAC-3’ |
Real-time PCR was conducted in a ViiA7 system (Thermo Fisher Scientific) using TaqMan Genotyping Master Mix (1 ×) (Life Technologies Corp.), forward and reverse primers, and specific probes. SNP genotyping was conducted by the allelic discrimination method. NUDT15 (rs186364861, Val18Ile; rs116855232, Arg139Cys) and TPMT (rs1800462, rs1800460, and rs1142345) genotyping primers and probes were purchased from Thermo Fisher Scientific. NUDT15 SNP typing was validated by the Sanger sequencing results. The GenBank sequences of human NUDT15 (accession number: NP_060753.1) and TPMT (accession number: NP_000358.1) were accessed at the NCBI Reference Sequence Database.
All statistical analyses were performed using R version 3.3.3. The statistical significance of differences in mean values between two cohorts was assessed by Student’s t-test if the variances were equal in an F test, or by the nonparametric Mann-Whitney test if the variances were not equal.
Genetic variants of exon 1 and exon 3 of NUDT15 were identified in 24 of 96 patients (25.0%) (Table 3). All mutated cases were heterozygotes. C.52G > A (p.Val18Ile) in exon 1 was found in three patients (Group A, Table 3). All three patients with c.36_37insGGAGTC (p.Val18_Val19insGlyVal) in exon 1 were heterozygotes for c.415C>T (p.Arg139Cys) in exon 3 (Group B, Table 3).
| Patients | Exon1 | Exon 3 | TPMT |
| Group A (n = 3) | |||
| #1-#3 | c.52G > A (p.Val18Ile) | Wild | Wild |
| Group B (n = 3) | |||
| #4-#6 | c.36_37insGGAGTC (p.Val18 Val19insGlyVal) | c.415C>T (p.Arg139Cys) | Wild |
| Group C (n = 18) | |||
| #7-#24 | Wild | c.415C>T (p.Arg139Cys) | Wild |
| Group D (n = 72) | |||
| #25-#96 | Wild | Wild | Wild |
Eighteen patients had c.415C>T (p.Arg139Cys) in exon 3 alone (Group C, Table 3). The mutations p.Val18Ile and p.Val18_Val19insGlyVal were mutually exclusive. The mutation c.416G > A (p.Arg139His) in exon 3 was not observed in any of the patients.
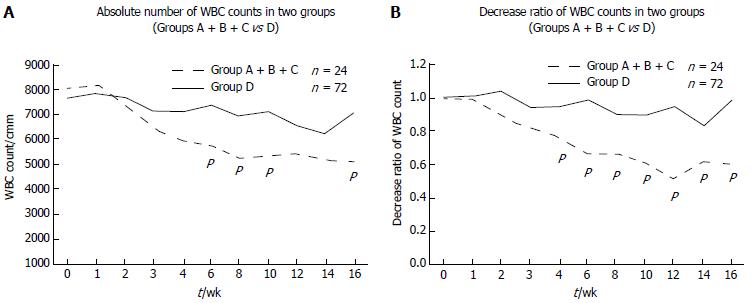
We investigated the changes in the WBC count. The WBC count gradually decreased after thiopurine treatment was started in both the mutated (n = 24) and wild-type (n = 72) cases (Figure 1A). The WBC count in the mutated cases was significantly lower at 6, 8, 10 and 16 wk (P = 0.0271, 0.0037, 0.0051 and 0.0185, respectively). To examine the decrease rates in the WBC count, we set the WBC count at the beginning of thiopurine treatment at 1.0. The decrease rate was higher in the mutated cases (n = 24) than in the wild-type cases (n = 72), and showed significant differences at 4, 6, 8, 10, 12, 14 and 16 wk (P = 0.004, 0.0001, 0.0012, 0.0022, 0.00001, 0.0264, and 0.0031, respectively, Figure 1B).
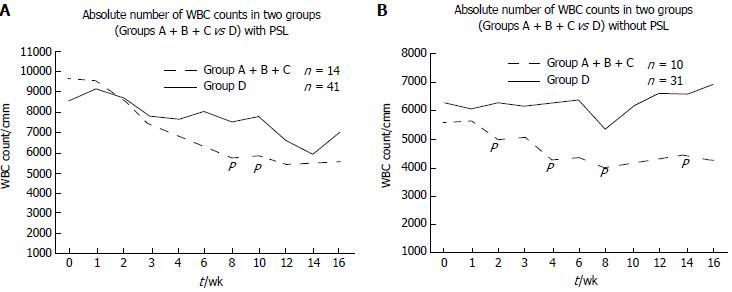
We also analyzed the WBC count in the patients with and without prednisolone treatment. In the patients with prednisolone treatment, the WBC count tended to show a greater decrease in the mutated cases (Group A + B + C), with significant differences at 8 and 10 wk (P = 0.012 and 0.029, respectively; Figure 2A). Prednisolone induced dynamic change of WBC counts which varied in each case. Statistical difference was only obtained at 8 and 10 wk. In the patients without prednisolone treatment, the WBC count was significantly lower at 2, 4, 8 and 14 wk in the mutated cases (Group A + B + C) compared with the wild-type cases (Group D; P = 0.0196, 0.0182, 0.0237, and 0.0241, respectively; Figure 2B).
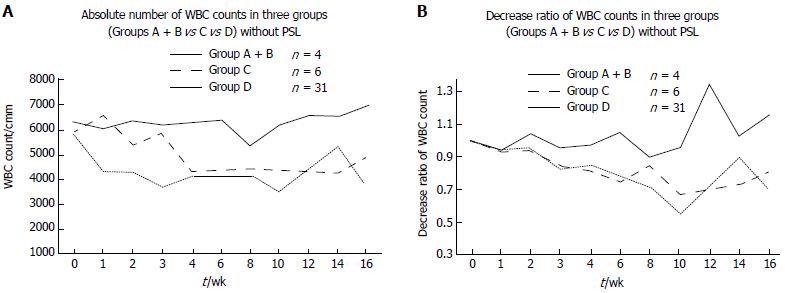
Next, we divided the cases into three categories: Group A + B, Group C and Group D (Figure 3). Group C was already reported cases in IBD with c.415C>T in exon 3 of NUDT15. Group A + B did not show any significant differences from Group C, but had a lower WBC count compared with that in Group D.
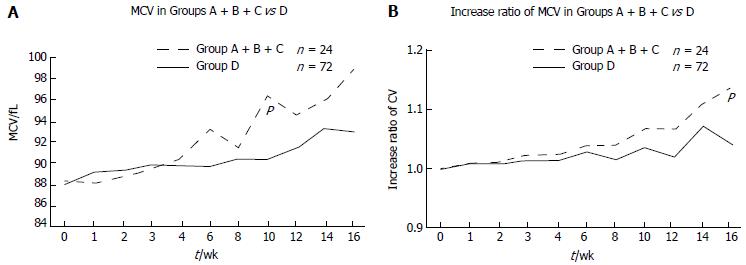
As it is well known that thiopurines increase MCV[17], we analyzed the changes in MCV after initiation of thiopurine treatment. MCV increased after starting 6MP in both the mutated (Group A + B + C) and wild-type (Group D) cases (Figure 4). MCV was significantly higher at 10 wk in the mutated cases compared with the wild-type cases (P = 0.0085; Figure 4A). To analyze the increase rate in MCV, we set the MCV at the beginning of thiopurine treatment at 1.0. The increase rate was higher in the mutated cases compared with the wild-type cases, and the difference was significant at 16 wk (P = 0.00198; Figure 4B).
We also investigated the changes in platelet count, hemoglobin, CRP, amylase, albumin, AST, ALT and ESR, but did not observe any significant differences between the mutated and wild-type cases. TPMT mutations were not observed in any of the 96 patients.
The genotypes of NUDT15 vary worldwide, according to the 1000 Genomes Project (http://www.1000genomes.org/category/frequently-asked-questions/population), 5000 Exomes Project (NHLBI ESP; https://esp.gs.washington.edu/drupal/), and The Exome Aggregation Consortium (ExAC; http://exac.broadinstitute.org). However, detailed information on the different types of NUDT15 SNPs is not necessarily available for individual diseases.
Recently, new genotypes of NUDT15 were reported in patients with ALL, including three genetic variants of NUDT15 that induced leukopenia[12]. Until now, there have been several reports on analyses of NUDT15 in patients with IBD. However, these studies only evaluated one SNP site (c.415C>T, encoding p.Arg139Cys) in exon 3[6,7,18]. Therefore, we analyzed the three additional genetic variants of NUDT15, namely c.36_37insGGAGTC (encoding p.Val18_Val19insGlyVal) and c.52G > A (encoding p.Val18Ile) in exon 1 and c.416G > A (encoding p.Arg139His) in exon 3, in patients with IBD.
In our 96 patients with IBD, we found six cases with exon 1 mutations. Three exon 1 mutated cases with c.36_37insGGAGTC also had the c.415C>T mutation in exon 3. However, the other three exon 1 mutated cases with c.52G > A had the wild-type in exon 3. Consequently, without analysis of exon 1, 3.1% (3 of 96) of the patients at risk of thiopurine-induced leukopenia would have been missed. Regarding the mutations among the 24 patients with SNPs in either exon 1 or exon 3, 6 (25%) had mutations in exon 1. Thus, to fully evaluate thiopurine-induced leukopenia and other side effects, investigation of both exon 1 and exon 3 of NUDT15 is necessary.
We also examined MCV and other variables. MCV tended to be higher in the NUDT15 mutated cases than in the wild-type cases, with a significant difference at 10 wk after the start of thiopurine treatment. We didn’t measure the concentrations of folate and vitamin B12, which affect MCV. Before starting thiopurines, the MCV was in normal range. Previously it is reported that salazosulfapyridine, one of 5ASAs, decreased the absorption of folate, but the only nine patients were taking salazosulphapyridine and the MCV was also in normal range at the initiation of 6MP. MCV was previously shown to be positively correlated with the 6-thioguanine nucleotide (6-TGN) concentration in red blood cells[19]. The role of NUDT15 SNPs is not totally understood. It was reported that NUDT15 inactivates thiopurine metabolites and decreases thiopurine cytotoxicity in vitro, and that patients with defective NUDT15 alleles had excessive levels of thiopurine active metabolites and toxicity[12]. Another study that evaluated 6-TGN levels found that thiopurine-induced leukopenia was independent of the 6-TGN concentration[7]. However, that study measured the total amount of 6-TGN and did not differentiate among thiopurine active metabolites (TGTP and DNA-6TG incorporation). Therefore, further investigations are necessary to examine the correlations of NUDT15 SNPs and thiopurine metabolites, and hence the induction of side effects.
We also tested three SNPs of TMPT, and as previously reported, no TPMT variant was found. So it is not necessarily performed in Asian patients. Our results support previous data that TPMT variant is low in Asian patients.
Our study has several limitations. Firstly, the number of patients was too small to have definite conclusions. For example, the WBC count in patients with prednisolone treatment was not significant at time points other than 8wk and 10 wk. We have not encountered any patients with agranulocytosis and severe hair loss. If we were able to recruit more patients we may be able to obtain definite conclusions. Secondly, our study was a retrospective in nature, and therefore clinical utility of SNP analysis is not assured to avoid complications related to use of 6MP. Thirdly, we only observed the patients for 16 wk after initiation of thiopurine treatment, the long-term effects of thiopurines remain unclear. We are planning further studies to clarify these limitations.
Recently, three other NUDT15 variants, c.101G > C (p.R34T), c.103A > G (p.K35E), and c.37_42delGGAGTC (p.G17_V18del), were reported in ALL patients[20]. By using next-generation sequencing, it will become easier to provide information on NUDT15 SNPs despite changes in SNP numbers. Analysis of NUDT15 should be routinely performed before starting thiopurine treatment in patients with IBD.
Previous study demonstrated that single nucleotide polymorphism (SNP) in NUDT15 c.415C>T (encoding p.Arg139Cys) in exon 3 affects thiopurine-induced leukopenia in Asian patients with inflammatory bowel disease (IBD). In acute lymphoblastic leukemia (ALL), there are other variants of NUDT15 in exon 1 and exon 3. We demonstrated the variants of c.36_37insGGAGTC (encoding p.Val18_Val19insGlyVal) and c.52G > A (encoding p.Val18Ile) in exon 1 also affect the thiopurine-induced leukopenia. To present thiopurine-induced leukopenia and other side effects, checking both exons 1 and exon 3 of NUDT15 is definitely needed.
It is well known that leukopenia is one of the most important adverse effects of thiopurines. To distinguish the high risk group of the adverse effects is clinically very important. Thus we investigated other NUDT15 variants than NUDT15 c.415 C > T in exon 3.
The main of this paper is to investigate other NUDT15 variants than c.415 > T have an effect on hematological pictures including WBC count.
We enrolled 96 Japanese patients with IBD. Genotyping for NUDT15 and TPMT genes was performed using Custom TaqMan SNP genotyping assays or Sanger sequencing. The changes of white blood cell (WBC) count, mean corpuscular volume (MCV), platelet count, hemoglobin, CRP, amylase, albumin, AST, ALT and ESR were analyzed.
In 24 out of 96 patients (25.0%), genetic variants of exons 1 and 3 were identified. C.52G > A and c.36_37insGGAGTC in exon 1 was found in 3 cases each. All 3 cases of c.36_37insGGAGTC in exon 1 had heterozygote of p.Arg139Cys in exon 3. Eighteen patients showed p.Arg139Cys in exon 3 alone. WBC count gradually decreased after thiopurine was started in the mutant (n = 24). The WBC count of the mutant was statistically significantly lower at 6, 8, 10 and 16 wk (P = 0.0271, 0.0037, 0.0051 and 0.0185, respectively). We also analyzed WBC count in the cases with and without prednisolone. In the cases with prednisolone, WBC count tended to decrease more in the mutant cases and was significantly lower at 8 and 10 wk (P = 0.012 and 0.029, respectively). In the cases without prednisolone, WBC count was significantly lower at 2, 4, 8 and 14 wk in the mutant than the wild cases (P = 0.0196, 0.0182, 0.0237 and 0.0241, respectively). MCV increased after starting thiopurine in the mutant. MCV was significantly higher at 10 wk in the mutant than the wild cases (P = 0.0085). Platelet count, hemoglobin, CRP, amylase, albumin, AST, ALT, and ESR was not different between the wild and the mutant cases. TPMT mutation was not found in any of our Japanese patients.
We reported NUDT15 variant in exon 1 also affect thiopurine-induced leukopenia in patients with IBD. Before starting the treatment with thiopurines for patients with IBD, NUDT15 variant in exon 1 and 3 will be routinely performed for preventing adverse events of thiopurines in the near future.
There are other NUDT15 variants which are reported in patients with ALL and near future their role on IBD patients will be investigated.
| 1. | Amin J, Huang B, Yoon J, Shih DQ. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis. 2015;21:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2451] [Article Influence: 153.2] [Reference Citation Analysis (1)] |
| 3. | Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641-649. [PubMed] |
| 4. | Meijer B, Mulder CJ, Peters GJ, van Bodegraven AA, de Boer NK. Efficacy of thioguanine treatment in inflammatory bowel disease: A systematic review. World J Gastroenterol. 2016;22:9012-9021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 5. | Sandborn WJ. Pharmacogenomics and IBD: TPMT and thiopurines. Inflammatory bowel diseases. 2004;10 Suppl 1:S35-S37. [PubMed] |
| 6. | Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, Haritunians T, Ye BD, Kim KJ, Park SH. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 439] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 7. | Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Chiengthong K, Ittiwut C, Muensri S, Sophonphan J, Sosothikul D, Seksan P, Suppipat K, Suphapeetiporn K, Shotelersuk V. NUDT15 c.415C>T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica. 2016;101:e24-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, Pei D, Chen Y, Crews KR, Kornegay N. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Shah SA, Paradkar M, Desai D, Ashavaid TF. Nucleoside diphosphate-linked moiety X-type motif 15 C415T variant as a predictor for thiopurine-induced toxicity in Indian patients. J Gastroenterol Hepatol. 2017;32:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Ailing Z, Jing Y, Jingli L, Yun X, Xiaojian Z. Further evidence that a variant of the gene NUDT15 may be an important predictor of azathioprine-induced toxicity in Chinese subjects: a case report. J Clin Pharm Ther. 2016;41:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 13. | Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya K, Mochizuki H, Omata M. Detection of BRCA1 and BRCA2 germline mutations in Japanese population using next-generation sequencing. Mol Genet Genomic Med. 2015;3:121-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Sakamoto I, Hirotsu Y, Nakagomi H, Ouchi H, Ikegami A, Teramoto K, Amemiya K, Mochizuki H, Omata M. BRCA1 and BRCA2 mutations in Japanese patients with ovarian, fallopian tube, and primary peritoneal cancer. Cancer. 2016;122:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Hirotsu Y, Nakagomi H, Amemiya K, Oyama T, Inoue M, Mochizuki H, Omata M. Intrinsic HER2 V777L mutation mediates resistance to trastuzumab in a breast cancer patient. Med Oncol. 2017;34:3. [PubMed] |
| 16. | Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya K, Oyama T, Mochizuki H, Omata M. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med. 2015;3:459-466. [PubMed] |
| 17. | Kopylov U, Battat R, Benmassaoud A, Paradis-Surprenant L, Seidman EG. Hematologic indices as surrogate markers for monitoring thiopurine therapy in IBD. Dig Dis Sci. 2015;60:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, Endo K, Negoro K, Kinouchi Y, Shimosegawa T. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Meijer B, Wilhelm AJ, Mulder CJJ, Bouma G, van Bodegraven AA, de Boer NKH. Pharmacology of Thiopurine Therapy in Inflammatory Bowel Disease and Complete Blood Cell Count Outcomes: A 5-Year Database Study. Ther Drug Monit. 2017;39:399-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Moriyama T, Yang YL, Nishii R, Ariffin H, Liu C, Lin TN, Yang W, Lin DT, Yu CH, Kham S. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood. 2017;130:1209-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: De Silva AP, Osawa S, Skok P S- Editor: Chen K L- Editor: A E- Editor: Li D