Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5445
Peer-review started: March 18, 2016
First decision: March 31, 2016
Revised: April 8, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 21, 2016
Processing time: 89 Days and 17.8 Hours
AIM: To evaluate the applicability of nonbismuth concomitant quadruple therapy for Helicobacter pylori (H. pylori) eradication in Chinese regions.
METHODS: A systematic review and meta-analysis of randomized controlled trials was performed to evaluate the efficacy of nonbismuth concomitant quadruple therapy between sequential therapy or triple therapy for H. pylori eradication in Chinese regions. The defined Chinese regions include China, Hong Kong, Taiwan, and Singapore. The primary outcome was the H. pylori eradication rate; the secondary outcome was the compliance with therapy. The PubMed, Embase, Scopus, and Cochrane databases were searched for studies published in the period up to March 2016 with no language restriction.
RESULTS: We reviewed six randomized controlled trials and 1616 patients. In 3 trials comparing concomitant quadruple therapy with triple therapy, the H. pylori eradication rate was significantly higher for 7-d nonbismuth concomitant quadruple therapy than for 7-d triple therapy (91.2% vs 77.9%, risk ratio = 1.17, 95%CI: 1.09-1.25). In 3 trials comparing quadruple therapy with sequential therapy, the eradication rate was not significant between groups (86.9% vs 86.0%). However, higher compliance was achieved with concomitant therapy than with sequential therapy.
CONCLUSION: The H. pylori eradication rate was higher for nonbismuth concomitant quadruple therapy than for triple therapy. Moreover, higher compliance was achieved with nonbismuth concomitant quadruple therapy than with sequential therapy. Thus, nonbismuth concomitant quadruple therapy should be the first-line treatment in Chinese regions.
Core tip:Helicobacter pylori (H. pylori) infection is highly prevalent in Chinese regions and associated with peptic ulcers. Currently, triple and sequential therapies have been widely used to eradicate H. pylori. Nonbismuth concomitant quadruple therapy is an alternative treatment with high efficacy. Our meta-analysis revealed that a higher H. pylori eradication rate was achieved with 7-d concomitant therapy than with 7-d triple therapy. The eradication rates of concomitant and sequential therapies were similar. However, the compliance with concomitant therapy was higher. Therefore, nonbismuth concomitant quadruple therapy should be the first-line treatment for H. pylori infection.
- Citation: Lin LC, Hsu TH, Huang KW, Tam KW. Nonbismuth concomitant quadruple therapy for Helicobacter pylori eradication in Chinese regions: A meta-analysis of randomized controlled trials. World J Gastroenterol 2016; 22(23): 5445-5453
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5445.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5445
Helicobacter pylori (H. pylori) infection has been proven to be the major cause of chronic gastritis, gastric and duodenal ulcers, gastric adenocarcinoma, and gastric mucosa-associated lymphoma[1-3]. Moreover, H. pylori eradication has become the standard and most widely adopted therapy for curing peptic ulcers[4-6].
According to most international guidelines, conventional triple therapy, involving the use of a proton pump inhibitor (PPI) with amoxicillin and clarithromycin for 7-10 d, is the first-line therapy for H. pylori eradication[7-10]. However, the eradication rate of triple therapy has decreased to 80% in many countries worldwide[11-14]. By contrast, studies have shown a high eradication rate for sequential therapy, which entails administering a PPI and amoxicillin for 5 d, followed by a PPI, clarithromycin, and metronidazole (or tinidazole) for another 5 d[15-18]. However, compliance may be poor because of the complexity of sequential therapy[19]. In addition, nonbismuth concomitant quadruple therapy, involving the simultaneous administration of a PPI, amoxicillin, clarithromycin, and metronidazole for 7 or 10 d, is more convenient than sequential therapy, although its efficacy is yet to be determined[20-26].
Peptic ulcer is a common disease in Chinese regions. In Taiwan, the overall prevalence of H. pylori infection is 54%, and it increases with age[27]. However, the infection rate of H. pylori is only 31% in Singapore[28]. Because antibiotic resistance is a critical reason for H. pylori eradication failure, studies on H. pylori eradication are needed within specific region[14]. However, most meta-analyses of H. pylori eradication have been performed in Europe and Korea, and the optimal treatment for H. pylori eradication in Chinese regions is still unknown[29,30]. Therefore, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate whether nonbismuth concomitant quadruple therapy is the first-line therapy for H. pylori eradication in Chinese regions.
The PubMed, Embase, Scopus, and Cochrane databases were searched for studies published in the period up to March 2016 without language restrictions. The following medical search heading terms, words, and combinations of words were used in the systematic search: Helicobactor pylori or H. pylori, eradication, peptic or gastric or duodenal ulcer, concomitant or quadruple, and China or Chinese or Hong Kong or Taiwan or Singapore. All included studies were also entered into the PubMed “similar articles” function and the science citation index. Moreover, we identified additional studies by manually searching the reference sections of these papers and by contacting known experts in the field. Finally, unpublished trials were retrieved from the ClinicalTrials.gov registry (http://clinicaltrials.gov/). The systematic review described herein was accepted by the online PROSPERO international prospective register of systematic reviews of the National Institute for Health Research (CRD42016032668).
The following studies were selected for analysis: RCTs evaluating the efficacy of nonbismuth concomitant quadruple therapy versus standard triple or sequential for H. pylori eradication; those performed in Chinese regions including China, Hong Kong, Taiwan, and Singapore; patients aged 18 years or over; those clearly describing the inclusion and exclusion criteria used for patient selection; those adequately describing the administration of antibiotics and PPIs; and trials that precisely defined and evaluated H. pylori infection. Triple therapy was defined as a PPI plus amoxicillin and clarithromycin given for 7-14 d. Sequential therapy was defined as a PPI plus amoxicillin given for the first 5-7 d, followed by a PPI plus nitroimidazole derivatives and clarithromycin for the next 5-7 d. Nonbismuth concomitant quadruple therapy was defined as a PPI plus amoxicillin, clarithromycin, and nitroimidazole derivatives given for 7-14 d. The Studies were excluded from the analysis if one or both of the following criteria were present: patients enrolled in the trials who were proven to have had previous H. pylori infection with a history of bacterial eradication, and an overlap occurred between patient cohorts evaluated in two or more studies.
Two independent reviewers (Lin LC and Hsu TH) extracted the data of the trials, including the participants, inclusion and exclusion criteria, administration of experimental drugs, prevalence and assessment of H. pylori infection, and complications. Discrepancies and any disagreements were resolved through discussion with a third reviewer (Tam KW). The authors of the studies were contacted for additional information when necessary.
The risk of bias in the included trials was assessed using the following domains: adequacy of the randomization, allocation concealment, blinding, duration of follow-up, numbers of drop-outs, and performance of intention-to-treat (ITT) analysis.
The H. pylori eradication rate was the primary outcome used to evaluate the efficacy of nonbismuth concomitant quadruple therapy. The occurrence of H. pylori infection was determined using assessments of histology, culture, rapid urease tests, or breath tests. The secondary outcome was the compliance with treatment.
The analysis was performed using the statistical package Review Manager, version 5.3 (Cochrane Collaboration, Oxford, England). The meta-analysis was performed according to the recommendations of the PRISMA statement[31]. For dichotomous data, the results were summarized as risk ratios (RRs) with 95%CIs. A pooled estimate of the RR was calculated using the DerSimonian and Laird random effect model[32]. This approach provides a more appropriate estimate of the average treatment effect when trials are statistically heterogeneous and usually yields wider CIs, thereby resulting in a more conservative statistical claim. χ2 statistics tests (Q statistics) and the I2 test were used to test for heterogeneity among controlled trials.
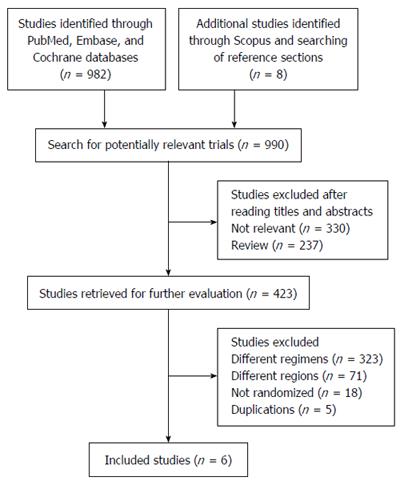
The review process is outlined in Figure 1. The initial search yielded 990 studies, 567 of which were deemed ineligible through screening of titles and abstracts. Subsequently, the full text of 423 studies was screened. Of these, five did not meet the eligibility criteria because of duplicate publication, 18 were not randomized studies, 71 evaluated H. pylori eradication in different regions, and 323 included different comparisons. Thus, only six eligible trials were included in this meta-analysis[20,22,33-36].
The main characteristics of the studies are listed in Table 1. Four of the six studies were performed in Taiwan, and the remaining two were conducted in China and Singapore. The publication dates of the studies were between 2012 and 2015, and the sample sizes ranged from 169 to 462. All trials evaluated patients diagnosed with H. pylori infection. In our included studies, three indicated that their patients had gastritis or peptic ulcers[33-35]. Only one study did not report the tests for diagnosing H. pylori infection[34], and other studies reported the following diagnostic tests: rapid urease test, histology, urea breath test, and culture. The timing of evaluation for the H. pylori infection status ranged from 4 to 12 wk after the treatment course. Treatment strategies for H. pylori eradication varied among the studies. Regarding the PPIs administered, three studies used esomeprazole[20,34,35], one used pantoprazole[33], one used lansoprazole[22], and one did not control the choice of the PPI[36]. Regarding concomitant and sequential regimens, all studies used metronidazole, except for Wang et al[35], who substituted metronidazole with tinidazole. In all included studies, a PPI, amoxicillin, and clarithromycin were administered as triple therapy for 7 d[33-35] or 10 d[35,36]. Regarding sequential therapy, all treatment regimens entailed administering a PPI and amoxicillin for 5 d, followed by a PPI, clarithromycin, and metronidazole for another 5 d[20,22,33,36]. Finally, concomitant therapy involved administering a PPI, clarithromycin, amoxicillin, and metronidazole for 7 d[33-35] or 10 d[20,22,36]. Huang et al[22] prolonged PPI maintenance therapy to 10 wk. Baseline characteristics were balanced and similar between groups in the six included RCTs.
| Ref. | Inclusion criteria | Region | Diagnostic test | No. of patients (male %) | Age, yr(mean ± SD) | Intervention |
| Ang et al[36] (2015) | Age > 21 yr | Singapore | RUT, H, UBT | C10: 153 (47.1) | C10: 46.9 ± 14.8 | 10-d concomitant therapy |
| S10: 154 (59.7) | S10: 47.5 ± 12.7 | 10-d sequential therapy | ||||
| T10: 155 (58.1) | T10: 49.8 ± 14.6 | 10-d triple therapy | ||||
| Hsu et al[33] (2014) | Age ≥ 20 yr, PU or gastritis | Taiwan | RUT, Cu, H | C7: 102 (59.8) | C7: 53.9 ± 12.3 | 7-d concomitant therapy |
| S10: 102 (50.9) | S10: 55.0 ± 12.0 | 10-d sequential therapy | ||||
| T7: 103 (60.2) | T7: 56.1 ± 14.0 | 7-d triple therapy | ||||
| Huang et al[22] (2012) | Dyspepsia or epigastric discomfort | Taiwan | RUT, Cu, H | C10: 84 (57.1) | C10: 53.8 ± 15.2 | 10-d concomitant therapy |
| S10: 85 (56.7) | S10: 51.3 ± 15.0 | 10-d sequential therapy | ||||
| Tai et al[34] (2015) | Age ≥ 20 yr, PU or gastritis | Taiwan | Not reported | C7: 92 (50.0) | C7: 47.8 ± 11.6 | 7-d concomitant therapy |
| T7: 92 (49.0) | T7: 52.8±12.8 | 7-d triple therapy | ||||
| Wang et al[35] (2014) | PU and gastritis | China | UBT | C7: 81 (45.7) | C7: 51 ± 13 | 7-d concomitant therapy |
| T7: 82 (42.7) | T7: 51 ± 15 | 7-d triple therapy | ||||
| T10: 83 (45.8) | T10: 52 ± 14 | 10-d triple therapy | ||||
| Wu et al[20] (2010) | Patients visited GI clinics with HP infection | Taiwan | RUT, Cu, H | C10: 115(52.2) | C10: 51.8 ± 11 | 10-d concomitant therapy |
| S10:117(52.1) | S10: 51.7 ± 12 | 10-d sequential therapy |
Table 2 presents the details of the six included RCTs. The use of random allocation was clearly documented in all studies. The treatment group allocation was concealed from the patients in three studies[33,34,36]. Only one reported the blinding of the investigators who assessed the outcomes[20]. In all studies, outcomes were evaluated using both ITT and per-protocol analyses. The percentage of patients lost to follow-up was acceptable (< 20%) in all studies. All studies had a bias attributable to insufficient data on antibiotic susceptibility[20,22,33,36].
| Ref. | Region | Allocation generation | Allocation concealment | Blinding of patients and assessors | Data analysis | Loss to follow up | Selective reporting | Other bias |
| Ang et al[36] (2015) | Singapore | Sealed envelope | Adequate | Open-label | ITT/PP | 10.0% | Low risk | Not all patients underwent antibiotic susceptibility testing |
| Hsu et al[33] (2014) | Taiwan | Compute generated | Adequate | Open-label | ITT/PP | 0.3% | Low risk | Not all patients underwent antibiotic susceptibility testing |
| Huang et al[22] (2012) | Taiwan | Computer generated | Unclear | Open-label | ITT/PP | 6.5% | Low risk | No patient underwent antibiotic susceptibility testing |
| Tai et al[34] (2015) | Taiwan | Computer generated | Adequate | Unclear | ITT/PP | 8.0% | Low risk | Not all patients underwent antibiotic susceptibility testing |
| Wang et al[35] (2014) | China | Computer generated | Unclear | Unclear | ITT/PP | 1.2% | Low risk | No patient underwent antibiotic susceptibility testing |
| Wu et al[20] (2010) | Taiwan | Computer generated | Unclear | Outcome assessor blinded | ITT/PP | 0.4% | Low risk | Not all patients underwent antibiotic susceptibility testing |
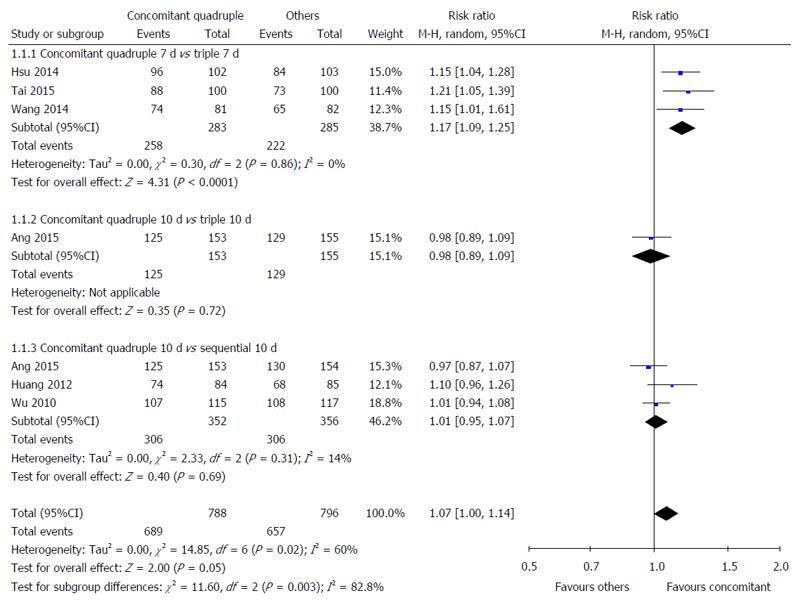
Nonbismuth concomitant quadruple therapy vs triple therapy: Three studies compared the H. pylori eradication rates of 7-d nonbismuth concomitant quadruple and triple therapies[33-35]. The timing of H. pylori infection status assessment was different among these studies: 4[35], 6[33], and 8 wk[34] after treatment. A significant difference was observed in the overall H. pylori eradication rate of nonbismuth concomitant quadruple and triple therapies (91.2% vs 77.9%). Fewer patients receiving nonbismuth concomitant quadruple therapy experienced H. pylori infection after treatment (RR = 1.17, 95%CI: 1.09-1.25) (Figure 2). The results demonstrated low heterogeneity among the studies (I2 = 0%).
One study compared the H. pylori eradication rates of 10-d nonbismuth concomitant quadruple and triple therapies[36]. The timing of H. pylori infection status assessment was 4 wk after treatment. No significant difference was observed in the H. pylori eradication rates of nonbismuth concomitant quadruple and triple therapies (81.7% vs 83.2%, RR = 0.98, 95%CI: 0.89-1.09) (Figure 2).
Nonbismuth concomitant quadruple therapy vs sequential therapy: Three studies compared the H. pylori eradication rate of 10-d nonbismuth concomitant quadruple and sequential therapies[20,22,36]. The timing of H. pylori infection status assessment was different among the studies: 4[36], 6[20], and 12 wk[22] after treatment. No statistically significant difference was observed in the overall H. pylori eradication rates of nonbismuth concomitant quadruple and sequential therapies (86.9% vs 86.0%, RR = 1.01, 95%CI: 0.95-1.07) (Figure 2).
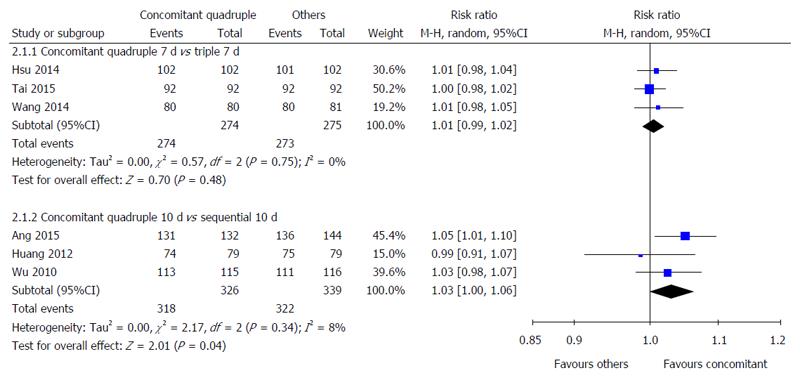
Nonbismuth concomitant quadruple therapy vs triple therapy: Three studies compared the compliance with 7-d nonbismuth concomitant quadruple and triple therapies[33-35]. No statistically significant difference was observed in the compliance with these therapies (100% vs 99.3%, RR = 1.01, 95%CI: 0.99-1.02) (Figure 3).
Nonbismuth concomitant quadruple therapy vs sequential therapy: Three studies compared the compliance with 10-d nonbismuth concomitant quadruple and sequential therapies[20,22,36]. Although no significant difference was observed in the compliance with these therapies (97.5% vs 95.0%, RR = 1.03, 95%CI: 1.00-1.06), more patients tended to comply with nonbismuth concomitant quadruple therapy (Figure 3).
Two studies compared adverse events including abdominal pain, gastrointestinal disturbance, nausea and vomiting, skin rash, dizziness, and fatigue between 10-d nonbismuth concomitant quadruple and sequential therapies[20,22]. Patients receiving these two therapies showed a similar adverse event rate. Moreover, three studies compared the adverse event rate between 7-d nonbismuth concomitant quadruple and triple therapies[33-35]. Among two studies, patients receiving these two therapies showed a similar incidence of adverse events[33,35]. One study reported more adverse events after 7-d nonbismuth concomitant quadruple therapy than after triple therapy[34]. However, these effects were mild and did not markedly interfere with the patients’ daily activities.
Because antibiotic resistance is a critical reason for H. pylori eradication failure, we conducted a systematic review and meta-analysis of RCTs to evaluate whether nonbismuth concomitant quadruple therapy is the optimal first-line therapy for H. pylori eradication in Chinese regions. Our meta-analysis revealed that a higher H. pylori eradication rate was achieved with 7-d concomitant therapy than with 7-d triple therapy. The eradication rates of concomitant and sequential therapies were similar. However, the compliance with concomitant therapy was higher. Therefore, nonbismuth concomitant quadruple therapy should be the first-line treatment for H. pylori infection.
Recently, Li et al[37] conducted a network meta-analysis of treatment for H. pylori infection. They showed that nonbismuth concomitant quadruple treatment is effective in H. pylori eradication. However, ethnicity and region play pivotal roles in antibacterial treatments; thus, investigating H. pylori eradication in different regions is necessary[38]. In South Korea, two RCTs showed that a much higher H. pylori eradication rate was achieved with nonbismuth concomitant quadruple therapy than with standard triple therapy or sequential therapy[26,39]. In Japan, an RCT also reported a higher eradication rate for nonbismuth concomitant quadruple therapy than that for triple therapy[40]. Our study revealed a similar outcome in Chinese regions.
Although the included studies used different PPIs, the same PPI was administered to the experimental groups in all studies, except for Ang et al[36]. Nevertheless, a meta-analysis revealed that different PPI types did not have different efficacies for H. pylori eradication[41]. Moreover, regarding concomitant therapy, Wang et al[35] substituted metronidazole with tinidazole; the eradication rate of that study is similar to that of other studies using metronidazole. These results are compatible with the trial that compared the efficacy of tinidazole and metronidazole for H. pylori eradication[42].
The optimal dosage of metronidazole remains undetermined. All RCTs used 500 mg of metronidazole twice daily, except for Ang et al[36], who used 400 mg twice daily. Nevertheless, Ang et al[36] still obtained a high eradication rate; this finding indicated that metronidazole doses from 400 to 500 mg are acceptable for H. pylori eradication.
To determine the effectiveness of the treatments in practice, we considered the compliance rate. Although the eradication rates of nonbismuth concomitant and sequential therapies were not statistically different, higher compliance was achieved with nonbismuth concomitant therapy than with sequential therapy. Generally, the compliance rate may be higher in RCTs than in clinical settings. Thus, nonbismuth concomitant therapy may be a superior choice for H. pylori eradication because higher compliance was achieved with this therapy than with sequential therapy.
The value of I2 was 0%-14% for each therapy, revealed that mild heterogeneity existed among our selected studies. This could be attributed to heterogeneity among patients’ demographics and characteristics and the inclusion and exclusion criteria, dose and route of administration of H. pylori treatment, and time of outcome assessment.
Our study has several limitations. First, all our studies were open label, except for the study of Wu et al[20], which was outcome assessor blinded. However, we believe that this is not a major concern because the treatment outcomes were mainly objective. Second, not all trials evaluate antibiotic susceptibility. Third, because China has the highest population globally, more RCTs conducted in China may be required to determine the optimal treatment for H. pylori infection in Chinese regions.
In conclusion, the evidence reviewed in the present meta-analysis indicated that nonbismuth concomitant quadruple therapy achieved a higher H. pylori eradication rate than that of standard triple therapy and higher compliance than that of sequential therapy. Therefore, nonbismuth concomitant quadruple therapy should be the first-line treatment for H. pylori infection in Chinese regions.
Peptic ulcer is a common disease in Chinese regions, and Helicobacter pylori (H. pylori) eradication has become the standard and most widely adopted therapy. However, eradication rate of standard triple therapy has decreased to 80% in many countries worldwide, and compliance of sequential therapy may be poor due to the complexity. Therefore, there is a need to evaluate whether nonbismuth concomitant quadruple therapy is the first-line therapy for H. pylori eradication in Chinese regions.
Due to increasing antibiotic resistance, current H. pylori eradication therapies may be poor. In this study, the authors compared eradication rate and compliance rate of three different types of therapies in Chinese region.
This study is the first meta-analysis to compare nonbismuth concomitant quadruple therapy with triple therapy and sequential therapy in Chinese region. Based on this study, nonbismuth concomitant quadruple therapy showed high H. pylori eradication rate and good compliance rate in Chinese region.
This study showed high efficacy and compliance in nonbismuth concomitant quadruple therapy. Thus, nonbismuth concomitant quadruple therapy is a good choice for first-line H. pylori eradication therapy in Chinese region.
Nonbismuth concomitant quadruple therapy consist of proton pump inhibitor plus amoxicillin, clarithromycin, and nitroimidazole derivatives given for 7-14 d. Chinese regions in this study included China, Hong Kong, Taiwan, and Singapore.
The results of this meta-analysis showed that treatment with nonbismuth concomitant quadruple therapy resulted in high H. pylori eradication rate in Chinese region. In addition, good compliance rate and mild adverse effects were also noted in this study. Consequently, the study provided a better choice for first-line eradication therapy of H. pylori in Chinese region.
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1936] [Article Influence: 80.7] [Reference Citation Analysis (3)] |
| 2. | Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 3. | Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137:1641-1648.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 4. | Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 622] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Sung JJ, Chung SC, Ling TK, Yung MY, Leung VK, Ng EK, Li MK, Cheng AF, Li AK. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. N Engl J Med. 1995;332:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Wong CS, Chia CF, Lee HC, Wei PL, Ma HP, Tsai SH, Wu CH, Tam KW. Eradication of Helicobacter pylori for prevention of ulcer recurrence after simple closure of perforated peptic ulcer: a meta-analysis of randomized controlled trials. J Surg Res. 2013;182:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 431] [Article Influence: 25.4] [Reference Citation Analysis (1)] |
| 8. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K; Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 298] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (7)] |
| 10. | Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 838] [Article Influence: 44.1] [Reference Citation Analysis (3)] |
| 11. | Irwin J, Terrault N. Cognitive impairment in hepatitis C patients on antiviral therapy. Gastroenterol Hepatol (N Y). 2008;4:65-67. [PubMed] |
| 12. | Huang AH, Sheu BS, Yang HB, Huang CC, Wu JJ, Lin XZ. Impact of Helicobacter pylori antimicrobial resistance on the outcome of 1-week lansoprazole-based triple therapy. J Formos Med Assoc. 2000;99:704-709. [PubMed] |
| 13. | Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 14. | Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Zullo A, Rinaldi V, Winn S, Meddi P, Lionetti R, Hassan C, Ripani C, Tomaselli G, Attili AF. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Gisbert JP, Calvet X, O’Connor A, Mégraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010;44:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 20. | Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | McNicholl AG, Marin AC, Molina-Infante J, Castro M, Barrio J, Ducons J, Calvet X, de la Coba C, Montoro M, Bory F. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014;63:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 22. | Huang YK, Wu MC, Wang SS, Kuo CH, Lee YC, Chang LL, Wang TH, Chen YH, Wang WM, Wu DC. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis. 2012;13:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, Perez-Gallardo B, Dueñas-Sadornil C, Hernandez-Alonso M, Gonzalez-Garcia G, Mateos-Rodriguez JM, Fernandez-Bermejo M, Gisbert JP. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Georgopoulos SD, Xirouchakis E, Martinez-Gonzalez B, Sgouras DN, Spiliadi C, Mentis AF, Laoudi F. Clinical evaluation of a ten-day regimen with esomeprazole, metronidazole, amoxicillin, and clarithromycin for the eradication of Helicobacter pylori in a high clarithromycin resistance area. Helicobacter. 2013;18:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Georgopoulos S, Papastergiou V, Xirouchakis E, Laoudi F, Lisgos P, Spiliadi C, Papantoniou N, Karatapanis S. Nonbismuth quadruple “concomitant” therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication: a randomized trial. J Clin Gastroenterol. 2013;47:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 26. | Heo J, Jeon SW, Jung JT, Kwon JG, Kim EY, Lee DW, Seo HE, Ha CY, Kim HJ, Kim ES, Park KS, Cho KB, Lee SH, Jang BI; Daegu-Gyeongbuk Gastrointestinal Study Group. A randomised clinical trial of 10-day concomitant therapy and standard triple therapy for Helicobacter pylori eradication. Dig Liver Dis. 2014;46:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Teh BH, Lin JT, Pan WH, Lin SH, Wang LY, Lee TK, Chen CJ. Seroprevalence and associated risk factors of Helicobacter pylori infection in Taiwan. Anticancer Res. 1994;14:1389-1392. [PubMed] |
| 28. | Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 29. | Kim JS, Kim BW, Ham JH, Park HW, Kim YK, Lee MY, Ji JS, Lee BI, Choi H. Sequential Therapy for Helicobacter pylori Infection in Korea: Systematic Review and Meta-Analysis. Gut Liver. 2013;7:546-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7886] [Article Influence: 463.9] [Reference Citation Analysis (3)] |
| 32. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 31212] [Article Influence: 780.3] [Reference Citation Analysis (1)] |
| 33. | Hsu PI, Wu DC, Chen WC, Tseng HH, Yu HC, Wang HM, Kao SS, Lai KH, Chen A, Tsay FW. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother. 2014;58:5936-5942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Tai WC, Liang CM, Lee CH, Chiu CH, Hu ML, Lu LS, Kuo YH, Kuo CM, Yen YH, Kuo CH. Seven-Day Nonbismuth Containing Quadruple Therapy Could Achieve a Grade “A” Success Rate for First-Line Helicobacter pylori Eradication. Biomed Res Int. 2015;2015:623732. [PubMed] |
| 35. | Wang S, Wang W, Chu Y, Teng G, Hu F. [Non-bismuth quadruple therapy versus standard triple therapy for Helicobacter pylori eradication: a randomized controlled study]. Zhonghua Yi Xue Zazhi. 2014;94:576-579. [PubMed] |
| 36. | Ang TL, Fock KM, Song M, Ang D, Kwek AB, Ong J, Tan J, Teo EK, Dhamodaran S. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J Gastroenterol Hepatol. 2015;30:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Li BZ, Threapleton DE, Wang JY, Xu JM, Yuan JQ, Zhang C, Li P, Ye QL, Guo B, Mao C. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis. BMJ. 2015;351:h4052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 38. | Wu IT, Chuah SK, Lee CH, Liang CM, Lu LS, Kuo YH, Yen YH, Hu ML, Chou YP, Yang SC. Five-year sequential changes in secondary antibiotic resistance of Helicobacter pylori in Taiwan. World J Gastroenterol. 2015;21:10669-10674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, Chung WC, Kim BW, Kim SS. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21:351-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Nagahara A, Miwa H, Yamada T, Kurosawa A, Ohkura R, Sato N. Five-day proton pump inhibitor-based quadruple therapy regimen is more effective than 7-day triple therapy regimen for Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Vergara M, Vallve M, Gisbert JP, Calvet X. Meta-analysis: comparative efficacy of different proton-pump inhibitors in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;18:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Berrutti M, Pellicano R, Astegiano M, Smedile A, Saracco G, Morgando A, De Angelis C, Repici A, Fagoonee S, Leone N. Helicobacter pylori eradication: metronidazole or tinidazole? Data from Turin, Italy. Minerva Gastroenterol Dietol. 2008;54:355-358. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Slomiany BL, Tovey FI S- Editor: Gong ZM L- Editor: A E- Editor: Ma S