Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5436
Peer-review started: March 11, 2016
First decision: March 21, 2016
Revised: April 4, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: June 21, 2016
Processing time: 135 Days and 19.8 Hours
AIM: To compare previously reported randomized controlled studies (RCTs) of cold and hot polypectomy, we systematically reviewed and clarify the utility of cold polypectomy over hot with respect to efficacy and adverse events.
METHODS: A meta-analysis was conducted to evaluate the predominance of cold and hot polypectomy for removing colon polyps. Published articles and abstracts from worldwide conferences were searched using the keywords “cold polypectomy”. RCTs that compared either or both the effects or adverse events of cold polypectomy with those of hot polypectomy were collected. The patients’ demographics, endoscopic procedures, No. of examined lesions, lesion size, macroscopic and histologic findings, rates of incomplete resection, bleeding amount, perforation, and length of procedure were extracted from each study. A forest plot analysis was used to verify the relative strength of the effects and adverse events of each procedure. A funnel plot was generated to assess the possibility of publication bias.
RESULTS: Ultimately, six RCTs were selected. No significant differences were noted in the average lesion size (less than 10 mm) between the cold and hot polypectomy groups in each study. Further, the rates of complete resection and adverse events, including delayed bleeding, did not differ markedly between cold and hot polypectomy. The average procedural time in the cold polypectomy group was significantly shorter than in the hot polypectomy group.
CONCLUSION: Cold polypectomy is a time-saving procedure for removing small polyps with markedly similar curability and safety to hot polypectomy.
Core tip: We conducted a meta-analysis to evaluate the predominance of cold and hot polypectomy for removing colon polyps. The patients’ demographics, endoscopic procedures, No. of examined lesions, lesion size, macroscopic and histologic findings, rates of incomplete resection, bleeding, and perforation, and length of procedure were extracted from six randomized controlled studies. The rates of complete resection and adverse events did not markedly differ between cold and hot polypectomy. However, the procedural time was significantly shorter in the cold polypectomy group. These results suggest that cold polypectomy is a time-saving procedure for removing small polyps with similar curability and safety to hot polypectomy.
- Citation: Fujiya M, Sato H, Ueno N, Sakatani A, Tanaka K, Dokoshi T, Fujibayashi S, Nomura Y, Kashima S, Gotoh T, Sasajima J, Moriichi K, Watari J, Kohgo Y. Efficacy and adverse events of cold vs hot polypectomy: A meta-analysis. World J Gastroenterol 2016; 22(23): 5436-5444
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5436.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5436
Colon cancer is an extremely common cancer with high mortality in both Asia and the West. Because colon cancer develops due to an accumulation of genetic alterations prompting the transformation of the normal colon epithelium to adenoma and subsequently adenocarcinoma[1,2], removal of colon adenomas is considered crucial for preventing the development of cancer[3]. Indeed, several trials have demonstrated that removal of colon adenoma successfully decreases the mortality rate of colon cancer[3,4].
Polypectomy is a minimally invasive and easy-to-learn procedure for removing colon adenomas, particularly elevated-shaped ones. The polypectomy procedure for colon neoplasms generally uses a snare to enclose the lesion, which is then cut using a high-frequency generator. This procedure is known as hot polypectomy. Cutting and coagulation of the lesion with a high-frequency generator is generally believed to help prevent bleeding after polypectomy, although this procedure can occasionally cause perforation after lesion removal due to the burning of the intestinal wall.
An alternative procedure, known as cold polypectomy, simply removes lesions using a snare with no high-frequency generator and is believed to be as effective as hot polypectomy for removing colon neoplasms, but with lower risk of complications, such as perforation. In their examination of 210 constitutive patients after cold polypectomy for polyps 5 mm or less in size, Tappero et al[5] observed no adverse effects, including immediate or delayed bleeding or perforation. In addition, several uncontrolled studies have further confirmed the safety of cold polypectomy for removing small polyps[5-8]. However, 2 uncontrolled studies reported incomplete resection rates of 5% and 11% for cold polypectomy[9,10], which could still allow for progression to colon cancer. Any advantages of cold polypectomy over hot with respect to efficacy and adverse events therefore remain unclear.
To clarify the utility of cold polypectomy concerning efficacy and adverse events, we systematically reviewed previously-reported randomized controlled studies (RCTs) comparing cold and hot polypectomy procedures.
Articles posted on PubMed (as of March 2015) and abstracts of worldwide conferences described in Gastrointestinal Endoscopy and Gut were searched using the keywords “cold polypectomy”. The language was limited to studies published in English.
The results were reviewed by two evaluators. The abstracts were not blinded for authors, institutions, or journals during review. All studies comparing the effects and adverse events of cold polypectomy with those of hot polypectomy were collected, regardless of whether or not the data were part of the primary or secondary endpoint. When multiple articles were published from the same institution or study group, the most recent one was selected for our analysis. Even if the study group was included in multiple articles, the data were applied when the study population was different in each study. Reviews, case reports, abstracts, and presentations from meetings were excluded.
The risk of bias was evaluated in accordance with the Cochrane Handbook for Systematic Reviews of Interventions using the following parameters: adequacy of random sequence generation; allocation concealment; blinding of the participants, personnel and outcome assessors; incomplete outcome data and selective outcome reporting. The Jadad score was used to evaluate the quality of each study[11]. Briefly, the details of the randomization and blinding procedures and information regarding withdrawals were first evaluated. One or two points were then awarded for specifying the randomization and blinding procedures and one point for a statement of information regarding withdrawals (Table 1). A funnel plot was generated to assess the possibility of any publication bias[12].
| Ref. | Risk of bias | Jadad score | ||||||||
| Adequacy of random sequence generation | Allocation concealment | Blinding of the participants, personnel and outcome assessors | Blind outcome assessment | Incomplete outcome data | Selective outcome reporting | Details of randomization | Blinding procedures | Information regarding withdrawals | Total score | |
| Horiuchi et al[15], 2010 | High | High | Unclear | Unclear | Unclear | Low | 1 | 1 | 0 | 2 |
| Ichise et al[16], 2011 | Unclear | Low | Unclear | Unclear | Unclear | Low | 2 | 1 | 0 | 3 |
| Paspatis et al[17], 2011 | Low | Unclear | Unclear | Unclear | Unclear | Low | 2 | 0 | 1 | 3 |
| Aslan et al[18], 2013 | High | High | Unclear | Unclear | High | Unclear | 1 | 1 | 0 | 2 |
| Horiuchi et al[21], 2013 | Low | Low | Unclear | Unclear | Unclear | Unclear | 2 | 2 | 0 | 4 |
| Gomez et al[20], 2014 | Low | Low | Unclear | Unclear | Low | Unclear | 2 | 1 | 1 | 3 |
The patients’ demographics, endoscopic procedures, No. of examined lesions, lesion size, macroscopic and histologic findings, rates of incomplete resection, occurrence of immediate or delayed bleeding and perforation, and procedure duration were extracted from each study. The macroscopic and histologic findings were assessed based on Paris classification[13] and Vienna classification[14], respectively. Incomplete resection was defined as the clear presence of tumor cells in the margin of the removed specimen on histologic examination. The procedure duration was defined as the total time elapsed during the procedure. A forest plot analysis was used to verify the relative strength of the effects and adverse events of each procedure in multiple quantitative scientific studies. The between-study heterogeneity was tested and quantified using the Cochran Q statistic and the I2 statistic, respectively.
The relative risk (RR) and 95% confidence interval (CI) were calculated for each variable based on the data. Statistical analyses were performed using the Cochrane Collaboration’s Revman 5.0 software program (The Cochrane Collaboration, Oxford, Oxfordshire, United Kingdom). The inter-study heterogeneity was assessed using the Cochran Q test, with the significance level set at P = 0.1, and was quantified using the I2 statistic. If any obvious inter-study heterogeneity was noted (I2 > 50%), the random effects model was chosen; otherwise, the fixed effects model was chosen.
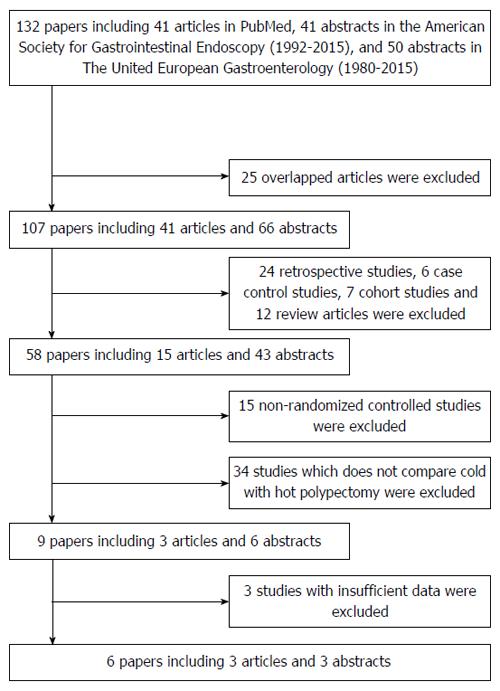
A total of 131 reports were initially retrieved (Figure 1). After screening the titles, abstracts, or full text and excluding reviews, case reports, uncontrolled tests, and basic research studies, seven studies were selected[15-21]. Of these seven, one study was excluded because some of the enrolled cases were believed to overlap with those of other reports[21]. The risk of bias was then assessed for the remaining six studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Table 1).
All six included studies were published from 2010 to 2014 (Table 2). The mean or median age of patients treated with cold polypectomy ranged from 59.4 to 67.0 years old, while those of patients treated with hot polypectomy ranged from 58.3 to 67.3 years old, which were described in 4 studies. The ratio of males to females in all described cases was 1.06 to 2.50 among cold polypectomy cases and 1.54 to 3.00 among hot polypectomy cases. Cold polypectomy with a snare only was used in 5 studies, while both snare and forceps were used in 1 study. The macroscopic appearances of the lesions in 2 studies were 0-I in 89 and 55 lesions and 0-II in 12 and 23 lesions among cold polypectomy cases, and 0-I in 95 and 62 lesions and 0-II in 9 and 19 lesions among hot polypectomy cases. The histologic diagnoses of the lesions in these 2 studies were 187 and 142 low-grade adenomas, 2 and 4 high-grade adenomas, and 16 and 13 hyperplastic polyps.
| Ref. | Patient, Age, mean ± SD or median (range) | Gender (M/F) | No. of polyps | Tumor size, mean ± SD (mm) | Endoscopic procedure for cold polypectomy | No. of all adverse events | Macroscopic appearances | Histologicalfindings | Complete retrieval rate | Post-polypectomy bleeding | Procedure time, mean ± SD (min) | |||
| 0-I | 0-II | High grade adenoma | Low grade adenoma | Hyperplastic polyp | ||||||||||
| Cold polypectomy | ||||||||||||||
| Horiuchi et al[15], 2010 | NA | NA | 94 | NA | Snare | 1 | NA | NA | NA | NA | NA | 96 | 0 | 18 |
| Ichise et al[16], 2011 | 65.1 ± 11 | 25/15 | 101 | 5.7 ± 4 | Snare | 1 | 89 | 12 | 1 | 94 | 6 | 96 | 0 | 18 ± 6 |
| Paspatis et al[17], 2011 | 59.4 ± 13.6 | 107/101 | 636 | 5.3 ± 1.4 | Snare | 19 | NA | NA | NA | NA | NA | 96 | 0 | 23.3 ± 4.8 |
| Aslan et al[18], 2013 | 59.5 ± 14.9 | 32/17 | 78 | 7.21 ± 1.4 | Snare | 1 | NA | NA | NA | NA | NA | 94.9 | 1 | NA |
| Horiuchi et al[21], 2013 | 67.0 ± 13 | 25/10 | 78 | 6.5 ± 1.2 | Snare | 4 | 55 | 23 | 2 | 70 | 6 | 94 | 0 | 16 ± 7 |
| Gomez et al[20], 2014 | NA | NA | 44 | NA | Snare or Forceps | 0 | NA | NA | NA | NA | NA | 90 (cold snare), 89 (cold forceps) | 0 | NA |
| Hot polypectomy | ||||||||||||||
| Horiuchi et al[15], 2010 | NA | NA | 92 | NA | - | 8 | NA | NA | NA | NA | NA | 97 | 0 | 25 |
| Ichise et al[16], 2011 | 65.5 ± 12 | 28/12 | 104 | 5.5 ± 6 | - | 8 | 95 | 9 | 1 | 93 | 10 | 96 | 0 | 25 ± 7 |
| Paspatis et al[17], 2011 | 61.3 ± 11 | 125/81 | 619 | 5.67 ± 1.3 | - | 2 | NA | NA | NA | NA | NA | 96 | 0 | 29.6 ± 7.4 |
| Aslan et al[18], 2013 | 58.3 ± 13.5 | 36/12 | 71 | 7.56 ± 1.45 | - | 1 | NA | NA | NA | NA | NA | 94.4 | 1 | NA |
| Horiuchi et al[21], 2013 | 67.3 ± 12 | 24/11 | 81 | 6.8 ± 1.3 | - | 16 | 62 | 19 | 2 | 72 | 7 | 93 | 5 | 26 ± 9 |
| Gomez et al[20], 2014 | NA | NA | 18 | NA | - | 0 | NA | NA | NA | NA | NA | 94 | 0 | NA |
Two and four studies investigated lesions measuring 10 mm or less[18,19] and 8 mm or less in diameter[15-17,20], respectively, indicating that all studies investigated the effect of cold and hot polypectomy on the treatment of small polyps. Four studies described the average size and standard deviation (SD) of the examined lesions[16-19], and no significant difference was noted in the average size (less than 10 mm) between the cold and hot polypectomy groups in each study (Table 2).
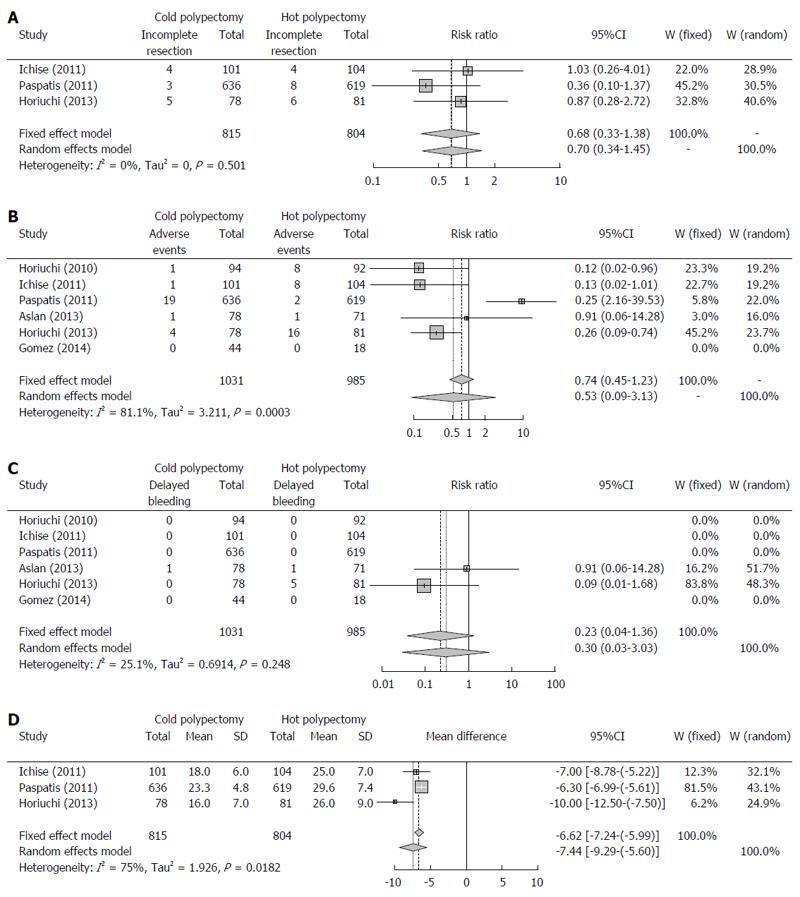
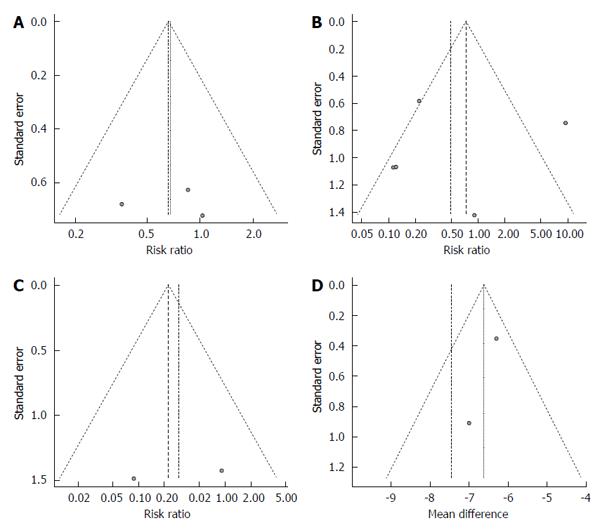
Three studies described the rate of curative resection[16,17,19]. These reports included a total of 815 lesions removed by cold polypectomy and 804 removed by hot polypectomy. Total rates of curative resection by cold and hot polypectomy were 98.5% and 97.8%, respectively. Because the between-study heterogeneity of these 3 studies was low (P = 0.5134, I2 = 0%), a fixed-effects model was used to analyze the rate of curative resection. The RR for all lesions was 0.68, and the 95%CI ranged from 0.33 to 1.38 (Figure 2A), findings which indicate that the rate of complete resection did not markedly differ between cold and hot polypectomy groups. Evaluation of publication bias was difficult due to the small No. of studies, as shown in the funnel plot (Figure 3A).
All six studies reported the rate of adverse events, including bleeding and abdominal pain or discomfort[15-20]. These six reports included a total of 1031 lesions removed by cold polypectomy and 985 removed by hot polypectomy. Total rates of adverse events by cold and hot polypectomy were 2.5% and 3.6%, respectively. Because the between-study heterogeneity of these six studies was high (P = 0.0002, I2 = 81.8%), a random-effects model was used to analyze the rate of adverse events. The RR for all lesions was 0.53, and the 95%CI ranged from 0.09 to 3.13 (Figure 2B), findings which indicated that the rate of adverse events did not markedly differ between cold and hot polypectomy procedures. An evaluation of publication bias was difficult to carry out due to the small No. of studies, as shown in the funnel plot (Figure 3B).
Delayed bleeding was observed in only two studies, with conflicting findings in both[18,19]. While Aslan et al[18] noted no marked differences in the rate of bleeding between cold and hot polypectomy groups, Horiuchi et al[19] observed a higher bleeding rate in the hot polypectomy group than in the cold procedure group among patients given anticoagulants. Total rates of delayed bleeding by cold and hot polypectomy were 0.1% and 0.6%, respectively. Because the between-study heterogeneity of these six studies was high (P = 0.2427, I2 = 26.7%), a fixed-effects model was used to analyze the rate of bleeding. The RR for all lesions was 0.23, and the 95%CI ranged from 0.04 to 1.36 (Figure 2C). An evaluation of publication bias was difficult to carry out due to the small No. of studies, as shown in the funnel plot (Figure 3C).
No perforation occurred in any of the six studies investigated.
Three studies described the average and SD of procedure duration[16,17,19]. The average durations for these three studies ranged from 16.0 to 23.3 min in the cold polypectomy group and from 25.0 to 29.6 min in the hot polypectomy group. The cold polypectomy procedures were significantly shorter in duration than those using hot polypectomy in all three studies. Because the between-study heterogeneity of these three studies was high (P = 0.0182, I2 = 75.0%), a random-effects model was used to analyze the length of the procedure. The mean difference for all lesions was -7.44, and the 95%CI ranged from -9.29 to -5.60 (Figure 2D), findings which indicate that the cold polypectomy procedure was shorter than the hot polypectomy procedure. An evaluation of publication bias was difficult to carry out due to the small No. of studies, as shown in the funnel plot (Figure 3D).
To our knowledge, the present systematic review is the first meta-analysis to compare the efficacy and safety of cold polypectomy for removing colon tumors with those of hot polypectomy procedures based on previously published RCTs. In our analysis, the rates of incomplete resection and adverse events in the cold polypectomy group were not significantly different from those in the hot polypectomy group, suggesting that cold polypectomy possesses the same efficacy and safety as hot polypectomy. Further, the procedural time was significantly shorter in the cold polypectomy group, suggesting that cold polypectomy is also a time-saving procedure compared to hot polypectomy.
The present analysis also investigated the sizes of polyps encountered in the six RCTs and confirmed a lack of any marked difference in size between the two polypectomy groups, which indicates low bias associated with tumor size. However, all polyps encountered in these RCTs were 10 mm or less in diameter, and most were 8 mm or less. The findings from the present analysis therefore apply only with respect to removal of small polyps, and the risks of incomplete resection and adverse events with cold polypectomy for removing large polyps remains unclear. Previous studies have reported that rates of incomplete resection and adverse events of endoscopic resection are proportional to polyp size[22,23]. Recently, Tribonias et al[24] developed a new method for removing flat polyps measuring larger than 10 mm in size using a pulling technique and proposed the potential utility of cold polypectomy in removing large polyps. Further analysis of studies involving patients with large polyps using novel methods will be needed to determine whether or not cold polypectomy is a practical option for removing polyps larger than 10 mm in size.
Cold polypectomy can be performed via two methods: cold snare polypectomy and cold forceps polypectomy. The cold snare procedure uses snares to encircle and cut polyps, while the cold forceps procedure uses large forceps to “bite” polyps. While cold forceps polypectomy is easier to perform than cold snare polypectomy, the rate of incomplete resection is believed to be higher for cold forceps polypectomy[25], coming in at approximately 20% even when using jumbo-sized forceps[26]. In the RCTs retrieved in the present meta-analysis, only one study used the cold forceps procedure (and only for a portion of cases)[20], while other five studies used only the cold snare procedure[15-19]. Because the RCT using the cold forceps procedure did not describe the rate of incomplete resection, our meta-analysis of the rate of incomplete resection was not influenced by any bias related to cold polypectomy procedure.
Lee et al[27] showed in their RCT that the rate of incomplete resection for cold snare polypectomy was significantly lower than that in the cold forceps polypectomy group (93.2% vs 75.9%). Similarly, Kim et al[28] showed in their RCT that the rate of incomplete resection for cold snare polypectomy removing 5- to 7-mm-sized adenomatous polyps was significantly lower than that for cold forceps polypectomy (93.8% vs 70.3%). These findings suggest that the cold snare procedure is indeed superior to the cold forceps procedure for removing colon polyps.
Several limitations to the present meta-analysis warrant mention. First, the rates of recurrence after cold and hot polypectomies were not investigated, although histological margins were assessed. Because histological findings do not always predict future recurrence, a true rate of incomplete resection should be assessed through follow-up studies. Second, the difference in the mortality rates of colon cancer in populations with and without cold polypectomy was not investigated. Because the goal of polypectomy for removing colon adenoma is to reduce risk of colon cancer-associated death, the efficacy of cold polypectomy should be assessed based on improvement in colon cancer-related mortality. Long-term follow-up studies are therefore needed to clarify whether or not cold polypectomy does indeed reduce the mortality of colon cancer. Third, the skill and experience of each physician is believed to be another source of bias affecting the rates of incomplete resection and adverse events as well as the procedural time, which should be resolved by future studies.
In conclusion, we conducted the first meta-analysis concerning the efficacy and adverse events of cold polypectomy in comparison to hot polypectomy. Our findings demonstrate that cold polypectomy possesses similar curability and safety to hot polypectomy. Further, cold polypectomy is a time-saving procedure compared to hot polypectomy and is thus recommended for use over the hot procedure in removing small polyps. However, further analyses are needed to assess the feasibility of cold polypectomy for removing large polyps as well as to evaluate any bias associated with the skill and experience of each physician and the type of endoscopic device used.
Interest in cold polypectomy for removing small polyps is growing among endoscopists worldwide. In several prospective randomized trials, cold polypectomy has been proven effective in removing colon polyps less than 10 mm in diameter. However, no systematic review concerning the efficacy and safety of cold polypectomy, versus conventional hot polypectomy, has been published.
Polypectomy is an easy-to-learn, minimally invasive procedure for removing colon polyps. Cold polypectomy is believed to be a safer procedure with fewer complications than hot polypectomy.
To confirm the safety and efficacy of cold polypectomy for removing colon polyps, the authors evaluated the findings from six prospective randomized controlled trials (RCTs). This is the first meta-analysis of RCTs comparing cold and hot polypectomy outcomes.
The present findings support the safety and efficacy of cold polypectomy for removing colon polyps less than 10 mm in diameter.
Cochrane Handbook for Systematic Reviews of Interventions: This book is an official guide describing in detail the process of preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions. Adequacy of random sequence generation: A simple statement such as ‘we randomly allocated’ or ‘using a randomized design’ is often insufficient to prove that the allocation sequence was genuinely randomized. Authors commonly use the term ‘randomized’ even when it is not justified, and many trials with declared systematic allocation are described by the authors as being randomized. When in doubt, the adequacy of sequence generation should be considered unclear. Allocation concealment: Proper allocation sequence concealment secures strict implementation of an allocation sequence without foreknowledge of intervention assignments. Methods for allocation concealment refer to techniques used to implement the sequence, not to generate it. However, most allocation sequences that are deemed inadequate, such as allocation based on day of admission or case record number, cannot be adequately concealed, and so fail on both counts. It is theoretically possible, yet unlikely, that an inadequate sequence is adequately concealed. However, it is not uncommon for an adequate allocation sequence to be inadequately concealed, such as if the sequence is posted on a staff room wall. Blinding of the participants, personnel, and outcome assessors: Study reports often describe blinding in broad terms, such as ‘double-blinded’. This term makes it impossible to know who was blinded. Such terms are often used inconsistently, and the frequency of explicit reporting of the blinding status of study participants and personnel remains low. A review of methods used for blinding highlights the variety of methods used in practice. The following may help review authors assess whether or not any blinding of participants and personnel in a study was likely to be sufficient to protect against bias when using the Collaboration’s tool: When considering the risk of bias from lack of blinding of participants and personnel, consider specifically (1) who was and was not blinded; and (2) risk of bias in actual outcomes due to lack of blinding during the study. Incomplete outcome data and selective outcome reporting: The risk of bias arising from incomplete outcome data depends on several factors, including the amount and distribution across intervention groups, the reasons for outcomes being missing, the likely difference in outcome between participants with and without data, what study authors have done to address the problem in their reported analyses, and the clinical context. Therefore, it is not possible to formulate a simple rule for judging a study to be at low or high risk of bias. The following may help review authors assess whether or not incomplete outcome data can be addressed in a way that protects against bias when using the Collaboration’s tool. Jadad score: The Jadad score assesses the quality of published clinical trials based on methods relevant to random assignment, double blinding, and the flow of patients. The score is assigned based on four items. Forest plot: A forest plot is a graphic display of estimated results from a No. of scientific studies addressing the same question, along with the overall results. This plot was developed for use in medical research as a means of graphically representing a meta-analysis of the results of RCTs. Funnel plot: A funnel plot is a graph designed to check for the existence of publication bias; funnel plots are commonly used in systematic reviews and meta-analyses.
This systematic review and meta-analysis of six RCTs provides valuable information supporting cold polypectomy as a safe and time-saving procedure for removing small polyps with no risk of additional complications.
| 1. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 2. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 2832] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 3. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2386] [Article Influence: 170.4] [Reference Citation Analysis (2)] |
| 4. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3173] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 5. | Tappero G, Gaia E, De Giuli P, Martini S, Gubetta L, Emanuelli G. Cold snare excision of small colorectal polyps. Gastrointest Endosc. 1992;38:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 6. | Repici A, Hassan C, Vitetta E, Ferrara E, Manes G, Gullotti G, Princiotta A, Dulbecco P, Gaffuri N, Bettoni E. Safety of cold polypectomy for & lt; 10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Aslan F, Camcı M, Alper E, Akpınar Z, Arabul M, Celik M, Unsal B. Cold snare polypectomy versus hot snare polypectomy in endoscopic treatment of small polyps. Turk J Gastroenterol. 2014;25:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Chukmaitov A, Bradley CJ, Dahman B, Siangphoe U, BouHaidar D, Warren JL. Polypectomy techniques, endoscopist characteristics, and serious gastrointestinal adverse events. J Surg Oncol. 2014;110:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Ellis KSM, Marquis S, Katon R. Efficacy of hot biopsy forceps, cold micro-snare and micro-snare with cautery techniques in the removal of diminutive colonic polyps. Gastrointest Endosc. 1997;45:AB107. [DOI] [Full Text] |
| 10. | Humphris JL, Kwok A, Katelaris PH. Cold snare polypectomy for diminutive polyps: An assessment of the risk of incomplete removal of small adenomas. Gastrointest Endosc. 2009;69:AB207. [DOI] [Full Text] |
| 11. | Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 755] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Derry S, Loke YK, Aronson JK. Incomplete evidence: the inadequacy of databases in tracing published adverse drug reactions in clinical trials. BMC Med Res Methodol. 2001;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 675] [Article Influence: 32.1] [Reference Citation Analysis (2)] |
| 14. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [PubMed] |
| 15. | Horiuchi A, Nakayama Y. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy. Gastointestinal Endosc. 2010;71:AB127. [DOI] [Full Text] |
| 16. | Ichise Y, Horiuchi A, Nakayama Y, Tanaka N. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion. 2011;84:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Paspatis GA, Tribonias G, Konstantinidis K, Theodoropoulou A, Vardas E, Voudoukis E, Manolaraki MM, Chainaki I, Chlouverakis G. A prospective randomized comparison of cold vs hot snare polypectomy in the occurrence of postpolypectomy bleeding in small colonic polyps. Colorectal Dis. 2011;13:e345-e348. [PubMed] |
| 18. | Aslan F, Alper E, Vatansever S, Akpinar Z, Camci M, Arabul M, Celik M, Kandemir A, Ipek S, Akay HS. Cold snare polypectomy versus standard snare polypectomy in endoscopic treatment of small polyps. Gastrointestinal Endosc. 2013;77:AB561. [DOI] [Full Text] |
| 19. | Horiuchi A, Nakayama Y, Kajiyama M, Tanaka N, Sano K, Graham DY. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417-423. [PubMed] |
| 20. | Gomez V, Krishna M, Cangemi JR, Picco MF, Raimondo M, Crook J, Diehl N, Wallace MB. Resection of diminutive colorectal polyps comparing hot snare, cold snare and cold foceps polypectomy. Results of a randomized, single center pilot study. Gastrontest Endosc. 2014;79:AB540. |
| 21. | Horiuchi A, Nakayama Y. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps in patients receiving anticoagulation therapy. Gastrointest Endosc. 2013;77:AB174. [DOI] [Full Text] |
| 22. | Moon HS, Park SW, Kim DH, Kang SH, Sung JK, Jeong HY. Only the size of resected polyps is an independent risk factor for delayed postpolypectomy hemorrhage: a 10-year single-center case-control study. Ann Coloproctol. 2014;30:182-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 24. | Tribonias G, Komeda Y, Voudoukis E, Bassioukas S, Viazis N, Manola ME, Giannikaki E, Papalois A, Paraskeva K, Karamanolis D. Cold snare polypectomy with pull technique of flat colonic polyps up to 12 mm: a porcine model. Ann Gastroenterol. 2015;28:141-143. [PubMed] |
| 25. | Efthymiou M, Taylor AC, Desmond PV, Allen PB, Chen RY. Biopsy forceps is inadequate for the resection of diminutive polyps. Endoscopy. 2011;43:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Draganov PV, Chang MN, Alkhasawneh A, Dixon LR, Lieb J, Moshiree B, Polyak S, Sultan S, Collins D, Suman A. Randomized, controlled trial of standard, large-capacity versus jumbo biopsy forceps for polypectomy of small, sessile, colorectal polyps. Gastrointest Endosc. 2012;75:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. Cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol. 2013;108:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Kim JS, Lee BI, Choi H, Jun SY, Park ES, Park JM, Lee IS, Kim BW, Kim SW, Choi MG. Cold snare polypectomy versus cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc. 2015;81:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Seow-Choen F S- Editor: Yu J L- Editor: A E- Editor: Wang CH