Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2286
Peer-review started: January 3, 2017
First decision: February 10, 2017
Revised: February 13, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 7, 2017
Processing time: 97 Days and 22.4 Hours
Tumor cells induce an immunosuppressive microenvironment which leads towards tumor immune escape. Understanding the intricacy of immunomodulation by tumor cells is essential for immunotherapy. Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme which mediates tumor immune escape in various cancers including hepatocellular carcinoma (HCC). IDO up-regulation in HCC may lead to recruitment of regulatory T-cells into tumor microenvironment and therefore inhibit local immune responses and promote metastasis. HCC associated fibroblasts stimulate natural killer cells dysfunction through prostaglandin E2 and subsequently IDO promotes favorable condition for tumor metastasis. IDO up-regulation induces immunosuppression and may enhance the risk of hepatitis C virus and hepatitis B virus induced HCC. Therefore, IDO inhibitors as adjuvant therapeutic agents may have clinical implications in HCC. This review proposes future prospects of IDO not only as a therapeutic target but also as a prognostic marker for HCC.
Core tip: Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme which mediates tumor immune escape in hepatocellular carcinoma (HCC). IDO up-regulation in HCC may lead to recruitment of regulatory T-cells into tumor microenvironment and therefore inhibit local immune responses and promote metastasis. IDO inhibitors as adjuvant therapeutic agents may have clinical implications in HCC.
- Citation: Asghar K, Farooq A, Zulfiqar B, Rashid MU. Indoleamine 2,3-dioxygenase: As a potential prognostic marker and immunotherapeutic target for hepatocellular carcinoma. World J Gastroenterol 2017; 23(13): 2286-2293
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2286
Hepatocellular carcinoma (HCC) is one of the top five malignancies and the second leading cause of cancer associated deaths worldwide[1,2]. The prognosis of HCC is poor, with overall survival rates of 3%-5%[3]. HCC patients are often diagnosed with advanced disease and therefore, the therapeutic options are limited[4]. HCC is also characterized as an inflammation associated cancer[5]. The infection of chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) are well-known etiologic agents of HCC[6]. The tumor progression has been observed among HCC patients in spite of the presence of tumor specific immune responses[7], thereby suggesting that HCC adopts tumor immune escape mechanisms. Hence, understanding the HCC-induced immunosuppressive mechanism will be important to potentially design HCC targeted immune based therapies[7].
HCV is one of the major causes of HCC[8]. Approximately 700000 people die every year from HCV induced liver diseases[9]. HCV causes both acute and chronic infections. About 15%-45% of HCV infected individuals spontaneously clear this infection without any treatment. Efficient clearance of acute viral infection is mediated by innate and adaptive immune responses[10]. The remaining 55%-85% individuals may develop chronic HCV infection leading to liver cirrhosis[11] and ultimately HCC[12]. Occult HCV infection is characterized as the presence of HCV-RNA in hepatocytes and absence of HCV in serum according to usual tests[13]. Occult cases of HCV infection occur as a consequence of infection in particularly non-hepatic immunologically protected sites or due to infection with HCV variant that is exclusively capable of infecting non-hepatic tissues[14]. Role of occult HCV infection in HCC is still under investigation. The mechanism by which chronic HCV infection progresses and sustains in infected patients is still unclear. Impairment of HCV-specific CD4+ and CD8+ T-cell responses[15-21] and abnormal dendritic cells (DCs) function has been observed[22]. Furthermore, elevated levels of regulatory T (T-reg) cells at the onset of infection have been noted among patients with chronic HCV infection as compared to those who cleared this infection[23-26]. Impaired immune responses may contribute to the development of HCV induced HCC.
Similar to HCV, convincing epidemiological data also support the HBV induced HCC[27]. Approximately 780000 individuals die every year from HBV induced liver diseases[28]. The integration of HBV DNA and expression of HBV protein may have direct influence on cellular function[27]. Increased rates of occult HBV infection have also been detected in HCC patients, specifically among HCV carriers[29,30]. Occult HBV infection may further deteriorate the course of HCV infection[31-33]. Several studies have shown that CD4+ and CD8+ T-cell mediated immune responses define the consequence of HBV acute infection. Chronic infection of HBV is associated with late, weak and transient CD4+ and CD8+ T-cell responses[34,35]. Among patients with chronic HBV infection increase in the number of T-regs and depletion of effector T-cells may inhibit tumor immune surveillance against HBV induced HCC[36].
Recently, an intracellular enzyme, indoleamine 2,3-dioxygenase (IDO/IDO-1) has been reported to play a vital role in regulation of liver immunity and inflammation[37,38]. It is involved in immune homeostasis and immune-related functions in infectious, chronic inflammatory diseases and tumor immune-escaping mechanisms[39-41]. This enzyme also induces maternal tolerance to fetal allograft[42]. IDO initiates the first and rate limiting step of tryptophan degradation of the kynurenine pathway[43]. Deprivation of tryptophan directly affects the cytotoxicity of T cells. In addition, the toxic metabolites produced from tryptophan degradation directly induce T-cell apoptosis in vitro[41]. IDO may inhibit T-cell immunity by inducing differentiation and maturation of T-regs[44]. IDO overexpression induces immunosuppression and tolerance[40]. IDO expression and regulation is mediated by numerous immune and inflammatory factors[45]. IFN-γ is the most potent inducer of IDO[45].
Among HCC cases, IDO overexpression was associated with poor prognosis[46]. IDO was expressed in human HCC derived cells following the stimulation of IFN-γ[46]. IFN-γ stimulated inflammatory mediators are involved in the progression of carcinogenesis[47]. IDO expression was found to be elevated during hepatic inflammation[48,49]. Persistent IDO expression within the liver microenvironment may play a critical role in declining HCV specific T-cell responses[50]. It is also responsible for immune tolerance against HBV[51]. The immunosuppressive role of IDO in tumor immunology has recently begun to be elucidated. The molecular mechanisms underlying tumor-immune escape are currently the topic of emerging interest. In this review we are addressing the immunomodulatory potential of IDO in HCC.
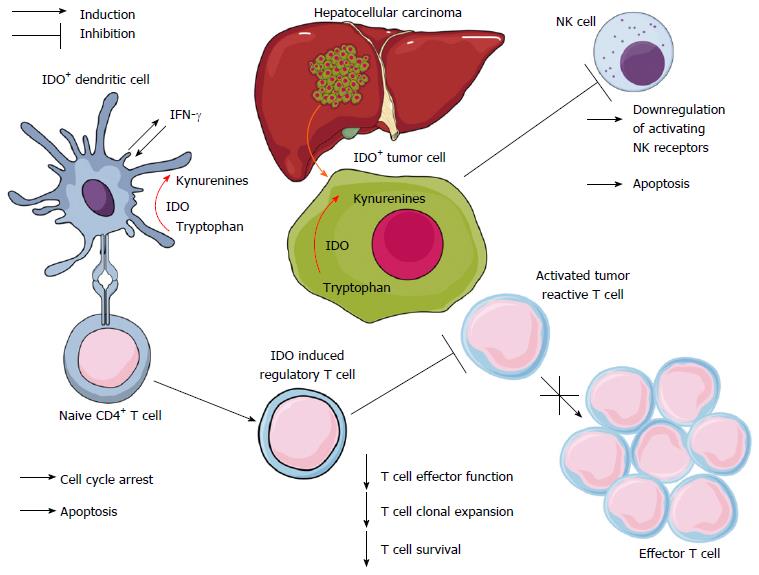
It is recognized that IDO emerging from tumor has the capacity to inhibit the antitumor immunity (Figure 1) and promote metastasis[52,53]. IDO plays a pivotal role in the pathogenesis of HCC (Table 1). Initially, IDO was demonstrated as a necessary enzyme for anticancer immune reactions of tumor infiltrating lymphocytes (TIL). The recurrence-free survival rate of IDO-positive HCC patients was higher than IDO-negative HCC patients[54]. Further studies were proposed to investigate the difference in recurrence-free survival rate between HCC cases with and without TIL[54]. IDO overexpression was associated with poor prognosis in HCC patients[46,55]. IDO expression in HCC cell lines was dependent on IFN-γ. IDO was proposed as a novel favorable prognostic marker and therapeutic target for HCC[46]. HCC associated fibroblasts stimulate NK cell dysfunction through PGE2 (prostaglandin E2) and IDO, and therefore create favorable condition for tumor metastasis[56].
| Year | Investigator | Description |
| 2004 | Ishio et al[54] | IDO is an essential enzyme for anticancer immune reactions of tumor-infiltrating cells. Authors also proposed to clarify the phenotype of IDO-producing cells in PBMC or tumor-infiltrating cells. |
| 2007 | Larrea et al[49] | Both human and chimpanzees express hepatic IDO that is directly correlated with CTLA-4. IDO induction may suppress the T-cell reactivity to viral antigens in chronic HCV infection. Hepatic IDO expression declined in animals who recovered from HCV infection. |
| 2008 | Pan et al[46] | IDO overexpression is significantly linked with metastasis. IDO may be a novel promising prognostic marker and candidate adjuvant therapeutic target for HCC. |
| 2008 | Iwamoto et al[67] | HBV infection helps the IDO induction in response to proinflammatory cytokines, specifically IFN-γ. |
| 2009 | Chen et al[51] | IDO associates with viral load and is accountable for immune tolerance against HBV. IDO inhibition can be a novel approach to break tolerance in chronic HBV infection. |
| 2012 | Li et al[56] | HCC associated fibroblasts stimulate NK cell dysfunction through PGE2 and IDO and therefore create favorable condition for tumor metastasis. |
| 2013 | Higashitani et al[48] | Elevated IDO activity is linked with liver inflammation and fibrosis in chronic HCV patients. In response to the inflammatory stimuli, DCs from patients tend to induce T-regs through IDO dependent mechanism. |
| 2013 | Lin et al[55] | IDO overexpression may be associated with poor prognosis in HCC patients. |
| 2014 | Han et al[57] | CD14+ CTLA4+ DCs suppressed T-cell response through IDO and IL-10. CD14+ DCs expansion induce systemic immunosuppression in HCC. |
| 2014 | Ohtaki et al[68] | IDO inhibition through 1-MT may facilitate in treatment of patients with fulminant hepatitis caused by HBV infection. |
| 2015 | Mukhopadhyay et al[58] | High IDO activity and consequent induction of immunosuppressive T-regs promote immune tolerance in tumor tissue. CB1R system is up-regulated in chemically induced HCC, resulting in the induction of various tumor-promoting genes, including, IDO. |
| 2015 | Lepiller et al[63] | Hepatic IDO performs a dual role during HCV infection by decelerating viral replication and regulating host immune responses. |
| 2015 | Barathan et al[64] | IDO is involved in the onset of immune exhaustion that eventually leads to HCC. |
| 2015 | Asghar et al[65] | IDO overexpression in the cirrhotic livers of HCV-infected patients may contribute to the development of HCC. IDO may also serve as therapeutic target against HCV. |
| 2016 | Shibata et al[62] | Elevation of L-kynurenine may play a critical role in both the early and late phases of liver carcinogenesis. IDO inhibition may act as an emerging approach for the prevention of liver cancer. |
| 2016 | Cheng et al[59] | IDO inhibitors can reverse hepatic CAF-DC regulatory function. IDO may be used as novel immunotherapeutic target for tumor immune escape. |
| 2016 | Salem et al[66] | IDO is overexpressed in IFN-α non-responder patient as compared with responder or healthy control. IDO induced immunosuppression may play role in non-responsiveness of chronic HCV patients to IFN-α based therapy. |
| 2016 | Zhao et al[60] | IDO is up-regulated in the hepatoma cells and serves as a counter regulatory mechanism stimulated by inflammatory response. IDO may be targeted as immunotherapeutic agent. |
| 2016 | Yoshio et al[69] | IDO activation in the early phase followed by a successive increase of chemokines and cytokines is involved in successful HBV clearance in patients with acute hepatitis. IDO possibly acts as a noncytopathic anti-HBV effector. |
| 2016 | Ye et al[61] | HIF-1α/CCL20/IDO axis in HCC is crucial for promoting tumor metastasis through both the induction of epithelial-to-mesenchymal transition and the establishment of an immunosuppressive tumor microenvironment. |
Another study added new insight into HCC induced immunosuppression mechanism which offered a previously unrecognized target for HCC immunotherapy. CD14+ CTLA4+ DCs suppressed T-cell responses through IDO, which may contribute towards the progression of HCC[57]. CB1R (cannabinoid receptor 1) system is up-regulated in chemically induced HCC, resulting in the induction of various tumor-promoting genes, including IDO. Peripheral CB1R blockade might play a role in the treatment of HCC[58].
A comprehensive study explicated the role of hepatic carcinoma associated fibroblasts (CAFs) mediated IDO-producing regulatory DCs. This was the first study to prove that CAFs in HCC recruit DCs and convert them to regulatory DCs through IL-6 mediated STAT3 activation. STAT3 activation in DCs as mediated by CAFs-derived IL-6, is crucial for IDO production. IDO inhibition can reverse the hepatic CAF-DC regulatory function. These findings provide new insights into the mechanism by which CAFs induce tumor immunosuppression and may be targeted by IDO[59]. IDO-1 expression in tumor cells of HCC showed distinct rather than uniform pattern. IDO-1 expression was induced only in tumor cells when co-cultured with both monocytes and T lymphocytes. This cooperation between T lymphocytes and monocytes played a crucial role in the IDO-1 expression in tumor microenvironment in immunocompromised mice as well. IDO up-regulation in hepatoma cells might serve as a counter regulatory mechanism. Further investigations are warranted to understand the clinical benefit of cancer immunotherapy targeting IDO-1 in HCC patients[60]. Recently, the role of IDO in metastasis of HCC has been investigated. Hypoxia inducible factor-1 α (HIF-1α)/CCL20/IDO axis plays a key role in tumor metastasis by promoting epithelial to mesenchymal transition as well as inducing an immunosuppressive microenvironemt[61]. IDO up-regulation may facilitate the progression of liver carcinogenesis. IDO overexpression might be responsible for creating inflammatory and immunosupressive tumor microenvironment. Hence, IDO inhibition might lead to the development of novel therapy for HCC[62].
IDO up-regulation has been reported in human and chimpanzee livers with chronic HCV infection. HCV infection was observed to promote IDO induction in response to the pro-inflammatory cytokines and activated T-cells. IDO was considered as a therapeutic target for HCV infection[49]. IDO activity was elevated in chronic HCV infected patients. Furthermore IDO-DCs from patients induced more T-regs in vitro as compared to healthy controls. T-regs induced by IDO-DCs were decreased with IDO specific inhibitor 1-methyl tryptophan (1-MT). 1-MT could serve as possible approach to improve the immune responses in HCV infection[48].
IDO plays a dichotomous role in HCV infection. Hepatic IDO might be beneficial in acute infection and damaging in chronic infection. HCV infection stimulates the IDO expression. IDO induction during HCV infection down-regulated the viral replication but with the course of disease it had significant inhibitory effect on CD4+ T cell proliferation. IDO induction in acute and chronic HCV infection may provide an insight into novel therapeutic intervention[63]. IDO is one of the factors that are involved in the onset of immune exhaustion during chronic HCV infection, ultimately leading to HCC[64]. Our group has already established that IDO overexpression in the cirrhotic livers of HCV-infected patients might contribute to the development of HCC[65]. IDO may also serve as a novel therapeutic target against HCV[65]. Another study added that IDO expression was higher in IFN-α non-responder patients as compared to responders or healthy controls. These findings opened a new avenue for targeting IDO in HCV therapy[66].
IDO expression and its activity was significantly higher in the chronic HBV infected patients than healthy controls[51]. IDO was responsible for immune tolerance against HBV. IDO has a potential to be used as therapeutic target in chronic HBV infection[51].Mice model study showed that HBV infection facilitated the induction of IDO particularly through IFN-γ in hepatocytes. IDO elevation was responsible for the transduction of cytotoxic T lymphocytes which ultimately inhibit the T-cells responses[67]. Another study reported that IDO in murine fulminant hepatitis model is induced by HBV-specific T lymphocytes. IDO inhibition can reduce liver injury[68]. IDO has an antiviral activity in early phase of HBV infection which is supported by various immune mediators. IDO activity is boosted by NK cells and plasmacytoid DCs through IFN-α and IFN-γ dependent mechanisms[69]. These findings demonstrated the promising role of IDO in HBV therapy.
Although several reviews have been published that explain the immunomodulatory role of IDO in chronic infection and neoplasia[70,71], the aim of the present paper is to summarize the recent data about IDO expression in HCV and HBV induced HCC and its role as a potential therapeutic target in HCC. IDO overexpression was associated with poor prognosis in HCC patients[46,55-62]. However a study by Ishio et al[54] also reported that recurrence-free survival rate of IDO-positive HCC patients was higher than that of IDO-negative HCC patients[54]. Serum tryptophan concentration was also lower in patients with chronic HCV and HBV infection than healthy controls[72]. Moreover an antiviral activity of IDO has been reported in early phase of HBV infection supported by various immune mediators[69]. Hepatic IDO might be beneficial in acute infection and damaging in chronic infection[63]. IDO overexpression in HCV and HBV infection is associated with impairment of immune response and diseases progression[48,49,51,63-68]. In view of the contradictory findings, further studies are warranted to understand the precise role of IDO in HCV and HBV induced HCC.
Recently, IDO inhibitors have been found as adjuvant therapeutic agents with clinical implications in HCC[62]. Investigating an efficient and less toxic IDO inhibitor is emergent. Targeted hepatic IDO inhibition using nanoparticles may provide a better outcome. Tryptophan-2,3-dioxygenase (TDO) has a biochemical activity similar to that of IDO[73,74]. IDO-2 is also a recently discovered isoform of IDO[75,76]. Both IDO-2 and TDO are involved in the degradation of tryptophan[77]. Future studies should explore the role of IDO as well as IDO-2 and TDO in HCC. Therapeutics of IDO are unquestionable but its potential as prognostic biomarker may also have significant outcomes.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4300] [Article Influence: 226.3] [Reference Citation Analysis (2)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20723] [Article Influence: 1883.9] [Reference Citation Analysis (23)] |
| 3. | Schmidt S, Follmann M, Malek N, Manns MP, Greten TF. Critical appraisal of clinical practice guidelines for diagnosis and treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1779-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, Klempnauer J, Galanski M, Manns MP. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Hepatitis Viruses. In. France: World Health Organization, International Agency for Research on Cancer (IARC) 1994; . |
| 7. | Korangy F, Höchst B, Manns MP, Greten TF. Immune responses in hepatocellular carcinoma. Dig Dis. 2010;28:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:105-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9742] [Article Influence: 695.9] [Reference Citation Analysis (0)] |
| 10. | Koziel MJ. Cellular immune responses against hepatitis C virus. Clin Infect Dis. 2005;41 Suppl 1:S25-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. Hepatitis C. In: Fact Sheet. Edited by WHO. USA: WHO; 2016. . |
| 12. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3129] [Article Influence: 208.6] [Reference Citation Analysis (0)] |
| 13. | Rezaee-Zavareh MS, Hadi R, Karimi-Sari H, Hossein Khosravi M, Ajudani R, Dolatimehr F, Ramezani-Binabaj M, Miri SM, Alavian SM. Occult HCV Infection: The Current State of Knowledge. Iran Red Crescent Med J. 2015;17:e34181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Attar BM, Van Thiel D. A New Twist to a Chronic HCV Infection: Occult Hepatitis C. Gastroenterol Res Pract. 2015;2015:579147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Koziel MJ, Dudley D, Wong JT, Dienstag J, Houghton M, Ralston R, Walker BD. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339-3344. [PubMed] |
| 16. | Koziel MJ, Dudley D, Afdhal N, Grakoui A, Rice CM, Choo QL, Houghton M, Walker BD. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Invest. 1995;96:2311-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | He XS, Rehermann B, López-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692-5697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Schirren CA, Jung MC, Gerlach JT, Worzfeld T, Baretton G, Mamin M, Hubert Gruener N, Houghton M, Pape GR. Liver-derived hepatitis C virus (HCV)-specific CD4(+) T cells recognize multiple HCV epitopes and produce interferon gamma. Hepatology. 2000;32:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 19. | Penna A, Missale G, Lamonaca V, Pilli M, Mori C, Zanelli P, Cavalli A, Elia G, Ferrari C. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: functional characterization in patients with chronic hepatitis C. Hepatology. 2002;35:1225-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Rico MA, Quiroga JA, Subirá D, Garcia E, Castañón S, Sällberg M, Leroux-Roels G, Weiland O, Pardo M, Carreño V. Features of the CD4+ T-cell response in liver and peripheral blood of hepatitis C virus-infected patients with persistently normal and abnormal alanine aminotransferase levels. J Hepatol. 2002;36:408-416. [PubMed] |
| 21. | Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, Irving WL, Robins RA. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Perrella A, Vitiello L, Atripaldi L, Conti P, Sbreglia C, Altamura S, Patarino T, Vela R, Morelli G, Bellopede P. Elevated CD4+/CD25+ T cell frequency and function during acute hepatitis C presage chronic evolution. Gut. 2006;55:1370-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Heeg MH, Ulsenheimer A, Grüner NH, Zachoval R, Jung MC, Gerlach JT, Raziorrouh B, Schraut W, Horster S, Kauke T. FOXP3 expression in hepatitis C virus-specific CD4+ T cells during acute hepatitis C. Gastroenterology. 2009;137:1280-1288.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR, Tester IA, Wang CC, Culbertson N, Vandenbark AA, Rosen HR. Functional suppression by FoxP3+CD4+CD25(high) regulatory T cells during acute hepatitis C virus infection. J Infect Dis. 2008;197:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 434] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 27. | Chemin I, Zoulim F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. 2009;286:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | World Health Organization. Hepatitis B. In: Fact Sheet. Edited by Organization WH. USA: WHO; 2016. . |
| 29. | Koike K, Shimotouno K, Okada S, Okamoto H, Hayashi N, Ueda K, Kaneko S, Koike K, Yokosuka O, Chiba T. Survey of hepatitis B virus co-infection in hepatitis C virus-infected patients suffering from chronic hepatitis and hepatocellular carcinoma in Japan. Jpn J Cancer Res. 1999;90:1270-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 31. | Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Stanzione M, Filippini P, Felaco FM, Piccinino F. Isolated anti-HBc in chronic hepatitis C predicts a poor response to interferon treatment. J Med Virol. 2001;65:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Squadrito G, Pollicino T, Cacciola I, Caccamo G, Villari D, La Masa T, Restuccia T, Cucinotta E, Scisca C, Magazzu D. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer. 2006;106:1326-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Mrani S, Chemin I, Menouar K, Guillaud O, Pradat P, Borghi G, Trabaud MA, Chevallier P, Chevallier M, Zoulim F. Occult HBV infection may represent a major risk factor of non-response to antiviral therapy of chronic hepatitis C. J Med Virol. 2007;79:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442-3449. [PubMed] |
| 35. | Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047-1058. [PubMed] |
| 36. | Zhang HH, Mei MH, Fei R, Liu F, Wang JH, Liao WJ, Qin LL, Wei L, Chen HS. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat. 2010;17 Suppl 1:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 37. | Ito H, Hoshi M, Ohtaki H, Taguchi A, Ando K, Ishikawa T, Osawa Y, Hara A, Moriwaki H, Saito K. Ability of IDO to attenuate liver injury in alpha-galactosylceramide-induced hepatitis model. J Immunol. 2010;185:4554-4560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, Nakamura N, Ohtaki H, Ito H, Tanaka T, Tsurumi H. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PLoS One. 2013;8:e73404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1801] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 40. | Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 568] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 41. | Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 42. | Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1939] [Cited by in RCA: 1922] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 43. | Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 593] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 44. | Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177:2391-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, Huang W, Li JJ, Song HF, Xia JC. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1664] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 48. | Higashitani K, Kanto T, Kuroda S, Yoshio S, Matsubara T, Kakita N, Oze T, Miyazaki M, Sakakibara M, Hiramatsu N. Association of enhanced activity of indoleamine 2,3-dioxygenase in dendritic cells with the induction of regulatory T cells in chronic hepatitis C infection. J Gastroenterol. 2013;48:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Larrea E, Riezu-Boj JI, Gil-Guerrero L, Casares N, Aldabe R, Sarobe P, Civeira MP, Heeney JL, Rollier C, Verstrepen B. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J Virol. 2007;81:3662-3666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 51. | Chen YB, Li SD, He YP, Shi XJ, Chen Y, Gong JP. Immunosuppressive effect of IDO on T cells in patients with chronic hepatitis B*. Hepatol Res. 2009;39:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1642] [Cited by in RCA: 1770] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 53. | Sakurai K, Amano S, Enomoto K, Kashio M, Saito Y, Sakamoto A, Matsuo S, Suzuki M, Kitajima A, Hirano T. [Study of indoleamine 2,3-dioxygenase expression in patients with breast cancer]. Gan To Kagaku Ryoho. 2005;32:1546-1549. [PubMed] |
| 54. | Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Lin L, Yang DH, Huang Y, Li XH, Zhang QL, Liu X, Liang JK, Zhong KB, He H. [Relationship between the expressions of indoleamine 2, 3-dioxygenase in hepatocellular carcinoma and clinicopathological parameters]. Zhonghua Yixue Zazhi. 2013;93:2186-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 57. | Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 58. | Mukhopadhyay B, Schuebel K, Mukhopadhyay P, Cinar R, Godlewski G, Xiong K, Mackie K, Lizak M, Yuan Q, Goldman D. Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology. 2015;61:1615-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 60. | Zhao Q, Wang PP, Huang ZL, Peng L, Lin C, Gao Z, Su S. Tumoral indoleamine 2, 3-dioxygenase 1 is regulated by monocytes and T lymphocytes collaboration in hepatocellular carcinoma. Oncotarget. 2016;7:14781-14790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, Sun X, Li GG, Hu QD, Fu QH. Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis. Cancer Res. 2016;76:818-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 62. | Shibata Y, Hara T, Nagano J, Nakamura N, Ohno T, Ninomiya S, Ito H, Tanaka T, Saito K, Seishima M. The Role of Indoleamine 2,3-Dioxygenase in Diethylnitrosamine-Induced Liver Carcinogenesis. PLoS One. 2016;11:e0146279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Lepiller Q, Soulier E, Li Q, Lambotin M, Barths J, Fuchs D, Stoll-Keller F, Liang TJ, Barth H. Antiviral and Immunoregulatory Effects of Indoleamine-2,3-Dioxygenase in Hepatitis C Virus Infection. J Innate Immun. 2015;7:530-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Barathan M, Gopal K, Mohamed R, Ellegård R, Saeidi A, Vadivelu J, Ansari AW, Rothan HA, Ravishankar Ram M, Zandi K. Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes. Apoptosis. 2015;20:466-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Asghar K, Ashiq MT, Zulfiqar B, Mahroo A, Nasir K, Murad S. Indoleamine 2,3-dioxygenase expression and activity in patients with hepatitis C virus-induced liver cirrhosis. Exp Ther Med. 2015;9:901-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Salem ML, Barakat LA, Elnakeeb NA, Zeidan AA. Some biochemical markers and expression of indoleamine-2, 3-dioxygenase in Egyptian patients with chronic Hepatitis C. J Bio Appl Res. 2016;2:7. |
| 67. | Iwamoto N, Ito H, Ando K, Ishikawa T, Hara A, Taguchi A, Saito K, Takemura M, Imawari M, Moriwaki H. Upregulation of indoleamine 2,3-dioxygenase in hepatocyte during acute hepatitis caused by hepatitis B virus-specific cytotoxic T lymphocytes in vivo. Liver Int. 2009;29:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Ohtaki H, Ito H, Ando K, Ishikawa T, Hoshi M, Ando T, Takamatsu M, Hara A, Moriwaki H, Saito K. Kynurenine production mediated by indoleamine 2,3-dioxygenase aggravates liver injury in HBV-specific CTL-induced fulminant hepatitis. Biochim Biophys Acta. 2014;1842:1464-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Yoshio S, Sugiyama M, Shoji H, Mano Y, Mita E, Okamoto T, Matsuura Y, Okuno A, Takikawa O, Mizokami M. Indoleamine-2,3-dioxygenase as an effector and an indicator of protective immune responses in patients with acute hepatitis B. Hepatology. 2016;63:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med (Berl). 2008;86:145-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985-6991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 72. | Cozzi A, Zignego AL, Carpendo R, Biagiotti T, Aldinucci A, Monti M, Giannini C, Rosselli M, Laffi G, Moroni F. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Thackray SJ, Mowat CG, Chapman SK. Exploring the mechanism of tryptophan 2,3-dioxygenase. Biochem Soc Trans. 2008;36:1120-1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Rafice SA, Chauhan N, Efimov I, Basran J, Raven EL. Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Biochem Soc Trans. 2009;37:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 76. | Löb S, Königsrainer A, Zieker D, Brücher BL, Rammensee HG, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 77. | Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Rezaee-Zavareh MS, Shimizu Y S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF