Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.5017
Peer-review started: November 10, 2014
First decision: December 11, 2014
Revised: December 22, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: April 28, 2015
Processing time: 168 Days and 21.2 Hours
AIM: To compare differences between volumetric interpolated breath-hold examination (VIBE) using two-point Dixon fat-water separation (Dixon-VIBE) and chemically selective fat saturation (FS-VIBE) with magnetic resonance imaging examination.
METHODS: Forty-nine patients were included, who were scanned with two VIBE sequences (Dixon-VIBE and FS-VIBE) in hepatobiliary phase after gadoxetic acid administration. Subjective evaluations including sharpness of tumor, sharpness of vessels, strength and homogeneity of fat suppression, and artifacts that were scored using a 4-point scale. The liver-to-lesion contrast was also calculated and compared.
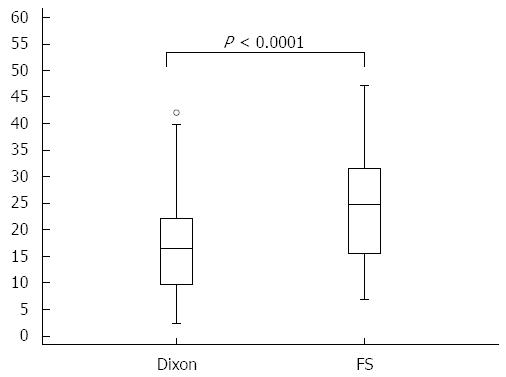
RESULTS: Dixon-VIBE with water reconstruction had significantly higher subjective scores than FS-VIBE in strength and homogeneity of fat suppression (< 0.0001) but lower scores in sharpness of tumor (P < 0.0001), sharpness of vessels (P = 0.0001), and artifacts (P = 0.034). The liver-to-lesion contrast on Dixon-VIBE images was significantly lower than that on FS-VIBE (16.6% ± 9.4% vs 23.9% ± 12.1%, P = 0.0001).
CONCLUSION: Dixon-VIBE provides stronger and more homogenous fat suppression than FS-VIBE, while has lower clarity of focal liver lesions in hepatobiliary phase after gadoxetic acid administration.
Core tip: The role of three dimensional gradient echo sequence with volumetric interpolated breath-hold examination (VIBE) by using chemically selective fat-saturation for abdominal magnetic resonance (MR) imaging is well established and it is now part of the standard clinical work-up, especially for dynamic contrast-enhanced liver MR imaging. The Dixon technique has been improved extensively in the aspects of phase errors, noise and artifacts. There are no reports yet on the potential value of two-point Dixon fat-water separation technique for image quality and focal liver lesions in hepatobiliary phase of gadoxetic acid-enhanced MR imaging. Therefore, we compare the image quality and liver-to-lesion contrast in hapatobiliary phase between VIBE using two-point Dixon fat-water separation and chemically selective fat saturation.
- Citation: Ding Y, Rao SX, Chen CZ, Li RC, Zeng MS. Usefulness of two-point Dixon fat-water separation technique in gadoxetic acid-enhanced liver magnetic resonance imaging. World J Gastroenterol 2015; 21(16): 5017-5022
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/5017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.5017
Gadoxetic acid is a hepatocyte-specific contrast agent for magnetic resonance (MR) imaging of the liver[1-4]. The major value of gadoxetic acid compared with other gadolinium-based agents is that it can be taken up by normal functional hepatocytes and excreted into the bile duct. Many studies have showed that gadoxetic acid-enhanced imaging can increase the detection of focal liver lesions, especially for small liver lesions[5-7], as well as improve the confidence for characterization of liver lesions[8-10]. The role of three dimensional (3D) gradient echo sequence with volumetric interpolated breath-hold examination (VIBE) by using chemically selective fat-saturation for abdominal MR imaging is well established and it is now part of the standard clinical work-up, especially for dynamic contrast-enhanced liver MR imaging[11-13]. Chemically selective fat-saturation imaging reduces the potential degradation of image quality resulting from motion-related artifacts, helps increase image contrast resolution and highlights lesions such as contrast-enhancing tissue, edema, and blood products by eliminating the high-intensity signal of fat[11,14]. However, this technique is susceptible to B0 and B1 inhomogeneities, which remains a challenge, particularly for imaging off-center, with large field of view, or anatomies with strong susceptibility effects[15,16]. Studies are therefore focusing on the potential added benefit of other methods for fat suppression like Dixon fat-water separation technique[17-21]. The Dixon technique, described for water and fat imaging in 1984[22-24], has been improved extensively in the aspects of phase errors, noise and artifacts. In addition, this technique is routinely used to find intracytoplasmic lipid (also referred to as microscopic fat)[25], as well as to have the potential for detection and grading of liver iron[26], based on comparison of signal intensity on in-phase and opposed-phase images. Ragan et al[19] demonstrated that 2-point Dixon fat separation with water reconstruction provided more reliable and homogenous fat suppression than chemical saturation in phantoms and mouse MR imaging. Previous studies also reported the similar results for abdominal and pelvic MR imaging in humans[21-23]. However, Rosenkrantz et al[23] showed that the contrast between focal liver lesions and liver parenchyma was slightly lower for Dixon imaging with water reconstruction than that for chemical saturation. To our knowledge, there are no reports yet on the potential value of two-point Dixon fat-water separation technique for image quality and focal liver lesions in hepatobiliary phase of gadoxetic acid-enhanced MR imaging.
The aim of this study was to compare the image quality and liver-to-lesion contrast in hapatobiliary phase between VIBE using two-point Dixon fat-water separation (Dixon-VIBE) and chemically selective fat saturation (FS-VIBE).
This study was approved by Institutional Review Board of our hospital. The requirement for informed consent was waived for our study. This study was performed at our department between December 2012 and July 2013. Inclusion criteria for patients consisted of (1) undergoing liver MR imaging with injection of gadoxetic acid; (2) detectable focal liver lesions; (3) breath-holding capacity for at least 18 s; and (4) normal liver function. Clinical data were obtained from medical records.
Liver examinations were performed on a 1.5-T MR system with a phased-array coil (Magneto Aera, Siemens Medical Solution, Erlangen, Germany). In routine liver MR protocol at our department, early dynamic contrast-enhanced MR imaging and hepatobiliary phase at 20 min were performed using FS-VIBE besides conventional MRI sequences. Dixon-VIBE was also routinely performed before gadoxetic acid administration. Besides conventional MR imaging sequences, Dixon-VIBE was performed for the hepatobiliary phase before FS-VIBE (no more than 2 min). The sequence parameters are displayed in Table 1. A parallel imaging technique (R factor of 2) was performed with generalized autocalibrating partially parallel acquisition for the 2 sequences. Four image sets were obtained by two-point Dixon-VIBE: an in-phase image, an out-of-phase image, a fat-reconstruction image, and a water-reconstruction image, with the water-reconstruction image being analogous to the standard FS-VIBE sequence. In all patients, 0.025 mmol/kg body weight of gadoxetic acid (Primovist; BayerSchering Pharma AG, Berlin, Germany) was injected manually at about 1 mL/s, as recommended, by one investigator through a 20-gange intravenous catheter placed in a cubical or cephalic vein. Immediately afterwards, a 20 mL saline flush was administered at the same injection rate.
| Dixon-VIBE | FS-VIBE | |
| Repetition time (ms) | 8.88 | 3.47 |
| Echo time (ms) | 2.39/4.77 | 1.36 |
| Section thickness (mm) | 3.5 | 3.5 |
| Field of view (mm) | 380-400 × 300-324 | 380-400 × 300-324 |
| Flip angle (degree) | 10 | 10 |
| Matrix | 320 × 240 | 320 × 195 |
| Bandwidth (Hz/pixel) | 430/490 | 400 |
| Acquisition time (s) | 18 | 14 |
MR imaging data were evaluated in the hospital’s Picture Archiving and Communication System. All MR images were assessed by a single reader (Ren-chen Li) with 5 years of experience in reading abdominal MR images. The reader was blinded to clinical data of the patients. For each patient, qualitative and quantitative analyses were performed on the largest available lesion.
The reader reviewed Dixon-VIBE and FS-VIBE sequences on 2 separate dates with 4-8 wk separating interpretation of the 2 sequences. For each data set, the reader scored the following parameters: sharpness of tumor, sharpness of vessels, strength and homogeneity of fat suppression, and artifacts, which were scored using the following 4-point scale: 4, excellent quality; 3, good quality, not impairing diagnostic performance; 2, fair quality, somewhat impairing diagnostic performance; 1, poor quality, impairing diagnostic performance.
The signal intensities (SIs) of focal liver lesions were measured on Dixon-VIBE with water reconstruction and FS-VIBE images in hepatobiliary phase. The reader manually traced the lesion boundary by placing free hand region of interest at the largest axial slice. The SI of liver parenchyma surrounding the tumor was also measured avoiding large vessels and artifacts. The liver-to-lesion contrast (%) was calculated according to the Michelson contrast formula[18]: 100 × (SIliver - SIlesion)/(SIliver + SIlesion), where SIliver is SI of liver parenchyma and SIlesion is SI of focal liver lesions.
The statistical methods of this study were reviewed by Ren-chen Li from Zhongshan Hospital Fudan University. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 18.0, SPSS, Chicago, IL, United States). The liver-to-lesion contrast was pared using a paired t-test when data were normally distributed or a Wilcoxon signed ranked test when data were not normally distributed. A Wilcoxon signed ranked test was also used to compare quality factors. Differences with a P-value less than 0.05 were considered statistically significant.
A total of 49 consecutive patients meeting the inclusion criteria were included in our study. Of the 49 patients, 41 were male and 8 were female (media age, 51 years; range, 26-84). Forty-one target lesions were confirmed by surgical pathologic assessment (33 hepatocellular carcinomas, 2 cholangiocaicinomas, 4 metastases from colorectal carcinoma, and 2 hemangiomas) and the remaining 8 lesions were hemangiomas which were confirmed by imaging findings combined with clinical data. Cirrhosis was diagnosed in 20 patients by pathologic assessment. The median size of the lesions was 15 mm (range, 5-70 mm); 12 (24.5%) were ≤ 10 mm, 24 (49.0%) were between 10 and 20 mm and 13 (26.5%) were > 20 mm.
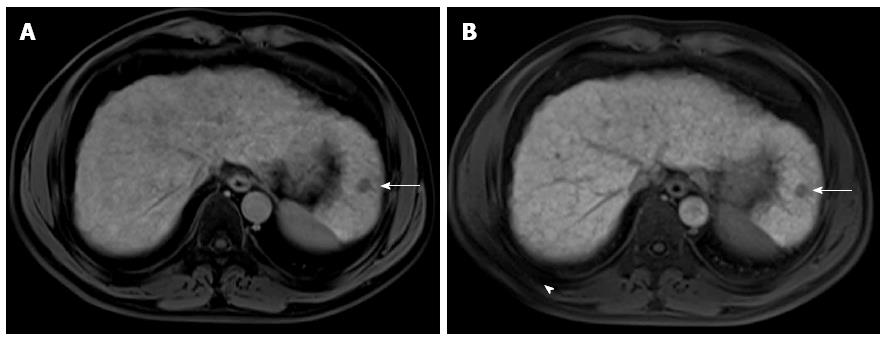
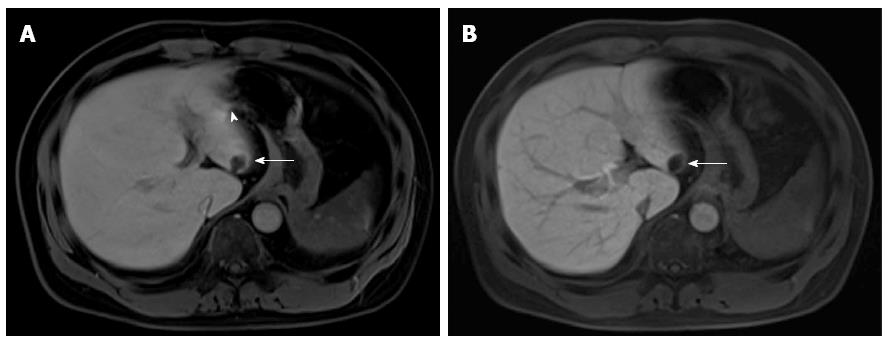
All the target lesions were detected as hypointense lesions on both Dixon-VIBE and FS-VIBE. The mean values for subjective scores (sharpness of tumor, sharpness of vessels, homogeneity of fat suppression, and artifacts) are shown in Table 2, Figures 1 and 2, and the results of liver-to-lesion contrast are displayed in Figure 3. Dixon-VIBE with water reconstruction had significantly higher subjective scores than FS-VIBE in strength and homogeneity of fat suppression (< 0.0001) but lower scores in sharpness of tumor (P < 0.0001), sharpness of vessels (P = 0.0001), and artifacts (P = 0.034). The liver-to-lesion contrast on Dixon-VIBE images was significantly lower than that on FS-VIBE (16.6% ± 9.4% vs 23.9% ± 12.1%, P = 0.0001).
| Parameter | FS-VIBE | Dixon-VIBE | P value |
| Sharpness of tumor | 3.22 ± 0.47 | 2.78 ± 0.47 | < 0.0001 |
| Sharpness of vessels | 3.20 ± 0.46 | 2.73 ± 0.49 | 0.0001 |
| Strength and homogeneity of fat suppression | 2.98 ± 0.25 | 3.6 ± 0.53 | < 0.0001 |
| Artifacts | 2.98 ± 0.25 | 2.86 ± 0.35 | 0.0340 |
The aim of this study was to compare Dixon-VIBE with conventional FS-VIBE sequence in gadoxetic acid-enhanced liver MR imaging. Our results indicated that Dixon-VIBE with water reconstruction demonstrated significantly stronger and more homogenous fat suppression but had lower clarity of hepatic vessels and lesions and increased artifacts, when compared with conventional FS-VIBE in hepatobiliary phase.
Inhomogeneous and incomplete fat suppression especially for the area near the edges of surface coils could degrade the image quality of conventional FS-VIBE for abdominal MR imaging, while Dixon methods are insensitive to B1 inhomogeneities and provide robust water-fat separation[16-18]. Our study showed that Dixon-VIBE with water reconstruction yielded fat suppression with superior quality than conventional FS-VIBE, which was in keeping with the results of previous studies[21-23]. We further found the improvement for homogeneous fat suppression adjacent to the lung and chest/abdominal wall by Dixon-VIBE with water reconstruction. This might have potential value for visualization of the uptake of gadoxetic acid by hepatocytes in the hepatobiliary phase. Unfortunately, our study failed to demonstrate that Dixon-VIBE with water reconstruction had the superiority for visualization of focal liver lesions with significantly lower subjective scores and liver-to-lesion contrast. The possible reason might be that Dixon fat separation techniques avoid the potentially severe suppression artifacts due to B0 inhomogeneities and frequency offsets among separate volumes of interest, including inadvertent suppression of the water signal, which can reduce image contrast[19,20]. This results also might be in part affected by earlier acquisition for Dixon-VIBE compared with FS-VIBE in our study. In theory, the peak liver parenchymal enhancement was observed to occur at 20 min after administration of gadoxetic acid. However, in our study there was only no more than 2 min between the two scans. A previous study also reported the lower, without reaching significant difference, liver-to-lesion contrast for Dixon-VIBE than FS-VIBE at about 3-min delayed gadolinium-enhanced liver MR imaging[27]. Our poor results for the increased artifacts on Dixon-VIBE may be due to the B0 inhomogeneities and relative longer acquisition time. Some researchers[28-30] modified the two-point Dixon method by acquiring a third image (three-point Dixon) that was used to compensate for B0 inhomogeneities, which have not been assessed for high resolution 3D VIBE breath-hold post-contrast liver MR imaging.
There are some limitations to our study. First, we did not change the routine default parameters for the 2 sequences, which were different in some parameters. Second, as above-mentioned we performed Dixon-VIBE a little earlier (≤ 2 min) than FS-VIBE, which might affect the calculation of the liver-to-lesion contrast. Finally, we did not evaluate the detection of the lesions. However, all the tumors (ranging from 5 mm to 70 mm) could be found in both sequences, which are consistent with the results of no difference for detection between the 2 sequences in the previous study[23].
In conclusion, Dixon-VIBE provides stronger and more homogenous fat suppression than FS-VIBE, while has lower clarity of focal liver lesions in hepatobiliary phase after gadoxetic acid administration. Dixon-VIBE has potential value when incomplete fat suppression is achieved by the FS-VIBE sequence.
The role of 3D gradient echo sequence with volumetric interpolated breath-hold examination (VIBE) by using chemically selective fat-saturation for abdominal magnetic resonance (MR) imaging is well established and it is now part of the standard clinical work-up, especially for dynamic contrast-enhanced liver MR imaging. Chemically selective fat-saturation imaging reduces the potential degradation of image quality resulting from motion-related artifacts, helps increase image contrast resolution and highlights lesions such as contrast-enhancing tissue, edema, and blood products by eliminating the high-intensity signal of fat. However, this technique is susceptible to B0 and B1 inhomogeneities, which remains a challenge, particularly for imaging off-center, with large field of view, or anatomies with strong susceptibility effects. Studies are therefore focusing on the potential added benefit of other methods for fat suppression like Dixon fat-water separation technique.
The Dixon technique has been improved extensively in the aspects of phase errors, noise and artifacts. In addition, this technique is routinely used to find intracytoplasmic lipid (also referred to as microscopic fat), as well as to have the potential for detection and grading of liver iron, based on comparison of signal intensity on in-phase and opposed-phase images.
Ragan et al demonstrated that 2-point Dixon fat separation with water reconstruction provided more reliable and homogenous fat suppression than chemical saturation in phantoms and mouse MR imaging. Previous studies also reported the similar results for abdominal and pelvic MR imaging in humans. However, Rosenkrantz et al showed that the contrast between focal liver lesions and liver parenchyma was slightly lower for Dixon imaging with water reconstruction than that for chemical saturation. To our knowledge, there are no reports yet on the potential value of two-point Dixon fat-water separation technique for image quality and focal liver lesions in hepatobiliary phase of gadoxetic acid-enhanced MR imaging. Therefore, the authors compared the image quality and liver-to-lesion contrast in hapatobiliary phase between VIBE using two-point Dixon fat-water separation (Dixon-VIBE) and chemically selective fat saturation (FS-VIBE).
Dixon-VIBE provides stronger and more homogenous fat suppression than FS-VIBE, while has lower clarity of focal liver lesions in hepatobiliary phase after gadoxetic acid administration. Dixon-VIBE has potential value when incomplete fat suppression is achieved by the FS-VIBE sequence.
Dixon-VIBE provides stronger and more homogenous fat suppression than FS-VIBE, while has lower clarity of focal liver lesions in hepatobiliary phase after gadoxetic acid administration.
The paper describes the comparison between VIBE using two-point Dixon-VIBE and chemically selective FS-VIBE in gadoxetic acid-enhanced liver MR imaging. Authors demonstrates that Dixon-VIBE provides stronger and more homogenous fat suppression than FS-VIBE, while has lower clarity of focal liver lesions in hepatobiliary phase after gadoxetic acid administration. The paper is scientifically accurate and complete. Many parameters (sharpness of tumor, sharpness of vessels, strength and homogeneity of fat suppression, artifacts and liver-to-lesion contrast) have been investigated to explore the differences between Dixon-VIBE and conventional FS-VIBE sequence.
| 1. | Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, Negishi C, Weinmann HJ, Speck U. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology. 1992;183:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 300] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Döhr O, Hofmeister R, Treher M, Schweinfurth H. Preclinical safety evaluation of Gd-EOB-DTPA (Primovist). Invest Radiol. 2007;42:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Saito S, Moriyama Y, Kobayashi S, Ogihara R, Koto D, Kitamura A, Matsushita T, Nishiura M, Murase K. Assessment of liver function in thioacetamide-induced rat acute liver injury using an empirical mathematical model and dynamic contrast-enhanced MRI with Gd-EOB-DTPA. J Magn Reson Imaging. 2012;36:1483-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007;6:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 5. | Liu X, Zou L, Liu F, Zhou Y, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e70896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Chen L, Zhang J, Zhang L, Bao J, Liu C, Xia Y, Huang X, Wang J. Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One. 2012;7:e48681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Sun HY, Lee JM, Shin CI, Lee DH, Moon SK, Kim KW, Han JK, Choi BI. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (& lt; or =2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010;45:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Lee JM, Zech CJ, Bolondi L, Jonas E, Kim MJ, Matsui O, Merkle EM, Sakamoto M, Choi BI. Consensus report of the 4th International Forum for Gadolinium-Ethoxybenzyl-Diethylenetriamine Pentaacetic Acid Magnetic Resonance Imaging. Korean J Radiol. 2011;12:403-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D, Ambrosino MM, Weinreb JC. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 412] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Conversano F, Franchini R, Demitri C, Massoptier L, Montagna F, Maffezzoli A, Malvasi A, Casciaro S. Hepatic vessel segmentation for 3D planning of liver surgery experimental evaluation of a new fully automatic algorithm. Acad Radiol. 2011;18:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Massoptier L, Casciaro S. A new fully automatic and robust algorithm for fast segmentation of liver tissue and tumors from CT scans. Eur Radiol. 2008;18:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Pokharel SS, Macura KJ, Kamel IR, Zaheer A. Current MR imaging lipid detection techniques for diagnosis of lesions in the abdomen and pelvis. Radiographics. 2013;33:681-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28:543-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 16. | Bley TA, Wieben O, François CJ, Brittain JH, Reeder SB. Fat and water magnetic resonance imaging. J Magn Reson Imaging. 2010;31:4-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 17. | Tanabe K, Nishikawa K, Sano T, Sakai O, Jara H. Fat suppression with short inversion time inversion-recovery and chemical-shift selective saturation: a dual STIR-CHESS combination prepulse for turbo spin echo pulse sequences. J Magn Reson Imaging. 2010;31:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kazama T, Nasu K, Kuroki Y, Nawano S, Ito H. Comparison of diffusion-weighted images using short inversion time inversion recovery or chemical shift selective pulse as fat suppression in patients with breast cancer. Jpn J Radiol. 2009;27:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ragan DK, Bankson JA. Two-point Dixon technique provides robust fat suppression for multi-mouse imaging. J Magn Reson Imaging. 2010;31:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Casciaro S, Franchini R, Massoptier L, Casciaro E, Conversano F, Malvasi A, Lay-Ekuakille A. Fully automatic segmentations of liver and hepatic tumors from 3-D computed tomography abdominal images: comparative evaluation of two automatic methods. IEEE Sens J. 2012;12:464-473. [DOI] [Full Text] |
| 21. | Low RN, Panchal N, Vu AT, Knowles A, Estkowski L, Slavens Z, Ma J. Three-dimensional fast spoiled gradient-echo dual echo (3D-FSPGR-DE) with water reconstruction: preliminary experience with a novel pulse sequence for gadolinium-enhanced abdominal MR imaging. J Magn Reson Imaging. 2008;28:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Cornfeld DM, Israel G, McCarthy SM, Weinreb JC. Pelvic imaging using a T1W fat-suppressed three-dimensional dual echo Dixon technique at 3T. J Magn Reson Imaging. 2008;28:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Rosenkrantz AB, Mannelli L, Kim S, Babb JS. Gadolinium-enhanced liver magnetic resonance imaging using a 2-point Dixon fat-water separation technique: impact upon image quality and lesion detection. J Comput Assist Tomogr. 2011;35:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Del Grande F, Santini F, Herzka DA, Aro MR, Dean CW, Gold GE, Carrino JA. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics. 2014;34:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 26. | Virtanen JM, Pudas TK, Ratilainen JA, Saunavaara JP, Komu ME, Parkkola RK. Iron overload: accuracy of in-phase and out-of-phase MRI as a quick method to evaluate liver iron load in haematological malignancies and chronic liver disease. Br J Radiol. 2012;85:e162-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Tolhurst DJ, Tadmor Y. Band-limited contrast in natural images explains the detectability of changes in the amplitude spectra. Vision Res. 1997;37:3203-3215. [PubMed] |
| 28. | Moriguchi H, Lewin JS, Duerk JL. Dixon techniques in spiral trajectories with off-resonance correction: a new approach for fat signal suppression without spatial-spectral RF pulses. Magn Reson Med. 2003;50:915-924. [PubMed] |
| 29. | He Q, Weng D, Zhou X, Ni C. Regularized iterative reconstruction for undersampled BLADE and its applications in three-point Dixon water-fat separation. Magn Reson Med. 2011;65:1314-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Doneva M, Börnert P, Eggers H, Mertins A, Pauly J, Lustig M. Compressed sensing for chemical shift-based water-fat separation. Magn Reson Med. 2010;64:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Casciaro S, Gao L, Storto G S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S