Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5430
Peer-review started: February 15, 2016
First decision: March 21, 2016
Revised: April 17, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: June 21, 2016
Processing time: 119 Days and 15 Hours
AIM: To compare the clinical efficacy of the second-generation H2RA lafutidine with that of lansoprazole in Japanese patients with mild gastroesophageal reflux disease (GERD).
METHODS: Patients with symptoms of GERD and a diagnosis of grade A reflux esophagitis (according to the Los Angeles classification) were randomized to receive lafutidine (10 mg, twice daily) or lansoprazole (30 mg, once daily) for an initial 8 wk, followed by maintenance treatment comprising half-doses of the assigned drug for 24 wk. The primary endpoint was the frequency and severity of heartburn during initial and maintenance treatment. The secondary endpoints were the sum score of questions 2 and 3 in the Gastrointestinal Symptom Rating Scale (GSRS), and the satisfaction score.
RESULTS: Between April 2012 and March 2013, a total of 53 patients were enrolled, of whom 24 and 29 received lafutidine and lansoprazole, respectively. After 8 wk, the frequency and severity of heartburn was significantly reduced in both groups. However, lafutidine was significantly inferior to lansoprazole with regard to the severity of heartburn during initial and maintenance treatment (P = 0.016). The sum score of questions 2 and 3 in the GSRS, and satisfaction scores were also significantly worse in the lafutidine group than the lansoprazole group (P = 0.0068 and P = 0.0048, respectively).
CONCLUSION: The clinical efficacy of lafutidine was inferior to that of lansoprazole, even in Japanese patients with mild GERD.
Core tip: The clinical efficacy of the second-generation H2RA lafutidine was inferior to that of lansoprazole, particularly during maintenance therapy, even in Japanese patients with mild gastroesophageal reflux disease.
- Citation: Takenaka R, Okada H, Kawano S, Komazawa Y, Yoshinaga F, Nagata S, Inoue M, Komatsu H, Onogawa S, Kushiyama Y, Mukai S, Todo H, Okanobu H, Manabe N, Tanaka S, Haruma K, Kinoshita Y. Randomized study of lafutidine vs lansoprazole in patients with mild gastroesophageal reflux disease. World J Gastroenterol 2016; 22(23): 5430-5435
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5430.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5430
Gastroesophageal reflux disease (GERD) is characterized by symptoms of heartburn and acid regurgitation. It is extremely common and has a chronic, relapsing disease course[1]. Standard pharmacological treatments for GERD comprise proton pump inhibitors (PPIs) and histamine receptor-2 antagonists (H2RAs), which act by suppressing gastric acid secretion. Of the two drug classes, PPIs are considered to be more cost-effective as initial treatment for GERD[2,3]. However, the cost-effectiveness of pharmacotherapy in Japanese patients with mild GERD has not been evaluated.
Lafutidine is a second-generation H2RA that has a potent and sustained anti-acid secretory effect. It acts by increasing plasma concentrations of calcitonin gene-related peptide and somatostatin, resulting in inhibition of postprandial acid secretion and gastroprotective activity[4]. In contrast to other H2RAs, the therapeutic action of lafutidine involves esophageal host-defense via capsaicin-sensitive afferent nerves[5]. The LAFORE trials conducted in Japanese patients with mild GERD (grade A in the Los Angeles classification) indicated that healing rates at 8 wk were 79.4% in the lafutidine groups and 68.3% in the famotidine group[6]. The cost of lafutidine treatment is 41.3 yen per day, and cheaper than half doses of PPIs (95.2 yen).
The aim of this study was to compare the clinical efficacy of lafutidine with that of lansoprazole as initial and maintenance treatment in Japanese patients with mild GERD.
This was a phase III, controlled study performed in 4 university hospitals and 11 of their affiliated hospitals in Japan between April 2012 and March 2013. The study was approved by the institutional review board of each participating hospital and was conducted in accordance to Good Clinical Practice guidelines. Written informed consent was obtained from all patients. The study is registered in the University Hospital Medical Network Clinical Trials Registry (unique trial number UMIN000006162).
Inclusion criteria: Patients who were ≥ 20 years old with symptoms of heartburn or regurgitation and a diagnosis of grade A reflux esophagitis according to the Los Angeles classification, as confirmed by endoscopic examination at least 1 wk prior to the observation period, were eligible for enrollment. Both the frequency and severity of symptoms were required to be ≥ 3 on question 2 or 3 of the Gastrointestinal Symptom Rating Scale (GSRS).
Exclusion criteria: Patients with any of the following conditions were excluded: (1) gastric or duodenal ulcers (excluding ulcer scars); (2) esophageal, gastric or duodenal cancer; (3) the concurrent presence of Barrett’s esophagus; (4) a history of upper gastrointestinal resection; (5) a history of receiving PPIs or H2RAs within the 2 wk prior to endoscopic examination; (6) comorbidity with severe cardiovascular, hepatic, or renal disease; (7) a history of allergy to lafutidine or lansoprazole; and (8) other conditions considered unsuitable for study participation by the attending physician.
Eligible patients were randomized in a 1:1 ration to receive lafutidine (10 mg, twice daily) or lansoprazole (30 mg, once daily) for an initial 8 wk according to assignment lists generated by a permuted-block procedure. Patients were questioned on the frequency of heartburn during the week prior to initial treatment. After initial treatment, the frequency of heartburn was recorded daily for 2 wk. The number of episodes and the severity of heartburn were also assessed using the visual analog scale (VAS) and GSRS until 24 wk after the initial treatment-period. During initial treatment, concomitant administration of the following drugs was not permitted: (1) PPI; (2) H2RA; (3) prostaglandins; (4) mucosal protection drugs; (5) antacids; and (6) drugs that may affect upper gastrointestinal symptoms. Unless symptoms became worse, the allocated drug was administrated as initial treatment for 8 wk, followed by maintenance treatment for 24 wk. In the lafutidine group, a half dose was selected for maintenance treatment if symptoms had improved or disappeared at the first assessment, whereas the full dose was continued if symptoms had not improved. An asymptomatic state was defined as ≤ 2 for both questions 2 and 3 on the GSRS. In the lansoprazole group, a half dose was administrated irrespective of symptoms, but was changed to the full dose if symptoms worsened. If symptoms had not improved at the first maintenance assessment, the attending physician was permitted to consider other treatment strategies. Patients underwent symptomatic evaluation every 8 wk for the duration of 32 wk. During the study, the number of episodes of heartburn was evaluated by reviewing patients’ diaries. In addition to endoscopy, physical examinations and laboratory tests were performed to confirm the eligibility and safety of the patients.
Patients’ diaries were used to assess the frequency and severity of heartburn. The severity of heartburn and patient satisfaction were graded with VAS scores.
The primary endpoint was the frequency and severity of heart burn. The secondary endpoints were the sum score of questions 2 and 3 of the GSRS, and the patient satisfaction score, during initial and maintenance treatment.
Statistical differences between the two groups were determined with the chi-squared test or Fisher’s exact test for discontinuous variables and the Mann-Whitney U test or Wilcoxon rank sum test for continuous variables. Repeated measures such as VAS and GSRS scores were analyzed by ANOVA. Statistical analyses were performed with the JMP (version 7) software package (SAS Institute, Cary, North Carolina, United States). P values ≤ 0.05 were considered to denote statistically significant differences between groups. The statistical methods of this study were reviewed by Ryuta Takenaka from Tsuyama Chuo Hospital.
Between April 2012 and March 2013, the study enrolled 53 patients with heartburn or regurgitation who underwent upper gastrointestinal endoscopy. Among the 53 patients, 24 and 29 were randomized to the lafutidine and lansoprazole group, respectively. There were no significant differences between the two groups in background characteristics, except for gender distribution (Table 1).
| Lafutidine group (n = 24) | Lansoprazole group (n = 29) | P value | |
| Age (median, range) | 64 (27-80) | 56 (26-85) | 0.24 |
| Gender (male/female) | 8/16 | 22/7 | 0.0025 |
| Hiatal hernia | 13 (54) | 19 (66) | 0.57 |
| H. pylori infection | 2 (8.3) | 3 (10.3) | > 0.99 |
| Habits | |||
| Alcohol | 7 (29.2) | 13 (44.8) | 0.27 |
| Tobacco | 3 (12.5) | 6 (20.7) | 0.49 |
| Coffee | 14 (58.3) | 15 (51.7) | 0.78 |
| Co-morbidity | |||
| Hypertension | 2 (8.3) | 4 (13.8) | 0.68 |
| Diabetes mellitus | 3 (12.5) | 3 (10.3) | > 0.99 |
| Cerebrovascular disease | 0 (0) | 0 (0) | - |
| Cardiovascular disease | 0 (0) | 0 (0) | - |
| Liver disease | 1 (4.2) | 3 (10.3) | 0.62 |
| Renal failure | 0 (0) | 0 (0) | - |
| Malignancy | 2 (8.3) | 2 (6.9) | > 0.99 |
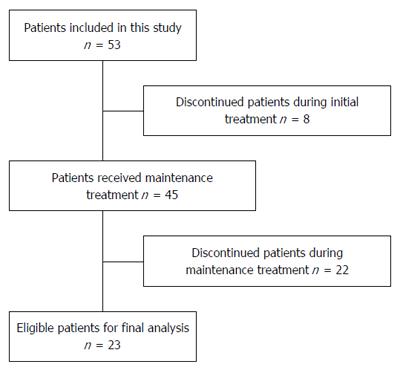
During initial treatment, 8 patients discontinued the study, with the most common reasons being withdrawal of informed consent. Of the 45 patients assigned to receive maintenance treatment, 22 were excluded from the final analysis, primarily because of missing to follow-up (Figure 1).
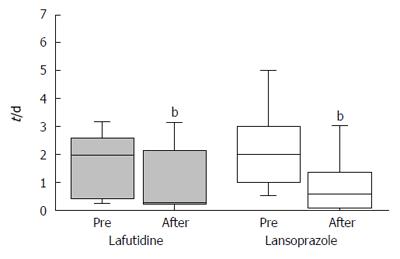
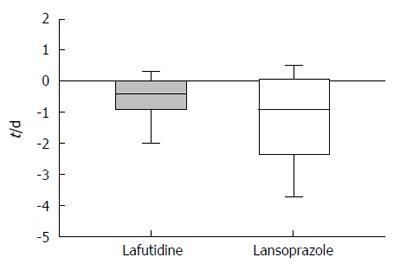
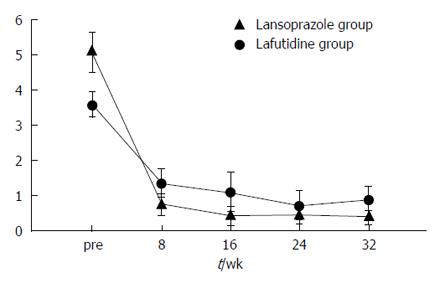
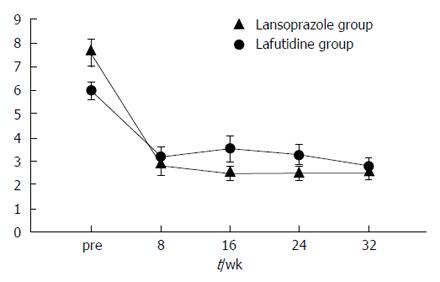
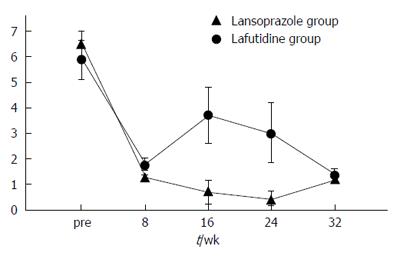
The frequency of heartburn according to the patients’ questionnaires was lower after initial treatment in both groups (Figure 2). However, the change in heartburn frequency was not statistically significantly different in either group (Figure 3). The severity of heartburn, evaluated using VAS, was also significantly reduced after initial treatment in both groups. Worsening of symptom during maintenance treatment did not occur in either group. However, lafutidine was significantly inferior to lansoprazole in reducing heartburn severity (Figure 4). Similarly, the sum score of questions 2 and 3 in the GSRS and the satisfaction score were both significantly worse in the lafutidine group than those in the lansoprazole group, particularly during maintenance therapy (Figures 5 and 6, respectively).
GERD is a chronic disorder and long-term acid-suppression therapy is necessary in most cases[7]. A meta-analysis of 34 trials that included 1,314 individuals demonstrated that PPIs were significantly more effective than H2RAs in relieving heartburn in GERD (RR = 0.66, 95%CI: 0.60-0.73) as well as in patients with non-erosive reflux disease on upper endoscopy (RR = 0.78, 95%CI: 0.62-0.97)[8]. Because no reports have been published concerning the cost-effectiveness of pharmacotherapy in Japanese patients with mild GERD, which is a common condition in Japan, the present study was designed to evaluate the efficacy of the second-generation H2RA lafutidine. However, lansoprazole was superior to lafutidine in Japanese patients with mild GERD, not only with respect to lowering the severity of heartburn, but also the satisfaction score. The GSRS score was also better in the lansoprazole than the lafutidine group. Thus, the hypothesis that lafutidine has similar efficacy and superior cost-effectiveness compared with lansoprazole in Japanese patients with mild GERD was not confirmed.
Limitations of PPIs include a higher cost than H2RAs, and potential side effects such as hypochlorhydria and hypergastrinemia. In particular, PPI-related hypochlorhydria is concern because it may increase susceptibility to infections, for example Clostridium difficile-associated diarrhea[9-11], as well as malabsorption leading to hypomagnesemia[12]. Hypochlorhydria may theoretically reduce calcium absorption[13]. A meta-analysis that included 11 cohort and case-control studies examined the risk of fractures associated with PPI use, and showed that the risk of hip fracture, spine fracture, and any-site fracture was increased among PPI users compared with nonusers (RR = 1.30, 95%CI: 1.19-1.43; RR = 1.56, 95%CI: 1.31-1.85; and RR = 1.16, 95%CI: 1.02-1.32, respectively)[14]. Therefore, once an asymptomatic state has been attained, tapering or cessation of medication should be considered in patients with GERD.
In the present study, initial therapy improved clinical outcomes such as heartburn and patient satisfaction score in both the lafutidine group and the lansoprazole group. However, the satisfaction score and GSRS score became worse during maintenance therapy in the lafutidine group. The effect of acid suppression in the lafutidine group was weak during the maintenance therapy. Two possible mechanisms may explain this observation. Firstly, patients may develop tolerance to lafutidine during maintenance therapy. As previously reported in a study assessing ranitidine and famotidine, the development of tachyphylaxis within 2 to 6 wk of initiation of H2RAs limits their use as maintenance therapy for GERD[15]. Secondly, the dose of lafutidine during maintenance therapy was low for symptomatic relief. If a 10-mg dose of lafutidine was administered twice daily during maintenance therapy, symptomatic severity may have been similar to that in the lansoprazole group. The costs of 10 mg of lafutidine twice daily and a half dose of lansoprazole were 82.6 yen and 95.2 yen, respectively. Therefore, future studies are planned to evaluate 10 mg of lafutidine twice daily for maintenance therapy, with the aim of proving our hypothesis that lafutidine has similar efficacy and superior cost-performance to lansoprazole in Japanese patients with mild GERD.
This study has some limitations. The sample size was relatively small. Furthermore, a substantial number of patients discontinued the study although the proportion of patients lost to follow-up was similar in the two treatment groups. In addition, the male/female ratio was significantly smaller in the lafutidine than the lansoprazole group, which may have had an influence on clinical outcomes.
In conclusion, the efficacy of a second-generation H2RA over a PPI in Japanese patients with mild GERD was not demonstrated, most notably during maintenance therapy.
Proton pump inhibitors (PPIs) are considered to be more cost-effective for patients with gastroesophageal reflux disease (GERD) than first-generation histamine receptor-2 antagonists (H2RAs). Lafutidine is a second-generation H2RA that has a potent and sustained anti-acid secretory effect. In LAFORE trials conducted in Japanese patients with mild GERD, lafutidine was superior to first-generation H2RA (famotidine). The aim of this study was to compare the clinical efficacy of lafutidine with that of PPI (lansoprazole) as initial and maintenance treatment in Japanese patients with mild GERD.
GERD is a chronic disorder and long-term acid-suppression therapy is necessary in most cases. Limitations of PPIs include a higher cost than H2RAs, and potential side effects related to hypochlorhydria and hypergastrinemia.
Lansoprazole was superior to lafutidine in Japanese patients with mild GERD, not only with respect to lowering the severity of heartburn, but also the satisfaction score. The hypothesis that lafutidine had similar efficacy and superior cost-effectiveness compared with lansoprazole in Japanese patients with mild GERD was not confirmed.
The efficacy of a second-generation H2RA over a PPI in Japanese patients with mild GERD was not demonstrated, most notably during maintenance therapy.
Lafutidine is a second-generation H2RA and shown to be superior to first-generation H2RA (famotidine).
Interesting and well written study.
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2519] [Article Influence: 126.0] [Reference Citation Analysis (2)] |
| 2. | Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev. 2007;CD003244. [PubMed] |
| 3. | Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Sato H, Kawashima K, Yuki M, Kazumori H, Rumi MA, Ortega-Cava CF, Ishihara S, Kinoshita Y. Lafutidine, a novel histamine H2-receptor antagonist, increases serum calcitonin gene-related peptide in rats after water immersion-restraint stress. J Lab Clin Med. 2003;141:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Nakano M, Ajioka H, Abe M, Kiniwa M. Possible involvement of host defense mechanism in the suppression of rat acute reflux esophagitis by the particular histamine H2 receptor antagonist lafutidine. Pharmacology. 2012;90:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ohara S, Haruma K, Kinoshita Y, Kusano M. A double-blind, controlled study comparing lafutidine with placebo and famotidine in Japanese patients with mild reflux esophagitis. J Gastroenterol. 2010;45:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kinoshita Y, Adachi K, Fujishiro H. Therapeutic approaches to reflux disease, focusing on acid secretion. J Gastroenterol. 2003;38 Suppl 15:13-19. [PubMed] |
| 8. | Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;5:CD002095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2844] [Article Influence: 948.0] [Reference Citation Analysis (0)] |
| 9. | Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 676] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 10. | Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Recker RR. Calcium absorption and achlorhydria. N Engl J Med. 1985;313:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 308] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519-526. [PubMed] |
| 15. | Komazawa Y, Adachi K, Mihara T, Ono M, Kawamura A, Fujishiro H, Kinoshita Y. Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Annese V S- Editor: Qi Y L- Editor: A E- Editor: Wang CH