Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.5009
Peer-review started: October 3, 2014
First decision: November 14, 2014
Revised: November 21, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 28, 2015
Processing time: 207 Days and 0 Hours
AIM: To investigate the pathophysiology of functional heartburn (FH) in Japanese patients.
METHODS: A total of 111 patients with proton pump inhibitor (PPI)-refractory non-erosive gastroesophageal reflux disease underwent intraesophageal pressure testing and 24-h multichannel intraluminal impedance-pH (24MII-pH) testing. The patients also completed several questionnaires while they were receiving the PPI treatment, including the questionnaire for the diagnosis of reflux disease (QUEST), the frequency scale for the symptoms of gastroesophageal reflux disease (FSSG), the gastrointestinal symptoms rating scale (GSRS), SF-36, and the Cornell Medical Index (CMI). The subjects were classified into FH and endoscopy-negative reflux disease (ENRD) groups based on the Rome III criteria.
RESULTS: Thirty-three patients with esophageal motility disorder were excluded from this study, while 22 patients with abnormal esophageal acid exposure time (pH-POS) and 34 with hypersensitive esophagus (HE) were included in the ENRD group. The FH group included 22 patients with no reflux involvement. Sex, age, and body mass index did not differ significantly between the groups. The mean SF-36 values were < 50 (normal) for all scales in these groups, with no significant differences. The GSRS scores in these groups were not different and showed overlap with other gastrointestinal symptoms. The QUEST and the FSSG scores did not differ significantly between the groups. Neuroticism was diagnosed using the CMI questionnaire in 17 of the 78 included subjects within the pH-POS (n = 4), HE (n = 8), and FH (n = 5) groups, with no significant differences.
CONCLUSION: Clinical characteristics of the FH and PPI-refractory ENRD groups were similar. Therefore, esophageal function should be examined via manometry and 24MII-pH testing to differentiate between them.
Core tip: The Rome III criteria define functional heartburn (FH) by normal esophageal acid exposure time, with no relationship between symptoms and reflux, and no response to proton pump inhibitor (PPI) treatment. However, in Japanese clinical practice, PPI-refractory non-erosive reflux disease is often treated as FH, thought the pathophysiology of these diseases is not clear. In this study, we found no differences in the clinical characteristics of FH and PPI-refractory endoscopy-negative reflux disease, and recommend using manometry and 24-h multichannel intraluminal impedance-pH testing to differentiate between these two conditions.
- Citation: Tamura Y, Funaki Y, Izawa S, Iida A, Yamaguchi Y, Adachi K, Ogasawara N, Sasaki M, Kaneko H, Kasugai K. Pathophysiology of functional heartburn based on Rome III criteria in Japanese patients. World J Gastroenterol 2015; 21(16): 5009-5016
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/5009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.5009
Non-erosive reflux disease (NERD) is characterized by the absence of esophageal mucosal damage during upper gastrointestinal endoscopy, despite the presence of classic symptoms of gastroesophageal reflux, such as heartburn and acid reflux[1]. In addition, NERD accounts for more than half of all cases of gastroesophageal reflux disease (GERD)[2]. Furthermore, acid reflux is known to have only a minor effect on the pathophysiologic mechanism of NERD[3]. For this reason, NERD patients who receive proton pump inhibitors (PPIs), which are the first-line therapy for GERD, show a low symptom-improvement rate, and almost 50% of NERD patients fail to respond to standard acid-suppression therapy that uses PPIs[2,4-6].
Heartburn symptoms that are defined as functional heartburn (FH) have been described as belonging to one of the functional gastrointestinal disorders, which present with chronic gastrointestinal symptoms despite the absence of organic gastrointestinal disease. According to the latest Rome III criteria[7], a diagnosis of FH must fulfill all of the following criteria: (1) burning retrosternal discomfort or pain; (2) no evidence that gastroesophageal acid reflux is the cause of the symptoms; (3) no histopathologic evidence of esophageal motility disorders; and (4) these criteria have been fulfilled for the past 3 mo, with symptom onset at least 6 mo before the diagnosis. It has also been shown that a presumptive diagnosis of heartburn can be made in cases of endoscopy-negative reflux disease (ENRD) if 24-h intraesophageal pH monitoring reveals abnormal acid reflux, or acid reflux within the normal range that is associated with symptoms that abate after a course of PPI treatment[8]. In all other cases, the diagnosis should be FH. Regarding the putative mechanism of FH, various mechanisms that are directly related, indirectly related, or unrelated to reflux can be cited. Possible reflux-related mechanisms include acidic factors, such as weak acid reflux, and other factors, such as weak alkaline (bile) reflux. The latter may result from hypersensitivity to physiologic stimuli, abnormal central processing of esophageal signals, hypervigilance, or emotional factors[7].
According to the Rome III criteria, FH might be involved in esophageal motility disorders that exhibit no histopathologic abnormalities. However, esophageal motility disorders are present in 4% of patients who have FH and NERD, and their prevalence increases according to the GERD severity[9]. We have reported that patients with PPI-refractory NERD have esophageal motility disorders, and that gastroesophageal reflux plays a role in the symptoms’ onset[10]. Therefore, it is important to differentiate esophageal motility disorders from diagnoses of FH during pathophysiologic investigation. We planned a prospective study of patients with PPI-refractory NERD, which is routinely diagnosed as FH, to investigate the pathophysiology of FH and to define rigorous diagnostic criteria for FH in Japanese patients.
This prospective study was conducted at Aichi Medical University Hospital between January 2007 and March 2014. All patients who complained of heartburn at least twice per week for more than 6 mo, and who did not receive antisecretory therapy, underwent upper gastrointestinal endoscopy. If no organic abnormalities (excluding esophageal hiatus hernia) were detected during the endoscopy, the patients were diagnosed as having NERD and were prescribed the standard dose of PPI. PPI-refractory NERD was defined as NERD with no improvements in heartburn symptoms after ≥ 8 wk of PPI treatment at the standard dose, and all patients with PPI-refractory NERD were enrolled in this study. All medical interviews and examinations were conducted while the patients were receiving a continuous course of PPI at the standard doses (30 mg of lansoprazole, 20 mg of rabeprazole, or 20 mg of omeprazole per day) for ≥ 8 wk.
This study was approved by the Ethics Committee of Aichi Medical University School of Medicine, and was performed with the written, informed consent of the patients.
Intraesophageal pressure was measured using a Polygraf ID multiparameter gastrointestinal motility function measurement system (Sierra Scientific Instruments, Los Angeles, CA, United States) and an 8-channel, water-perfused, Dent’s sleeve catheter that was inserted nasally into the esophagus. With the patient recumbent, the lower esophageal sphincter (LES) position from the nasal cavity, LES pressure, and the primary peristaltic wave was observed over ten water swallows. Esophageal motility disorders were classified as achalasia, diffuse esophageal spasm, nutcracker esophagus, hypertensive LES, ineffective esophageal motility, and nonspecific esophageal motility disorders, according to the classifications of Castell et al[11].
Patients in whom esophageal motility disorders were not detected after the intraesophageal pressure testing subsequently underwent twenty-four-hour multichannel intraluminal impedance-pH (24MII-pH) testing (Sleuth multi impedance pH monitoring system; Sandhill Scientific, Highlands Ranch, CO, United States)[12]. This system includes a portable data logger with impedance-pH amplifiers and a catheter with one antimony pH electrode and eight impedance electrodes at 2, 4, 6, 8, 10, 14, 16, and 18 cm from the tip of the catheter. Each pair of adjacent electrodes represents an impedance-measurement segment (length, 2 cm) that corresponds to one recording channel. The one pH signal and six impedance signals were recorded at a frequency of 50 Hz on a 128-MB CompactFlash memory card (SanDisk, Milpitas, CA, United States). The single-use MII-pH catheter was transnasally inserted into the esophagus and positioned with the pH electrode 5 cm above the upper margin of the LES. The MII-pH data were continuously recorded for 24 h while the patient was in the hospital. All patients ate three meals and were asked to record their posture (e.g., recumbent), the meals they consumed, and the occurrence of heartburn or other symptoms. Analysis of the data, excluding the meal times, was performed using BioVIEW Analysis software (version 5.3.4; Sandhill Scientific). This software is capable of automatically evaluating parameters such as reflux frequency for liquid, gas, and mixtures of the two, as well as liquid pH and the symptom indices (SI) of these parameters, with a high degree of reliability[13]. SI was defined as positive if the proportion of the symptoms due to reflux accounted for ≥ 50% of the overall symptoms in the 24 h period[14]. After the automatic analysis, an expert visually confirmed the results. In the group with clear intraesophageal prolongation of the esophageal acid reflux exposure time, based on the proportion of the 24-h intraesophageal pH monitoring when the pH was < 4 and the DeMeester scores[15], the reflux of non-acidic material (i.e., liquid or gas with pH ≥ 4) was evaluated based on the relationship with SI (subgroups with SI ≥ 50% and with SI < 50%). This method was chosen as the relationship between symptoms and liquid/gas reflux materials is difficult to evaluate using reflux frequencies and time ratios. In this study, reflux materials consisting of a mixture of liquid and gas was treated as liquid reflux.
During the medical examination, reflux symptoms were assessed using the questionnaire for the diagnosis of reflux disease (QUEST)[16] and the frequency scale for the symptoms of GERD (FSSG)[17]. Patients also completed the gastrointestinal symptoms rating scale (GSRS) as an indicator of the other gastrointestinal symptoms that involve acid reflux, the SF-36[18] to evaluate lifestyle and health, and the Cornell Medical Index (CMI) questionnaire to evaluate for the presence of neuroticism.
Data are reported as mean ± SD, and the analysis was performed using the Kruskal-Wallis test or the χ2 test, where appropriate. Differences were considered statistically significant at P < 0.05.
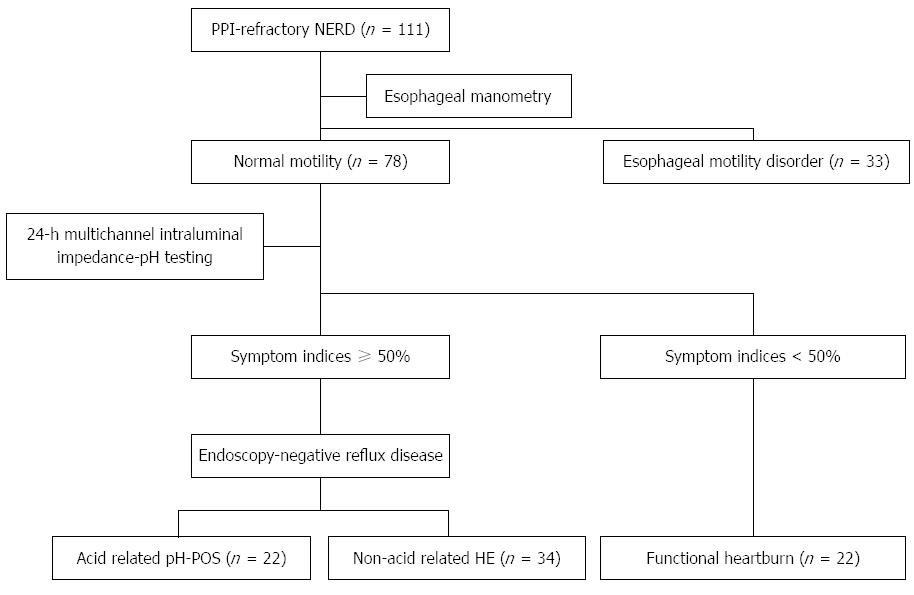
A total of 111 patients with PPI-refractory NERD were recruited and underwent intraesophageal pressure testing. Thirty-three patients were diagnosed with esophageal motility abnormalities, including achalasia (n = 4), ineffective esophageal motility (n = 8), nonspecific esophageal motility disorders (n = 13), hypertensive LES (n = 5), and nutcracker esophagus (n = 3). All patients with esophageal motility disorders were excluded, and the remaining 78 patients [40 men, 38 women; mean age: 55.5 ± 15.4 years; mean body mass index (BMI): 22.3 ± 3.1 kg/m2] were evaluated using 24MII-pH. Twenty-two patients showed a clear intraesophageal prolongation of esophageal acid exposure time based on the proportion of the 24 h when their intraesophageal pH was < 4 and on the DeMeester scores[15]. In addition, 34 patients were classified into the group with normal esophageal acid exposure time and SI associated with ≥ 50% non-acid reflux, and 22 patients were classified into the group in which reflux contributed to < 50% of the symptoms. Based on this classification, patients with PPI-refractory NERD were classified into 4 subgroups according to whether their symptom onset was associated with: (1) esophageal motility abnormality; (2) acid reflux; (3) non-acid reflux; or (4) no reflux (Figure 1).
Based on the results of the esophageal function testing, the three subgroups, except for those with esophageal motility abnormality, were classed as: the pH-positive group (pH-POS) with acid reflux, the hypersensitive esophagus group (HE) group with no acid, and the FH group with no reflux. The pH-POS group contained 22 patients (14 men, 8 women; mean age: 54.8 ± 3.9 years; BMI: 22.9 ± 3.5 kg/m2), the HE group contained 34 patients (17 men, 17 women; mean age: 54.9 ± 2.6 years; BMI: 22.0 ± 3.0 kg/m2), and the FH group contained 22 patients (9 men, 13 women; mean age: 57.7 ± 2.7 years; BMI: 21.3 ± 2.7 kg/m2). When these groups were compared, no differences in sex, age, or BMI were observed, and there was no causal relationship with alcohol or tobacco use (Table 1). The results of the symptom evaluations did not differ significantly between the FSSG and QUEST questionnaires, and there were no differences in any of the GSRS scores (Table 2). The SF-36 scores fell below the normal population values on all subscales, although no intergroup differences were observed. On the CMI health questionnaire, scores of grades III and IV indicate neuroticism. Four patients in the pH-POS group scored grades III or IV, compared to 8 patients in the HE group and 5 patients in the FH group; these differences were not statistically significant (Table 1).
| Characteristic | pH-POS | HE | FH | P value |
| (n = 22) | (n = 34) | (n = 22) | ||
| Age, yr | 54.8 ± 3.9 | 54.9 ± 2.6 | 57.7 ± 2.7 | 0.3 |
| (26–80) | (21–86) | (29–76) | ||
| Sex, male:female | 14:08 | 17:17 | 9:13 | 0.3 |
| Body mass index, kg/m2 | 21.9 ± 3.5 | 22.0 ± 3.0 | 21.3 ± 2.7 | 0.6 |
| Drinking | 8 (36.3) | 16 (47.0) | 8 (36.3) | 0.4 |
| Smoking | 7 (31.8) | 10 (29.4) | 5 (22.7) | 0.7 |
| Neuroticism (III or IV)1 | 4 (22.7) | 8 (23.5) | 5 (22.7) | 0.8 |
| Questionnaire | pH-POS | HE | FH | P value1 |
| (n = 22) | (n = 34) | (n = 22) | ||
| FSSG score (median) | 16.4 ± 7.2 | 18.4 ± 9.6 | 19.5 ± 10.3 | 0.6 |
| QUEST score (median) | 5.8 ± 4.7 | 5.1 ± 4.5 | 4.2 ± 4.2 | 0.5 |
| GSRS scale | ||||
| Over all | 2.5 ± 0.6 | 2.5 ± 0.8 | 2.2 ± 1.0 | 0.5 |
| Acid reflux | 3.4 ± 1.6 | 3.2 ± 1.5 | 3.0 ± 1.4 | 0.7 |
| Abdominal pain | 2.5 ± 1.1 | 2.4 ± 1.3 | 2.3 ± 1.3 | 0.7 |
| Indigestion | 2.9 ± 1.8 | 2.8 ± 1.1 | 2.5 ± 1.4 | 0.5 |
| Diarrhea | 1.6 ± 0.7 | 1.9 ± 1.2 | 1.7 ± 0.1 | 0.8 |
| Constipation | 2.3 ± 1.4 | 2.4 ± 1.2 | 2.0 ± 1.1 | 0.3 |
| SF-36 scale | ||||
| Physical Functioning | 46.5 ± 11.1 | 45.1 ± 16.2 | 44.7 ± 16.1 | 0.9 |
| Role Physical | 40.5 ± 13.2 | 41.0 ± 15.4 | 40.4 ± 15.1 | 0.9 |
| Bodily Pain | 43.6 ± 9.1 | 44.1 ± 10.1 | 43.5 ± 11.8 | 0.9 |
| General Health | 38.9 ± 9.8 | 42.1 ± 6.3 | 43.1 ± 10.5 | 0.5 |
| Vitality | 46.0 ± 10.6 | 45.5 ± 9.5 | 45.4 ± 11.1 | 0.9 |
| Social Functioning | 44.8 ± 10.6 | 39.2 ± 13.1 | 44.6 ± 11.2 | 0.2 |
| Role Emotional | 41.9 ± 14.3 | 42.5 ± 14.3 | 44.6 ± 14.2 | 0.8 |
| Mental Health | 43.9 ± 10.5 | 42.2 ± 11.0 | 41.8 ± 13.8 | 0.9 |
The distal and proximal numbers of gastroesophageal reflux episodes (total and acid) were significantly higher in the pH-POS group than those in the HE and FH groups, although there was no difference between the HE and FH groups (Table 3).
| Variable | pH-POS (n = 22) | HE (n = 34) | FH (n = 22) | P value1 |
| % acid exposure time | 16.1 ± 15.3b | 1.4 ± 2.4 | 0.7 ± 0.8 | < 0.01 |
| Number of reflux episodes | 77.8 ± 40.6b | 54.6 ± 30.3 | 33.9 ± 20.8 | < 0.01 |
| Acid reflux (pH < 4) | 34.1 ± 23.7b | 11.2 ± 11.1 | 7.1 ± 6.3 | < 0.01 |
| Non-acid reflux (pH ≥ 4) | 41.0 ± 35.1 | 37.7 ± 32.2 | 23.8 ± 17.3 | 0.2 |
| Number of proximal reflux episodes | 34.4 ± 26.7b | 22.2 ± 12.5 | 14.1 ± 9.0 | < 0.01 |
| % of proximal reflux Episodes | 42.5 ± 14.3 | 42.5 ± 13.3 | 41.4 ± 14.3 | 0.9 |
| % of gastric acid exposure time | 66.1 ± 20.5 | 50.6 ± 30.9 | 49.9 ± 28.7 | 0.1 |
The Japanese Society of Gastroenterology GERD Diagnosis Guideline[19], which was published in 2009, provides one flowchart for using PPI as a first-line therapy and another flowchart for using endoscopy as the initial phase of treatment for patients with symptoms indicative of GERD. Therefore, early treatment can be started for all patients, even at facilities where endoscopy is unavailable. In both cases, a pathophysiologic evaluation using 24-h pH monitoring at a specialist facility is recommended only if the symptoms persist after the PPI treatment. In Japan, pH monitoring and esophageal motility function tests are not widely used in clinical practice. As a result, cases in which no obvious organic lesions or mucosal damage are detected during endoscopy are typically classified as NERD, as ENRD if the symptoms respond to PPI treatment, or as FH if PPI treatment does not improve the symptoms. Thus, patients with PPI-refractory NERD are often treated as having FH. Therefore, to identify the characteristics of Japanese patients with FH, it is appropriate to target the broadly defined group of patients with FH who are characterized by a diagnosis of NERD based on endoscopy and lack of symptom response to PPI treatment.
One hundred and eleven patients with PPI-refractory NERD were grouped into those with esophageal motility abnormalities (n = 33), patients with ENRD and whose symptoms were related to some form of reflux (pH-POS and HE, n = 56), and patients with FH based on the Rome III criteria (n = 22). Regarding the ENRD pathophysiology, 22 patients exhibited insufficient suppression of acid secretion, despite taking a standard dose of PPI for 8 wk, and 34 patients had weak acid reflux or non-acidic reflux, which is considered a hypothetical diagnosis in the Rome III criteria. Previous studies have reported that patients with abnormal gastroesophageal reflux, as confirmed by pH monitoring and including GERD patients, are typically men and often have a high BMI[9,20-22]. Furthermore, a comparison of patients with FH and those with ENRD and some form of reflux-related symptoms (based on 24MII-pH monitoring) reported that ENRD was more common among men and was associated with a high BMI[23], whereas FH was more common in younger patients and among women[24,25]. Other studies have reported that age and lifestyle habits (i.e., alcohol or tobacco use) are not specific to either FH or ENRD[9,23,26]. The present study found no significant differences between the ENRD (pH-POS and HE) and FH groups when sex, age, BMI, and lifestyle habits (alcohol and tobacco use) were compared. The reason for this discrepancy remains unclear, although it may be related to the fact that BMI is lower in Japan than in the Western countries where the previous studies were conducted. In addition, a study investigating symptoms using questionnaires and 24MII-pH monitoring reported stronger reflux symptoms than heartburn symptoms in these groups[20], whereas a study that used pH monitoring to evaluate the occurrence rate of reflux symptoms reported a higher rate in patients with ENRD than in those with FH[25]. However, the present study found no significant intergroup differences in the symptoms or their occurrence rates using the FSSG and QUEST questionnaires. This discrepancy highlights the difficulty encountered when attempting to differentiate between ENRD and FH based on symptoms.
ENRD is associated with a marked deterioration in quality of life[27] and has a high rate of overlap with other functional gastrointestinal disorders[28]. In GERD, mild heartburn at least twice per week has a considerable effect on quality of life, whereas similar criteria for the frequency and severity of symptoms in FH have not yet been identified[29]. In the present study, the mean SF-36 scores in these groups fell below the normal population values on all subscales, although no significant intergroup differences were observed. This indicates that patients who have FH experience a similar deterioration in their quality of life compared to patients who have ENRD (pH-POS and HE). FH often occurs in tandem with symptoms that are normally considered to be indicative of dyspepsia, such as nausea, abdominal bloating, and early satiation[30,31]. In the study of 200 patients with NERD by Savarino et al, a questionnaire given to the 54 patients who were diagnosed with FH revealed that the prevalence of postprandial bloating, early satiation, and nausea was significantly higher in patients with FH, whereas in a study of 68 patients with NERD by Capasso et al[32], a significantly higher score for indigestion in functional dyspepsia (FD) symptoms was found among 25 patients with no abnormal acid reflux according to pH monitoring. In the patients with GSRS, a high degree of overlap in the gastrointestinal symptoms that were unrelated to acid was expected in the FH group, although no significant difference was observed between these groups. This difference might be explained by whether the subjects were or were not receiving PPI treatment. Patients with reflux esophagitis and who were treated with a PPI (pantoprazole) for up to 16 wk showed improvements in their reflux, FD, and irritable bowel syndrome symptoms[33]. However, when those patients were monitored for up to 6 mo after stopping the PPI treatment, their reflux symptoms returned, although their FD and irritable bowel syndrome symptoms did not. Similarly, the subjects in the present study had received PPI treatment at the standard oral dose for at least 8 wk, and it is possible that it suppressed the non-acid reflux dyspepsia symptoms.
In clinical practice, the psychologic profile of patients with reflux symptoms is characterized by higher scores for anxiety and depression than those of patients with no reflux[34]. Similarly, patients with heartburn that is poorly correlated with acid reflux during pH monitoring have higher scores for anxiety and hysteria than those in whom heartburn onset is correlated with acid reflux[35]. Therefore, many cases of FH are interpreted as esophageal neurosis. However, the present analysis of the CMI health questionnaire responses found no significant intergroup differences in the number of patients who were diagnosed with an emotional disturbance, and the scores were similar in the FH and ENRD (pH-POS and HE) groups. This finding suggests that the contribution of emotional factors to the putative factors that affect FH is not as high as was previously thought.
Savarino et al[23] reported that the increased number of weakly acidic reflux episodes and the higher rate of proximal reflux are the main causes of symptoms in HE patients who were evaluated with 24MII-pH. However, the data in the present indicate that the total reflux and proximal reflux time of the acid contents are significantly higher in the pH-POS group than in the HE and FH groups, which do not differ. This difference may be related to the presence or absence of PPI treatment. The present analysis was undertaken to evaluate the clinical practical situation of NERD, and to identify its pathophysiology, while avoiding the acid-related effects.
This study has several limitations. First, there may have been a case selection bias, as the subjects were patients with PPI-refractory NERD who were expertly examined at a specialist facility. Furthermore, the sample size was small, as the study was limited to one facility. Second, the subjects were patients who were receiving PPI at the standard dose set within the context of healthcare covered by Japanese national health insurance. Therefore, caution is required when comparing the present data with data from other countries, particularly regarding PPI dose and the duration of administration.
Despite this study’s limitations, the pathophysiologic classification of patients with PPI-refractory NERD, who are routinely encountered in clinical practice, was achieved by testing their esophageal function. In addition, the pathology of FH in Japanese patients, based on the Rome III criteria, was clarified through a comparison of patients with NERD who were divided into FH and ENRD (pH-POS and HE) groups. Furthermore, this study was conducted while the patients were receiving a continuous course of PPI at the standard doses to reflect the current Japanese clinical practice and to more clearly define the pathophysiology of FH, while avoiding the acid-related effects. A recently published review has mentioned that the use of 24MII-pH is the only functional method to reliably perform sub-classification of the complex population of patients with NERD. In addition, this technique is recommended to clearly separate the subsets of patients with real reflux disease from the subset with FH[36]. Our study supports this idea, and indicates that NERD is a markedly heterogeneous condition from the pathophysiologic and clinical points of view, and that it should be correctly classified by esophageal function testing to provide adequate relief from the related symptom. For example, patients with FH should be treated with non-PPI and non-reflux inhibitor medication, such as with pain modulators[36]. Our data might contribute to developing a therapeutic strategy for patients with PPI-refractory NERD.
In conclusion, Japanese patients with PPI-refractory NERD, who are typically treated as having FH in clinical practice, exhibit a mix of several pathologies and low rates of rigorously defined FD. The FH and PPI-refractory ENRD groups showed no differences in their clinical characteristics, such as their background, symptoms, and neuroticism, and it was difficult to differentiate between these groups. However, both manometry and MII-pH testing should be used to differentiate between patients with PPI-refractory ENRD and those with FH. Further studies with larger samples are needed to validate the diagnostic criteria for FH based on the Rome III criteria.
The Rome III criteria for functional heartburn (FH) suggest various possible factors that are involved in these mechanisms, and position FH as being primarily defined by a normal esophageal acid exposure time, with no relationship between symptoms and reflux, and no response to proton pump inhibitor (PPI) treatment. However, in Japanese clinical practice, PPI-refractory non-erosive reflux disease (NERD) is often treated as FH, and the pathophysiology of these diseases has not been adequately investigated.
This study was performed to elucidate the pathophysiology of FH in Japanese patients, and found that there were no differences between the clinical characteristics of FH and PPI-refractory endoscopy-negative reflux disease (ENRD) patients. It is difficult to differentiate these conditions, and therefore, both manometry and 24-h multichannel intraluminal impedance-pH (24MII-pH) testing should be used to obtain an accurate diagnosis.
Japanese patients with PPI-refractory NERD, who are typically treated as FH in clinical practice, demonstrate the combination of several pathologies and low rates of rigorously defined FH. The FH and PPI-refractory ENRD groups showed no differences in their clinical characteristics, such as their background, symptoms, and neuroticism, and it was difficult to differentiate between the two groups. Those data would contribute to develop a therapeutic strategy for patients with PPI-refractory NERD.
This is thre first prospective study of patients with PPI-refractory NERD, which is routinely diagnosed as FH, to investigate the pathophysiology of FH and to define rigorous diagnostic Rome III criteria for FH in Japanese patients. However, there may have been a case selection bias, and the sample size was small, as the study was limited to one facility. Further studies with larger samples are needed to validate the diagnostic criteria for FH based on the Rome III.
24MII-pH, which is different from conventional pH monitoring, can discriminate between the physical characteristics of the refluxate (liquid, gas or mixed) and non-acidic gastroesophageal reflux. NERD is characterized by the absence of esophageal mucosal damage seen in upper gastrointestinal endoscopy, despite the presence of typical symptoms of gastroesophageal reflux such as heartburn and acid reflux. According to the latest Rome III criteria, FH is defined by burning retrosternal pain or discomfort occurred for the past 3 mo with symptom onset ≥ 6 mo previously. The absence of gastroesophageal reflux and the absence of histopathologically defined esophageal motility disorders is also required.
This study was carried out to assess the pathophysiologic alterations of patients with FH and to compare them to those of the other subgroups of patients with NERD. The study is prospective and well done and an adequate number of patients were recruited.
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2520] [Article Influence: 126.0] [Reference Citation Analysis (2)] |
| 2. | Mishima I, Adachi K, Arima N, Amano K, Takashima T, Moritani M, Furuta K, Kinoshita Y. Prevalence of endoscopically negative and positive gastroesophageal reflux disease in the Japanese. Scand J Gastroenterol. 2005;40:1005-1009. [PubMed] |
| 3. | Fass R. Epidemiology and pathophysiology of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98:S2-S7. [PubMed] |
| 4. | Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656-664. [PubMed] |
| 5. | Uemura N, Inokuchi H, Serizawa H, Chikama T, Yamauchi M, Tsuru T, Umezu T, Urata T, Yurino N, Tanabe S. Efficacy and safety of omeprazole in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Miwa H, Sasaki M, Furuta T, Koike T, Habu Y, Ito M, Fujiwara Y, Wada T, Nagahara A, Hongo M. Efficacy of rabeprazole on heartburn symptom resolution in patients with non-erosive and erosive gastro-oesophageal reflux disease: a multicenter study from Japan. Aliment Pharmacol Ther. 2007;26:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Galmiche JP, Clouse RE, Bálint A, Cook IJ, Kahrilas PJ, Paterson WG, Smout AJ. Functional esophageal disorders. Gastroenterology. 2006;130:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 8. | Juul-Hansen P, Rydning A. Endoscopy-negative reflux disease: what is the value of a proton-pump inhibitor test in everyday clinical practice? Scand J Gastroenterol. 2003;38:1200-1203. [PubMed] |
| 9. | Savarino E, Zentilin P, Marabotto E, Bonfanti D, Inferrera S, Assandri L, Sammito G, Gemignani L, Furnari M, Dulbecco P. Overweight is a risk factor for both erosive and non-erosive reflux disease. Dig Liver Dis. 2011;43:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Izawa S, Funaki Y, Iida A, Tokudome K, Tamura Y, Ogasawara N, Sasaki M, Kasugai K. The role of gastroesophageal reflux in relation to symptom onset in patients with proton pump inhibitor-refractory nonerosive reflux disease accompanied by an underlying esophageal motor disorder. Digestion. 2014;89:61-67. [PubMed] [DOI] [Full Text] |
| 11. | Castell JA, Gideon RM, Castell DO. Esopahagus. Atlas of gastrointestinal motility in health and disease. Baltimore: Williams and Wilkins 1993; 135-157. |
| 12. | Bredenoord AJ, Tutuian R, Smout AJ, Castell DO. Technology review: Esophageal impedance monitoring. Am J Gastroenterol. 2007;102:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Roman S, Bruley des Varannes S, Pouderoux P, Chaput U, Mion F, Galmiche JP, Zerbib F. Ambulatory 24-h oesophageal impedance-pH recordings: reliability of automatic analysis for gastro-oesophageal reflux assessment. Neurogastroenterol Motil. 2006;18:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Wiener GJ, Richter JE, Copper JB, Wu WC, Castell DO. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83:358-361. [PubMed] |
| 15. | Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325-332. [PubMed] |
| 16. | Carlsson R, Dent J, Bolling-Sternevald E, Johnsson F, Junghard O, Lauritsen K, Riley S, Lundell L. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023-1029. [PubMed] |
| 17. | Kusano M, Shimoyama Y, Sugimoto S, Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H, Ino K. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Fukuhara S, Ware JE, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol. 1998;51:1045-1053. [PubMed] |
| 19. | Gakkai NS. Clinical practice guideline for gastroesophageal reflux disease.. Tokyo: Nankodo 2009; . [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Savarino E, Pohl D, Zentilin P, Dulbecco P, Sammito G, Sconfienza L, Vigneri S, Camerini G, Tutuian R, Savarino V. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009;58:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448-1456. [PubMed] |
| 22. | Labenz J, Jaspersen D, Kulig M, Leodolter A, Lind T, Meyer-Sabellek W, Stolte M, Vieth M, Willich S, Malfertheiner P. Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am J Gastroenterol. 2004;99:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Savarino E, Zentilin P, Tutuian R, Pohl D, Gemignani L, Malesci A, Savarino V. Impedance-pH reflux patterns can differentiate non-erosive reflux disease from functional heartburn patients. J Gastroenterol. 2012;47:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Matsuzaki J, Suzuki H, Iwasaki E, Yokoyama H, Sugino Y, Hibi T. Serum lipid levels are positively associated with non-erosive reflux disease, but not with functional heartburn. Neurogastroenterol Motil. 2010;22:965-70, e251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Hershcovici T, Zimmerman J. Functional heartburn vs. non-erosive reflux disease: similarities and differences. Aliment Pharmacol Ther. 2008;27:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Sarnelli G, De Giorgi F, Efficie E, Aprea G, Masone S, Savarese MF, Esposito I, Russo L, Cuomo R. Correlation between oesophageal acid exposure and dyspeptic symptoms in patients with nonerosive reflux disease. Eur J Gastroenterol Hepatol. 2008;20:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Fouad YM, Katz PO, Castell DO. Oesophageal motility defects associated with nocturnal gastro-oesophageal reflux on proton pump inhibitors. Aliment Pharmacol Ther. 1999;13:1467-1471. [PubMed] |
| 28. | Grande L, Lacima G, Ros E, García-Valdecasas JC, Fuster J, Visa J, Pera C. Lack of effect of metoclopramide and domperidone on esophageal peristalsis and esophageal acid clearance in reflux esophagitis. A randomized, double-blind study. Dig Dis Sci. 1992;37:583-588. [PubMed] |
| 29. | Dent J, Armstrong D, Delaney B, Moayyedi P, Talley NJ, Vakil N. Symptom evaluation in reflux disease: workshop background, processes, terminology, recommendations, and discussion outputs. Gut. 2004;53 Suppl 4:iv1-i24. [PubMed] |
| 30. | Shi G, Bruley des Varannes S, Scarpignato C, Le Rhun M, Galmiche JP. Reflux related symptoms in patients with normal oesophageal exposure to acid. Gut. 1995;37:457-464. [PubMed] |
| 31. | Small PK, Loudon MA, Waldron B, Smith D, Campbell FC. Importance of reflux symptoms in functional dyspepsia. Gut. 1995;36:189-192. [PubMed] |
| 32. | Capasso R, Borrelli F, Zjawiony J, Kutrzeba L, Aviello G, Sarnelli G, Capasso F, Izzo AA. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A reduce inflammation-induced hypermotility in mice. Neurogastroenterol Motil. 2008;20:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Mönnikes H, Schwan T, van Rensburg C, Straszak A, Theek C, Sander P, Lühmann R. Randomised clinical trial: sustained response to PPI treatment of symptoms resembling functional dyspepsia and irritable bowel syndrome in patients suffering from an overlap with erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2012;35:1279-1289. [PubMed] |
| 34. | Hu WH, Wong WM, Lam CL, Lam KF, Hui WM, Lai KC, Xia HX, Lam SK, Wong BC. Anxiety but not depression determines health care-seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population-based study. Aliment Pharmacol Ther. 2002;16:2081-2088. [PubMed] |
| 35. | Johnston BT, Lewis SA, Collins JS, McFarland RJ, Love AH. Acid perception in gastro-oesophageal reflux disease is dependent on psychosocial factors. Scand J Gastroenterol. 1995;30:1-5. [PubMed] |
| 36. | Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013;10:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Aghakhani A, Maleki I, Savarino V S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Ma S