Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2294
Peer-review started: November 29, 2016
First decision: January 10, 2017
Revised: February 6, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: April 7, 2017
Processing time: 129 Days and 9.4 Hours
To clarify the roles of TWEAK and its receptor Fn14 in 5-fluorouracil (5-FU)-induced diarrhea.
Diarrhea was induced in wild-type (WT), Fn14 knockout (KO), and IL-13 receptor (IL-13R)α1 KO BALB/c mice using a single injection of 5-FU. Histological analysis, cytokine analysis, and flow cytometry was performed on ileal tissues and cells. Murine colon carcinoma-bearing mice were co-treated with an anti-TWEAK antibody and 5-FU. Embryonic fibroblast response to cytokines was also analyzed.
5-FU induced high Fn14 expression in epithelial cells. The severity of 5-FU-induced diarrhea was lower in Fn14 KO mice compared with WT mice. Administration of anti-TWEAK antibody reduced 5-FU-induced diarrhea without affecting the antitumor effects of 5-FU in vivo. 5-FU-induced expression of IL-13, IL-17A, TNF-α, and IFN-γ in the ileum was Fn14 dependent. The severity of 5-FU-induced diarrhea was lower in IL-13Rα1 KO mice, indicating major role for IL-13 signaling via IL-13Rα1 in pathogenesis. We found that IL-13Rα2, an IL-13 neutralizing/cell protective receptor, was strongly induced by IL-33 in vitro and in vivo. IL-13Rα2 was upregulated in the ileum of 5-FU-treated Fn14 KO mice. Thus, the deletion of Fn14 upregulated IL-13Rα2 expression, which reduced IL-13 expression and activity.
Disruption of the TWEAK/Fn14 pathway affects several interconnected pathways, including those associated with IL-13, IL-33, and IL-13Rα2, to attenuate 5-FU-induced intestinal side effects.
Core tip: IL-13 signaling via IL-13 receptor (IL-13R)α1 plays a central role in developing diarrhea, a major side effect of 5-fluorouracil (5-FU). Disruption of the TWEAK/Fn14 pathway alleviated diarrhea by downregulating expression of IL-13 and upregulating expression of IL-13Rα2, a decoy IL-13 receptor. The IL-13Rα2 was induced by IL-33 in mesenchymal cells of 5-FU-treated intestines in vivo and fibroblasts in vitro. IL-33 expression was independent of TWEAK/Fn14 signaling, and its cell protective function in 5-FU-treated mice was enhanced in the absence of Fn14. Disruption of the TWEAK/Fn14 pathway affects several interconnected pathways to attenuate 5-FU-induced intestinal side effects.
- Citation: Sezaki T, Hirata Y, Hagiwara T, Kawamura YI, Okamura T, Takanashi R, Nakano K, Tamura-Nakano M, Burkly LC, Dohi T. Disruption of the TWEAK/Fn14 pathway prevents 5-fluorouracil-induced diarrhea in mice. World J Gastroenterol 2017; 23(13): 2294-2307
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2294
The chemotherapeutic antimetabolite agent 5-fluorouracil (5-FU) is used to treat numerous cancer types. However, chemotherapy with 5-FU often is associated with severe gastrointestinal side effects, such as diarrhea and anorexia. These adverse effects can be difficult to manage and lead to discontinuation of chemotherapy. Therefore, strategies to reduce or prevent adverse effects are needed to improve the efficacy of this cancer treatment to allow the patient to take the full course of treatment.
Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) is a member of the TNF superfamily. TWEAK mRNA is commonly expressed in several types of leukocytes, but it has been detected in other cell types, including endothelial cells and astrocytes. Fibroblast growth factor inducible 14 (Fn14) is the smallest member of the TNF receptor superfamily and the only known signaling receptor for TWEAK[1]. Fn14 expression levels are relatively low in healthy tissues; however, Fn14 expression is strongly upregulated in injured tissues. TWEAK/Fn14 signaling can activate several downstream pathways, including the nuclear factor-κB (NFκB), mitogen-activated protein kinase, and phosphatidylinositol 3-kinase/protein kinase B pathways[2]. The TWEAK/Fn14 pathway is involved in the repair of injured tissue; however, excessive activation contributes to tissue damage and pathological tissue remodeling[2]. Thus, the TWEAK/Fn14 pathway is a potential drug target for various diseases, including those that affect intestines, kidneys, liver, joints, nervous system, skeletal muscles, and vasculature[1].
This study focuses on the role of TWEAK/Fn14 in intestinal disease. Previously, we showed that Fn14 is expressed in injured epithelial cells (ECs). Treatment with a TWEAK-blocking antibody or knocking out TWEAK considerably ameliorated acute colitis in mice by reducing inflammation and limiting chemokine and matrix metalloproteinase expression in ECs[3]. Fn14 also mediates interleukin (IL)-13- and TNF-α-induced cell death in ECs[4]. TWEAK and TNF-α synergistically activate the canonical NF-κB pathway, which aggravates tissue damage in acute colitis[5]. In chronic colitis models, we showed that TWEAK and IL-13 cooperatively induce the expression of thymic stromal lymphopoietin in ECs. This expression is a key mechanism in the development of fibrosis[6]. In the context of γ-irradiation-induced injury, both TWEAK and Fn14 KO mice had more regenerating microcolonies in intestinal crypts than wild-type (WT) mice[3]. However, to our knowledge, no studies have addressed if TWEAK/Fn14 is involved in chemotherapy-induced damage of normal intestinal mucosa.
IL-33 is a member of the IL-1 family[7]. It is expressed constitutively in ECs of healthy intestine and is localized to the nucleus. Nucleus-localized IL-33 functions as a transcription factor[8,9] where it contributes to mucosal protection in various colitis models[10-13]. After EC injury or death, IL-33 undergoes cleavage and is released into the extracellular space[14]. Released IL-33 induces the expression of T helper 2 cytokines, such as IL-5 and IL-13, in various cells, including T helper 2 cells, group 2 innate lymphoid cells, eosinophils, and basophils[15]. These responses play major roles in parasite clearance and allergic reactions. The mechanisms behind the dual role of IL-33 as a protector of mucosal barriers and an alarmin[15] are not fully understood.
In this report, we investigated the effect of blocking TWEAK/Fn14 signaling on chemotherapy-induced intestinal side effects. Disruption of the TWEAK/Fn14 pathway using genetic or pharmacological approaches reduced 5-FU-induced diarrhea without impacting the antitumor effect of 5-FU. We also propose a novel protective mechanism for IL-33 in mucosal tissues.
Male BALB/c mice and Fn14 KO mice[16] with a BALB/c background that were 8-12-wk-old were purchased from Clea Japan, Inc. (Tokyo, Japan). IL-13 receptor (IL-13R) α1 KO mice were generated from BALB/c mice in our facility using a CRISPR/Cas9-mediated genome-editing system. All experimental protocols were approved by the institutional animal care and use committee of the National Center for Global Health and Medicine, Japan (#16086).
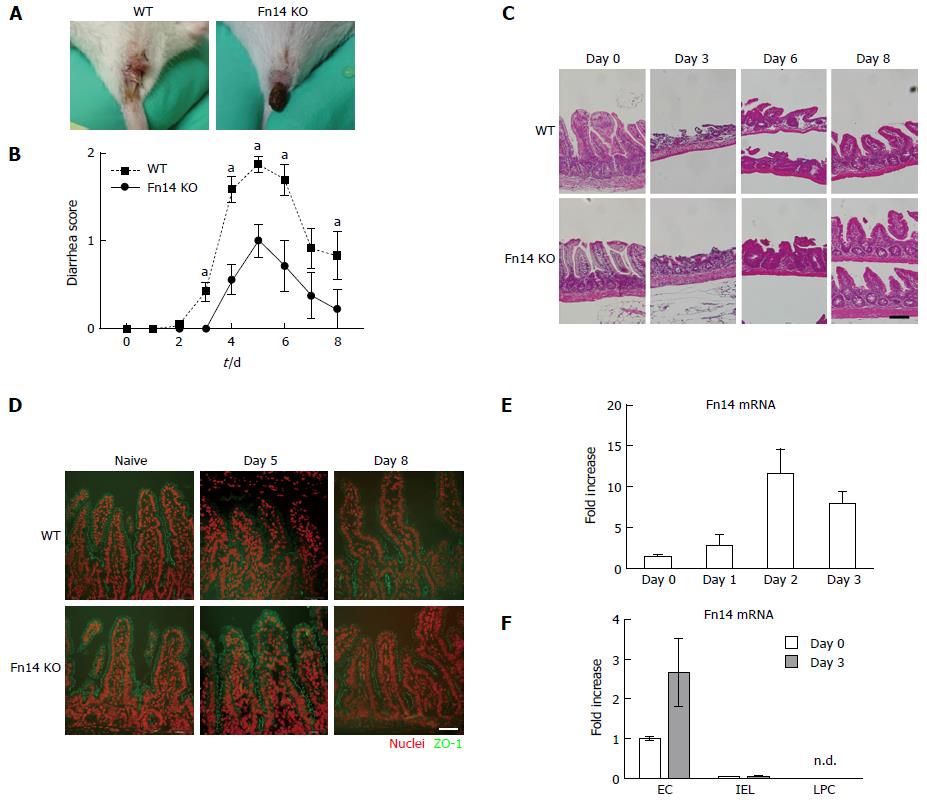
An intraperitoneal injection of 5-FU (250 mg/kg body weight, Sigma. Tokyo Japan) in phosphate-buffered saline pH 7.4 (PBS, Nacalai tesque. Kyoto, Japan) was administered to mice on day 0. The mice were monitored for diarrhea once a day. To assess diarrhea severity, individual mice were photographed daily during defecation and scoring of the photos was performed blindly to guarantee the objectivity of the scoring system (Figure 1A). Severity was scored as follows: 0, normal; 1, wet and unformed stool; 2, watery stool.
Total RNA of whole ileum was prepared using RNA-Bee RNA isolation solvent (Tel-Tests, Inc, Friendswood, TX). RNA from purified lamina propria cells (LPCs) or ECs was extracted using the RNeasy micro kit (QIAGEN, Tokyo, Japan). Complementary DNA (cDNA) was synthetized from the RNA using High Capacity cDNA Reverse Transcription Kits (Life Technologies). The resulting cDNA samples were used to perform quantitative real-time PCR reactions using a 7900HT Fast Real-Time PCR System (Applied Biosystems, Warrington, England). Primers and probes for murine genes were purchased from Applied Biosystems. The following TaqMan Gene Expression Assays were used in this study: TWEAK (Mm02583406_s), Fn14 (Mm00489103_m1), IL-33 (Mm00505403_m1), IL-13Rα1 (Mm00446726_m1), IL-13Rα2 (Mm00515166_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (4352932E). All experiments were performed according to their respective manufacturer’s protocols unless otherwise indicated. Results were normalized to GAPDH mRNA as an internal control. Threshold cycle numbers (Ct) were determined using Sequence Detector Software (version 2.3; Applied Biosystems) and transformed using the ΔCt/∆∆Ct method as described by the manufacturer.
Ileum tissues (the distal third of the small intestine) were opened longitudinally on filter paper and fixed overnight with Mildform (WAKO, Osaka, Japan). Tissues were rolled and embedded in paraffin. Paraffin-embedded tissues were sliced into 4-µm thick sections and stained with hematoxylin and eosin. To detect proliferating cells, sections were autoclaved at 121 °C for 5 min in 10 mmol/L citrate (pH 6.0) and stained with anti-Ki67 antibody (Vector Laboratories, Burlingame, CA, United States) and Envision+ dual Link System -HRP (DAKO, Glostrup, Denmark). To detect apoptotic cells, TdT-mediated dUTP nick end labeling was applied using DeadEndTM Colorimetric TUNEL System (Promega, Tokyo, Japan). For IL-33 and IL-13Rα2 staining, acetone-fixed frozen sections were used. Sections were incubated with anti-IL-33 antibody (1:20 dilution, R&D Systems) or anti-IL-13Rα2 antibody (1:50, R&D Systems) then incubated with FITC-conjugated anti-goat IgG antibody (1:200) or Alexa Fluor 488-conjugated anti-rat IgG antibody (1:300, Life Technologies), respectively. Zonula occludens-1 (ZO-1) immunohistochemistry was performed as described previously[4]. Briefly, cryosections were fixed with 4% paraformaldehyde, incubated with anti-ZO-1 antibody (1:25, Invitrogen, Yokohama, Japan) for 2h at RT, then incubated with FITC-conjugated anti-rabbit antibody (1:100, Santa Cruz Biotechnology, TX, United States) Images were collected using a BX50 fluorescence microscope (Olympus, Tokyo) with a DP72-cellSens Standard image capture system (Olympus, Tokyo). Sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI, Sigma). When images with green signals were merged, the blue DAPI signal was converted to red for clarity. Image merging was performed using Adobe Photoshop CS5 version 12.1. To quantify the fluorescence signal, images were collected using a fixed exposure condition, and the signal was measured using ImageJ software (NIH). The IL-13Rα2-positive area in ileal tissue was normalized to the longitudinal length of the measured intestine in the image’s field of view. In cell culture, IL-13Rα2-positive area was normalized to the number of nuclei (cells) in the measured area in the image’s field of view. In some in vitro experiments, determination of IL-13Rα2-positive and -negative cells was definitive. Those data are presented as the percent of positive cells for each experimental condition when more than 50 cells were observed.
Mice were inoculated subcutaneously in the left flank with 1 × 107 CT26 murine colon carcinoma cells 7 d prior to day 0. Tumor volume (calculated as volume = 0.52 × length × width2) was monitored throughout the experiment. Starting at 3 d prior to day 0, treatment with murine anti-TWEAK antibody (10 mg/kg; P2D10; Biogen, Cambridge, MA, United States) or control antibody (10 mg/kg, anti-hen egg lysozyme, mIgG2a, Biogen) was initiated. Antibodies were intraperitoneally injected every other day throughout the study period. On day 0, 250 mg/kg 5-FU was injected intraperitoneally.
The murine colon carcinoma cell line CT26 was obtained from American Type Culture Collection. CT26 cells were maintained in RPMI1640 medium (Sigma, Tokyo, Japan) with 10% fetal bovine serum. Mouse embryonic fibroblasts (MEFs) were cultured from WT BALB/c mice. MEFs were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. For IL-13Rα2 induction in MEFs, 8-well chamber slides (Matsunami glass, Kishiwada, Japan) were inoculated with 3 × 103 cells per well and cultured with IL-13 (40 ng/mL, R&D Systems, Minneapolis, MN) or IL-33 (20 ng/mL, R&D Systems) for 3 d. Cells were fixed with methanol at -20 °C for 20 min, blocked with BLOCKACE (DS Pharma Biomedical, Osaka, Japan), incubated with anti-IL-13Rα2 antibody (R&D Systems, Minneapolis, MN) for 1 h, then incubated with Alexa Fluor 488-conjugated anti-rabbit IgG antibody (Life Technologies, Tokyo, Japan) for 1 h. Nuclei were visualized using DAPI staining. Images were collected using a BX50 fluorescent microscope (OLYMPUS, Tokyo, Japan).
To dissociate ECs, ileal tissues were rinsed with PBS, suspended in 2 mmol/L EDTA in PBS, and stirred for 20 min at 37 °C. Single cell suspensions were stained using phycoerythrin (PE)-conjugated anti-EpCAM antibody (BioLegend, San Diego, CA, United States) and PE-Cy7-conjugated anti-CD45 antibody (BioLegend). Cells were sorted using a MoFlo XDP cell sorter (Beckman Coulter, Tokyo, Japan). ECs were EpCAM+ CD45-, and intraepithelial lymphocytes (IELs) were EpCAM-CD45+. To prepare LPCs, ileal tissues were rinsed with PBS, suspended in 2 mmol/L EDTA in PBS, and stirred for 20 min at 37 °C to dissociate the ECs. The non-dissociated cells were digested with a Lamina propria dissociation kit (Miltenyi Biotec, Tokyo, Japan) according to the manufacturer's protocol to obtain LPCs.
Cells were washed with PBS and lysed in 1% sodium dodecyl sulfate (Sigma) with a proteinase inhibitor cocktail (GE Healthcare, Little Chalfont, England) and a phosphatase inhibitor cocktail (Roche Diagnostics, Tokyo, Japan). Samples were electrophoresed and transferred onto PVDF membranes (Millipore). After blocking with 10% bovine serum albumin (Wako, Osaka, Japan), the membrane was incubated with anti-p-STAT6 antibody (Cell Signaling Technology, Tokyo, Japan) or anti-GAPDH antibody (Cell Signaling Technology) then incubated with HRP-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA, United States). The signal was detected with an ECL prime kit (GE Healthcare, Little Chalfont, United Kingdom) and imaged using a LAS 4000 analyzer (FUJIFILM, Tokyo, Japan).
Ileum was collected on day 0 (naïve tissue) and days 3, 5, and 8 after the administration of 5-FU or PBS. Tissues were homogenized with a Bio-Plex cell lysis kit (BIO-RAD, Hercules, CA, United States) and stored at -80°C until needed. Cytokines were quantified with the Bio-Plex Pro Mouse Cytokine 23-Plex and Bio-Plex Pro Mouse Th17 panels using a Bio-Plex 3D Suspension Array System (BIO-RAD) according to the manufacturer’s instructions.
Ileum-derived LPCs (1 × 106 cells/assay) were incubated for 44 h in RPMI1640 with 5% fetal bovine serum and IL-33 (40 ng/mL, R&D Systems) or vehicle (PBS). Protein Transport Inhibitor (0.33 µL, BD GolgiStop™, BD Biosciences, NJ) was added, and the cells were incubated for an additional 4 h. Cells were incubated with allophycocyanin (APC)-conjugated anti-lineage antibody cocktail (BD Biosciences). Following surface marker labeling, cells were treated with a Fixation/Permeabilization Solution Kit (BD Biosciences) and incubated with PE-conjugated anti-IL-13 antibody (eBioscience). Labeled cells were analyzed using the MoFlo XDP cell sorter.
Ileum tissue was collected from WT or Fn14 KO mice two days after 5-FU administration, and 28 mg was minced in 1 mL of MEF culture medium (described above) with Antibiotic-Antimycotic Mixed Solution (Nacalai tesque. Kyoto, Japan). The sample was centrifuged at 300 × g for 5 min. The supernatant fraction was collected as ileum homogenate. The homogenate was mixed in a 1:9 ratio with MEF culture medium. IL-33 was depleted from the homogenate by adding 2 µg of anti-IL-33 antibody or total goat IgG (Jackson ImmunoResearch, West Grove, PA, United States) to 1 mL of homogenate and incubating for 1 hour at 4 °C. Antibodies were removed by adding 20 µL of Protein G PLUS-Agarose (resuspended volume, Santa Cruz biotechnology) and incubating for 1 h at 4 °C. After centrifugation, the supernatant fraction (i.e., the IL-33-depleted homogenate) was used for cell culture.
All statistical analyses were performed in the Prism 5 software for Mac OS X, version5.0d. The data were expressed as the mean ± SD. P < 0.05 was considered to be statistically significant. Results of diarrhea score, cytokine measurement and IL-13Rα2 protein expression in time course experiments were analyzed with Two-way ANOVA adjusted with Bonferroni correction. IL-13Rα2 protein expression after stimulation with IL-33 in vivo and in vitro in comparison of WT and Fn14 KO was analyzed with Mann Whitney U test. IL-13Rα2 protein expression in various conditions of in vitro culture was compared with one-way ANOVA adjusted with Bonferroni correction.
All WT mice developed severe diarrhea on day 3 after 5-FU administration, and it persisted throughout the study period. By contrast, Fn14 KO mice developed milder diarrhea than WT mice (Figure 1A and B). Despite the difference in diarrhea severity between WT and Fn14 KO mice, histological analysis showed no difference in the small and large intestine between the two groups. Both mouse groups showed decreased numbers and height of ileal villi (Figure 1C), and there was no difference in the number of apoptotic or proliferating cells in the ileum and colon (Supplementary Figure 1). To investigate the mechanism for developing diarrhea, we measured the expression of transporter genes related to diarrhea, such as cystic fibrosis transmembrane conductance regulator (CFTR), Na-K-Cl cotransporter 1 (NKCC1), and downregulated in adenoma (DRA) (Supplementary Figure 2). 5-FU decreased CFTR expression and increased NKCC1 and DRA expression up to 3-fold; however, there were no differences between gene expression in WT and Fn14 KO mice. We concluded that expression of these molecules did not play a major role in 5-FU-induced diarrhea and hypothesized that the diarrhea was related to epithelial cell damage. On day 8 after 5-FU administration, both mouse groups had villi shortening. However, expression of ZO-1, a tight junction protein, persisted at days 5 and 8 in the villi of Fn14 KO mice, whereas its expression decreased in the villi of WT mice, especially in the upper part (Figure 1D). Thus, ZO-1 expression in the villi was parallel to diarrhea score. These results suggested that 5-FU can damage tight junctions and the epithelial cell barrier, which increases paracellular permeability and results in diarrhea.
Next, we measured Fn14 expression in the ileum. In WT mice, 5-FU administration strongly induced Fn14 mRNA expression in the ileum by day 2 (Figure 1E). 5-FU-induced Fn14 mRNA expression was observed in ECs but not IELs or LPC (Figure 1F). Collectively, these results indicate that Fn14 expression in ECs increases chemotherapeutic-induced diarrhea and disruption of the TWEAK/Fn14 pathway could potentially prevent 5-FU-induced diarrhea.
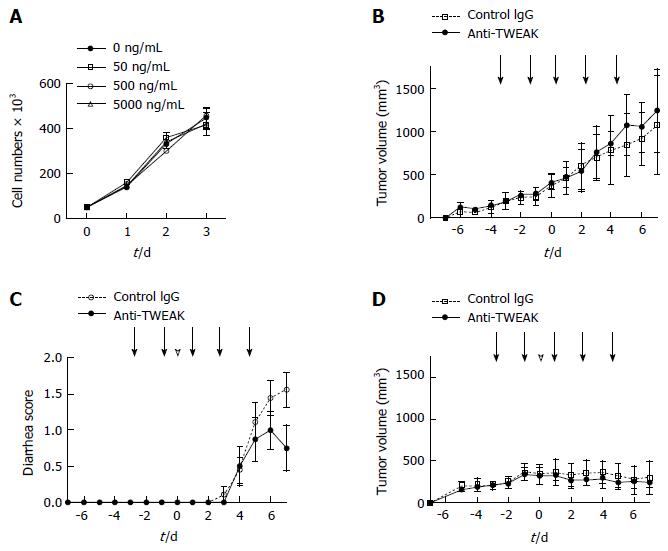
We used a mouse model bearing CT26 murine colon carcinoma tumors to investigate the effect of blocking the TWEAK/Fn14 signal pathway on 5-FU anticancer therapy. While CT26 expressed considerable levels of Fn14, addition of a TWEAK-neutralizing antibody in vitro did not change the cell growth rate (Figure 2A). The growth of CT26 tumors in the left inguinal region of WT mice was not altered by repeated anti-TWEAK or control antibody administration (Figure 2B). These results suggest that TWEAK/Fn14 signal disruption does not affect CT26 tumor growth in vitro or in vivo. Then, we treated CT26-tumor-bearing mice with a combination of 5-FU and anti-TWEAK antibody or a control antibody. Control antibody-treated mice had severe continuous 5-FU-induced diarrhea, whereas anti-TWEAK antibody treatment significantly suppressed 5-FU-induced diarrhea (Figure 2C, P = 0.0384). Importantly, tumor growth inhibition by 5-FU was effective with both control and anti-TWEAK antibody administration (Figure 2D). These results indicate that the anti-TWEAK antibody could be used to prevent 5-FU-induced diarrhea in anticancer chemotherapy.
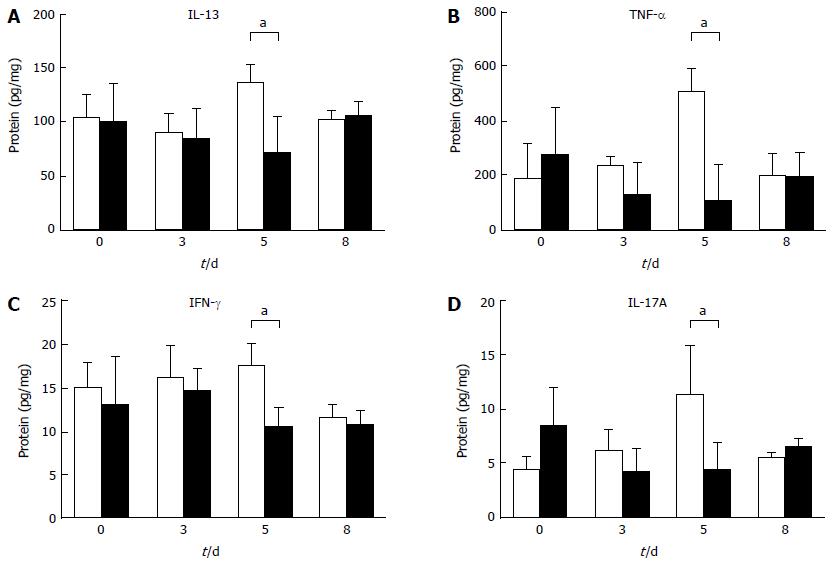
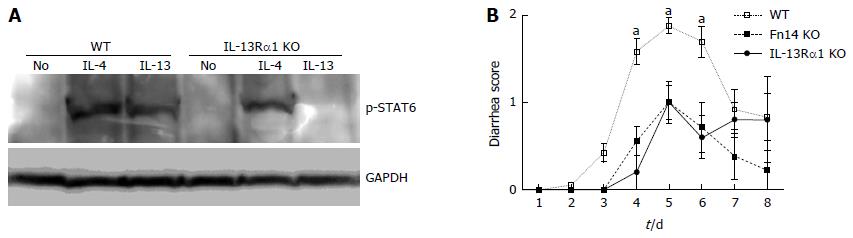
To investigate the mechanism by which diarrhea is prevented with Fn14 deficiency, we measured the expression levels of 5-FU-induced cytokines in the ileum. IL-13, TNF-α, IFN-γ, and IL-17A protein levels tended to increase on day 5 after 5-FU administration in WT mice compared with naïve ileum. These levels were higher than those in Fn14 KO mice (Figure 3). Previously, we showed that IL-13 is a pivotal mediator of intestinal epithelia damage following γ-irradiation[17]. Additionally, we showed that IL-13 works in concert with TNF-α to induce apoptosis in intestinal ECs in an Fn14-dependent manner[4]. To assess the role of IL-13 in 5-FU-induced diarrhea, we disrupted the IL-13Rα1 gene to generate IL-13Rα1 KO mice (Figure 4). Similar to the phenotype observed in Fn14 KO mice, the diarrhea score following 5-FU injection was lower in IL-13Rα1 KO mice through day 6. However, on days 7 and 8, there were no significant differences between the diarrhea score of IL-13Rα1 KO and WT mice (Figure 4). These results indicate that IL-13 is a major mediator of 5-FU-induced severe diarrhea, and its impact peaks around days 5-6.
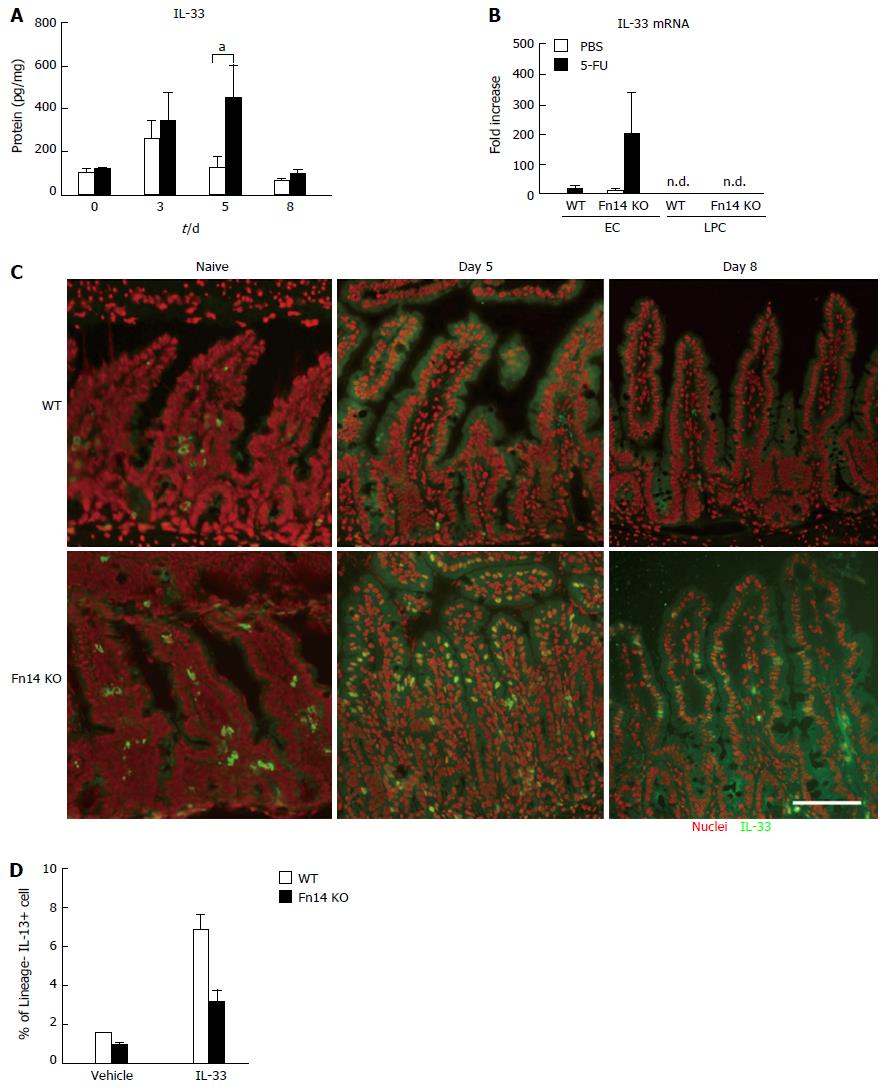
Because IL-33 is a potent inducer of IL-13, we initially hypothesized that IL-33 expression in ECs was Fn14 dependent. However, 5-FU induced IL-33 protein expression in the ileum of both WT and Fn14 KO mice by day 3 (Figure 5A). IL-33 was sustained at high levels in Fn14 KO ileum on day 5 (Figure 5A). IL-33 mRNA levels were elevated in ECs of Fn14 KO mice but not detected in LPCs, which is consistent with our observations of IL-33 protein levels (Figure 5B). Immunostaining with anti-IL-33 antibody confirmed that the number of IL-33-positive ileal ECs in Fn14 KO mice was greater than that in WT mice on day 5 after 5-FU administration (Figure 5C). IL-33 localized to the cytosol in WT mice but was found in both the cytosol and nucleus in Fn14 KO mice on day 5. The IL-33 staining pattern remained the same in Fn14 KO mice on day 8 (Figure 5C). These results indicate that 5-FU induced IL-33 in both WT and Fn14 KO mice but at much higher levels and with different subcellular localization in Fn14 KO mouse.
Because IL-13 expression in Fn14 KO mice was low compared with that in WT mice despite higher IL-33 expression (Figure 3A), we investigated whether IL-33-induced IL-13 expression was impaired in Fn14 KO LPCs. In vitro stimulation of WT LPCs with recombinant IL-33 induced IL-13 expression in lineage-negative (Lin-) fractions as expected. By contrast, this induction was attenuated in Fn14 KO LPCs (Figure 5D). These results indicate that IL-33-induced expression of IL-13 is dependent on an Fn14-mediated signal pathway. However, WT LPCs do not express Fn14 (Figure 1F). Thus, in Fn14 KO mice, IL-33 signaling in LPCs is reduced likely due to an Fn14 deficiency in an Fn14-expressing cell type, such as ECs. Overall, these results suggest that the presence of Fn14 in ECs affects IL-33-induced IL-13 expression in LPCs through an unknown mechanism.
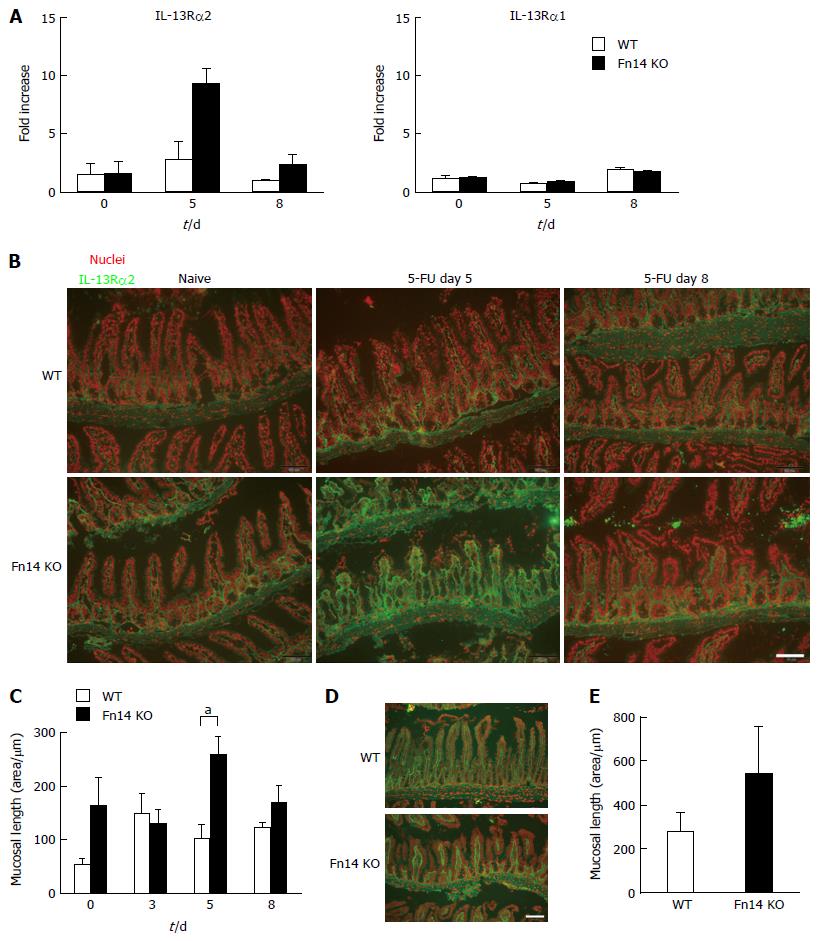
We observed that IL-13 expression was higher in WT mice than Fn14 KO mice on day 5. However, when comparing WT mice at day 5 following 5-FU administration with naïve mice (Day 0), induction of IL-13 expression was modest (Figure 3). Therefore, we hypothesized that there was an additional mechanism to reduce IL-13 activity in the absence of Fn14. This mechanism could potentially explain the dramatic diarrhea reduction in Fn14 KO mice. Previously, we showed that expression of IL-13Rα2, the IL-13 neutralizing receptor, promoted intestinal EC regeneration following γ-irradiation[17]. Thus, we hypothesized that IL-13Rα2 expression increased in Fn14 KO mice and contributed to the reduction of IL-13-induced diarrhea. As expected, on day 5 following 5-FU administration, IL-13Rα2 mRNA expression transiently increased 5-fold in the ileum of Fn14 KO mice but not WT mice (Figure 6A). IL-13Rα1 was not upregulated in either the WT or Fn14 KO ileum (Figure 6A). Consistent with the mRNA expression, immunostaining for IL-13Rα2 confirmed a transient but strong upregulation of protein expression in mesenchymal cells of Fn14 KO mice on day 5 but not in those of WT mice (Figure 6B and C). These results indicate that increased IL-13Rα2 expression in Fn14 KO mice may efficiently neutralize IL-13. Reduced IL-13 expression by Fn14-deficient cells combined with IL-13 neutralization through IL-13Rα2 could account for the attenuated diarrhea observed in 5-FU-treated KO mice.
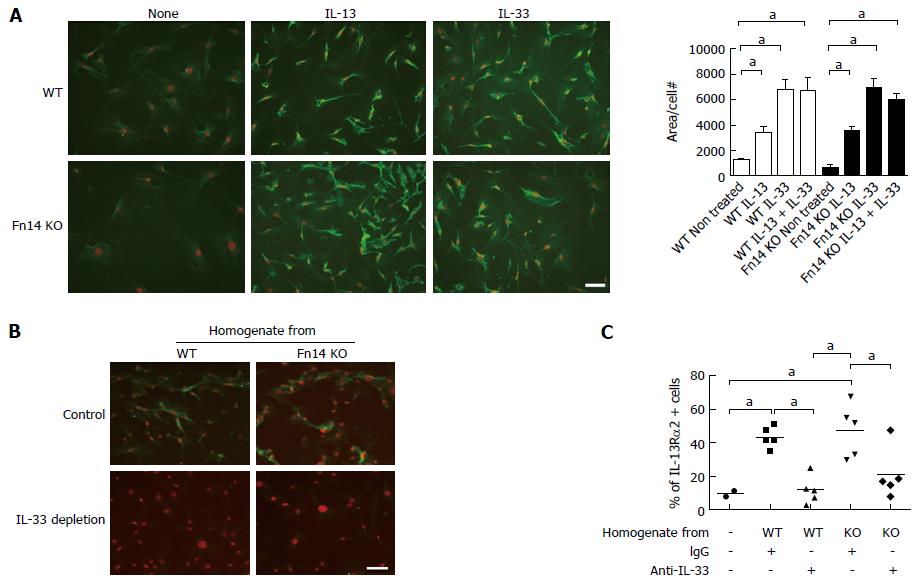
IL-13 is known to induce IL-13Rα2 expression; however, IL-13 protein levels did not increase in Fn14 KO mice following 5-FU administration. Because IL-33 was highly expressed in Fn14 KO mice after 5-FU administration, we investigated if IL-33 induces IL-13Rα2 expression. Administration of recombinant IL-33 to mesenchymal cells of the ileum induced IL-13Rα2 expression at a similar level in both WT and Fn14 KO mice (Figure 6D and E). Therefore, the high levels of IL-33 produced by ECs of Fn14 KO mice following 5-FU administration could have induced IL-13Rα2 upregulation. This mechanism was confirmed with in vitro assays using MEFs. In vitro stimulation of MEFs showed that recombinant IL-33 alone and in combination with recombinant IL-13 induced IL-13Rα2 expression in both WT and Fn14 KO cells (Figure 7A). To confirm that IL-33 was active in 5-FU-treated tissue, WT MEFs were cultured with ileal tissue homogenate from WT or Fn14 KO mice collected 2 d after 5-FU treatment. At this time point, WT and Fn14 KO mice have similar IL-33 levels in their tissues. Both WT and Fn14 KO ileum homogenate induced IL-13Rα2 expression in WT MEFs; however, this induction was not observed when MEFs were treated with IL-33-depleted homogenate (Figure 7B and C). These data suggest that IL-33 was responsible for the induction. Overall, these results indicate that IL-33 expression, which is enhanced in the absence of Fn14, could upregulate IL-13Rα2 expression, neutralize IL-13 signal, and ameliorate 5-FU-induced diarrhea.
Blocking the TWEAK/Fn14 pathway suppressed 5-FU-induced diarrhea, but did not affect tumor growth in the CT26 tumor-bearing model or the antitumor effects of 5-FU. In our mechanistic studies, we found that 5-FU-induced diarrhea was associated with IL-13, and its expression was Fn14-dependent. IL-13Rα2, the IL-13 neutralizing receptor, was induced by IL-33, which was upregulated in the absence of Fn14. Thus, an Fn14 deficit limited both IL-13 expression and activity. The TWEAK/Fn14 pathway coordinates these factors, thereby contributing to 5-FU-induced diarrhea.
Fn14 is often upregulated in malignant tumors, and high Fn14 levels are related to higher tumor grades and poor prognosis in esophageal[18] and gastric cancers[19]. Previous studies reported that TWEAK/Fn14 signaling promotes invasion, migration, and survival in many types of cancer cells, including glioblastoma, neuroblastoma, and breast cancer cells[20-22]. One study demonstrated therapeutic benefits when using a TWEAK-blocking antibody for cancer therapy[23]. These reports indicated that TWEAK/Fn14 signaling relates to tumor progression and blocking the TWEAK/Fn14 pathway could be beneficial in cancer treatment. Another recent report showed that anti-Fn14 antibody treatment prevented cancer-induced cachexia and prolonged survival[24]. By contrast, some reports show that TWEAK/Fn14 signaling has an antitumorigenic function[25]. Therefore, role of TWEAK/Fn14 signaling in cancer seems to be cell-type dependent. Careful investigation of the effects of blocking TWEAK/Fn14 signaling on each cancer cell type is needed.
In Fn14 KO mice, IL-13, TNF-α, IFN-γ, and IL-17A levels were low after 5-FU administration compared with WT mice. All these cytokines are involved in intestinal epithelial cell damage and diarrhea. Specifically, IL-13 is an important mediator of allergen-induced diarrhea[26]. This study demonstrated the critical role that IL-13 plays in 5-FU-induced diarrhea using IL-13Rα1 KO mice. IL-13 disrupts tight junctions, which affects intestinal barrier function[27,28]. In this study, ZO-1 expression levels in 5-FU-treated WT mice appeared to coincide with diarrhea score. Our previous study showed that IL-13 combined with TNF-α induced damage of tight junctions and intestinal EC death in an Fn14-dependent manner[4]. All these results support our hypothesis that 5-FU-induced diarrhea is primarily mediated by the IL-13 pathway, which affects the barrier function of ECs.
We hypothesized that IL-33 was responsible for induction of IL-13 in 5-FU-induced diarrhea. IL-13-producing cells were induced when WT LPCs were stimulated with IL-33. By contrast, IL-33 failed to induce IL-13 expression in Fn14 KO LPCs both in vitro and in vivo despite exposure to sustained high levels of IL-33. This observation indicates that IL-33-stimulated IL-13 expression is Fn14 dependent; however, LPCs did not express Fn14. This result suggests that the expression of IL-13 by LPCs in response to IL-33 requires some indirect priming, likely through soluble factors, by Fn14-expressing cells, such as ECs. In this situation, IL-33 functions as an alarmin in WT mice to induce IL-13 expression and diarrhea. In an Fn14-deficient microenvironment, LPCs could not induce IL-13 expression in response to IL-33. This result could be explained by IL-33 having a different function depending on the presence or absence of Fn14.
Previous studies reported that IL-33 is synthesized as a full-length protein that is localized in the nucleus of cells in epithelial barrier tissue[8,29]. Full-length IL-33 is not fully bioactive with respect to IL-33 receptor binding and NF-κB activation potential until it undergoes cleavage by caspase-1[14]. Full-length IL-33 can also be cleaved by inflammatory proteases from neutrophils, such as cathepsin and elastase. This cleavage results IL-13 expression or IL-6 secretion[30,31]. The nuclear localization sequence is located in the N-terminus of IL-33 and is removed following cleavage. Therefore, IL-33 leaves the nucleus after cleavage. In this study, IL-33 localized to the cytosol on day 5 of 5-FU treatment in WT ECs, whereas IL-33 was highly expressed and localized to both the nucleus and cytosol in Fn14 KO ECs on day 5. This observation could indicate that in WT mice, IL-33 was fully cleaved and bioactive (i.e., capable of inducing IL-13 expression in LPCs) but in Fn14 KO mice, the nuclear-localized IL-33 was in a different molecular form that served some other function with respect to IL-13 expression. Further studies are needed to investigate IL-33 processing, cellular localization, and function.
In addition to reducing IL-13 secretion, we found that upregulation of IL-33 in Fn14 KO mice actively protected ECs from damage. We demonstrated for the first time that IL-33 potently induced IL-13Rα2, an IL-13 neutralizing receptor. IL-13Rα2 plays an important protective role in cases of mucosal damage, such as oxazolone-induced colitis[32], and EC damage after γ-irradiation[17]. In these cases, IL-13Rα2 expression or IL-13 neutralization prevents damage in the intestine. Recently, IL-13Rα2 was reported to bind chitinase-like protein chitinase 3-like 1 and regulate oxidant injury, apoptosis, pyroptosis, inflammasome activation, and antibacterial responses[33]. This mechanism may also contribute to the protective effect of the IL-33-IL-13Rα2 axis in 5-FU-induced damage of the epithelial barrier. In contrast to IL-13 expression, recombinant IL-33-induced IL-13Rα2 expression seems to be similar between WT and Fn14 KO mice. Therefore, sustained exposure to high levels of IL-33 could explain the enhanced induction of IL-13Rα2 expression in Fn14 KO mice. Collectively, these results indicate that induction of IL-13Rα2 expression by IL-33 is an important mechanism to ameliorate 5-FU-induced diarrhea and intestinal damage in Fn14 KO mice.
In our study, blocking the TWEAK/Fn14 pathway did not affect tumor growth in the CT26 tumor-bearing model or the antitumor effects of 5-FU. Our results demonstrated that disrupting TWEAK/Fn14 signaling alters multiple processes in various cell types, including expression of IL-33 and tight junction proteins in ECs, induction of IL-13 expression in LPCs, and the induction of IL-13Rα2 expression in mesenchymal cells. These changes affected the sensitivity of normal intestinal cells to chemotherapeutic agents and ameliorated 5-FU-induced diarrhea. Therefore, we believe that disrupting the TWEAK/Fn14 pathway could reduce the side effects of 5-FU, thereby making chemotherapy more effective.
The chemotherapeutic antimetabolite agent 5-fluorouracil (5-FU) is frequently used to treat a wide range of cancer types. However, chemotherapy with 5-FU is often associated with severe gastrointestinal side effects, such as diarrhea and anorexia. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14 form a signaling pathway that is involved in the repair of injured tissue. The TWEAK/Fn14 pathway affect inflammation and epithelial cell death, especially in the intestine. In this study, we investigated the effect of blocking TWEAK/Fn14 signaling on chemotherapy-induced diarrhea.
The TWEAK/Fn14 pathway has been shown to play major roles in various pathological situations, including inflammatory bowel diseases, nephritis, myositis, and cancer. Developing new diagnostic methods and therapies targeting TWEAK/Fn14 signaling could improve outcomes of numerous conditions.
This is the first study reporting the prevention of 5-FU side effects using anti-TWEAK antibody injection. In mechanistic studies, we found that IL-33 induced IL-13 receptor α2 expression and its expression was enhanced in the absence of Fn14. This effect protected mice from severe diarrhea induced by the IL-13-receptor α1 pathway. These results also potentially identified a new tissue-protective function of IL-33 that was regulated by the presence of Fn14.
Because an anti-TWEAK antibody has already been developed for humans, it could be used to treat the severe side effects of 5-FU in the near future. This treatment could be used to improve the efficacy of anticancer drugs.
The manuscript can be evaluated as an excellent (A) work concerning about an innovative molecular study: this study could improve significantly the common use of 5FU into clinical practise with the possibility of reducing adverse effects that may decrease patient’s compliance to therapy. The study reflects a great “equipe” work with a solid scientific contribution.
| 1. | Burkly LC, Dohi T. The TWEAK/Fn14 pathway in tissue remodeling: for better or for worse. Adv Exp Med Biol. 2011;691:305-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev. 2011;244:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Dohi T, Borodovsky A, Wu P, Shearstone JR, Kawashima R, Runkel L, Rajman L, Dong X, Scott ML, Michaelson JS. TWEAK/Fn14 pathway: a nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis. Gastroenterology. 2009;136:912-923. [PubMed] |
| 4. | Kawashima R, Kawamura YI, Oshio T, Son A, Yamazaki M, Hagiwara T, Okada T, Inagaki-Ohara K, Wu P, Szak S. Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice-a pathway associated with ulcerative colitis. Gastroenterology. 2011;141:2119-2129.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Dohi T, Kawashima R, Kawamura YI, Otsubo T, Hagiwara T, Amatucci A, Michaelson J, Burkly LC. Pathological activation of canonical nuclear-factor κB by synergy of tumor necrosis factor α and TNF-like weak inducer of apoptosis in mouse acute colitis. Cytokine. 2014;69:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Son A, Oshio T, Kawamura YI, Hagiwara T, Yamazaki M, Inagaki-Ohara K, Okada T, Wu P, Iseki M, Takaki S. TWEAK/Fn14 pathway promotes a T helper 2-type chronic colitis with fibrosis in mice. Mucosal Immunol. 2013;6:1131-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2958] [Article Influence: 140.9] [Reference Citation Analysis (6)] |
| 8. | Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 963] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 9. | Choi YS, Park JA, Kim J, Rho SS, Park H, Kim YM, Kwon YG. Nuclear IL-33 is a transcriptional regulator of NF-κB p65 and induces endothelial cell activation. Biochem Biophys Res Commun. 2012;421:305-311. [PubMed] |
| 10. | Zhu J, Wang Y, Yang F, Sang L, Zhai J, Li S, Li Y, Wang D, Lu C, Sun X. IL-33 alleviates DSS-induced chronic colitis in C57BL/6 mice colon lamina propria by suppressing Th17 cell response as well as Th1 cell response. Int Immunopharmacol. 2015;29:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Waddell A, Vallance JE, Moore PD, Hummel AT, Wu D, Shanmukhappa SK, Fei L, Washington MK, Minar P, Coburn LA. IL-33 Signaling Protects from Murine Oxazolone Colitis by Supporting Intestinal Epithelial Function. Inflamm Bowel Dis. 2015;21:2737-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 2015;112:10762-10767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 444] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 13. | Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3+ regulatory T-cell responses in mice. Mol Med. 2012;18:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021-9026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 563] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 15. | Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 16. | Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang B. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115:2330-2340. [PubMed] |
| 17. | Kawashima R, Kawamura YI, Kato R, Mizutani N, Toyama-Sorimachi N, Dohi T. IL-13 receptor alpha2 promotes epithelial cell regeneration from radiation-induced small intestinal injury in mice. Gastroenterology. 2006;131:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Wang S, Zhan M, Yin J, Abraham JM, Mori Y, Sato F, Xu Y, Olaru A, Berki AT, Li H. Transcriptional profiling suggests that Barrett’s metaplasia is an early intermediate stage in esophageal adenocarcinogenesis. Oncogene. 2006;25:3346-3356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kwon OH, Park SJ, Kang TW, Kim M, Kim JH, Noh SM, Song KS, Yoo HS, Wang Y, Pocalyko D. Elevated fibroblast growth factor-inducible 14 expression promotes gastric cancer growth via nuclear factor-κB and is associated with poor patient outcome. Cancer Lett. 2012;314:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, Hoelzinger DB, Beaudry C, Coons SW, Berens ME. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol. 2003;162:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Pettersen I, Baryawno N, Abel F, Bakkelund WH, Zykova SN, Winberg JO, Moens U, Rasmuson A, Kogner P, Johnsen JI. Expression of TWEAK/Fn14 in neuroblastoma: implications in tumorigenesis. Int J Oncol. 2013;42:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Willis AL, Tran NL, Chatigny JM, Charlton N, Vu H, Brown SA, Black MA, McDonough WS, Fortin SP, Niska JR. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res. 2008;6:725-734. [PubMed] |
| 23. | Meulendijks D, Lassen UN, Siu LL, Huitema AD, Karanikas V, Mau-Sorensen M, Jonker DJ, Hansen AR, Simcox ME, Schostack KJ. Exposure and Tumor Fn14 Expression as Determinants of Pharmacodynamics of the Anti-TWEAK Monoclonal Antibody RG7212 in Patients with Fn14-Positive Solid Tumors. Clin Cancer Res. 2016;22:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, Faou P, Weston R, Jayatilleke KM, Schloegel J, Talbo G. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell. 2015;162:1365-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1664] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 26. | Brandt EB, Munitz A, Orekov T, Mingler MK, McBride M, Finkelman FD, Rothenberg ME. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. J Allergy Clin Immunol. 2009;123:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3:e977176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037-12046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488-3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 30. | Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 31. | Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502-15507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 32. | Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629-638. [PubMed] |
| 33. | He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep. 2013;4:830-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Peluso I, Sguinzi R S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF