Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3746
Peer-review started: October 6, 2014
First decision: October 29, 2014
Revised: November 12, 2014
Accepted: December 19, 2014
Article in press: December 22, 2014
Published online: March 28, 2015
Processing time: 175 Days and 4.8 Hours
Monoclonal antibodies against epidermal growth factor receptor (EGFR) are used in the treatment of advanced colorectal cancer. However, these agents can induce severe dermatological side effects that discourage their administration in patients with chronic dermatological disease. EGFR plays a key role in normal skin development and immunological function, and is expressed in various tissues and organs, although contrarily, it is overexpressed in psoriasis-related skin lesions. Thus, discussion is ongoing regarding the putative pathological role and therapeutic potential of this protein. We herein report on a patient with advanced colon cancer and concomitant long-standing psoriasis vulgaris who received anti-EGFR antibody monotherapy as a third-line treatment for metastatic disease. One week after the initiation of treatment, the patient’s skin lesions dramatically subsided and the improvement was sustained during therapy. Based on this case, we propose that anti-EGFR antibody therapy is not necessarily contraindicated in patients with psoriasis vulgaris. Moreover, the findings reaffirmed that EGFR is an important molecule in the pathology of psoriasis.
Core tip: Anti- epidermal growth factor receptor (EGFR) antibodies are effective in treating advanced colorectal cancer. However, anti-EGFR antibodies are not generally used in patients with concomitant chronic skin disease due to dermatological toxicities. In this case report, we present a patient with psoriasis vulgaris whose symptoms lessened during treatment with anti-EGFR antibody monotherapy for metastatic colon cancer. Based on this result, we consider that patients with concomitant skin disease should still be considered for anti-EGFR antibody therapy.
- Citation: Okamoto K, Maeda H, Shiga T, Shiga M, Dabanaka K, Hanazaki K, Kobayashi M. Cetuximab and panitumumab in a patient with colon cancer and concomitant chronic skin disease: A potential beneficial effect on psoriasis vulgaris. World J Gastroenterol 2015; 21(12): 3746-3749
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3746.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3746
Psoriasis vulgaris is a relatively common chronic inflammatory skin disease, which prevalence is ranging from 2.2% to 2.6% in the United States and 0.4% in Asia[1]. Psoriasis is characterized pathognostically by well-demarcated and slightly elevated red plaques with silver or white scale, reflecting the typically increased vascularity and keratinocyte hyper-proliferation[2]. Local therapies are prescribed for mild forms of the disease, while phototherapy and systemic therapies such as immunosuppressive drugs and anti-tumor necrosis factor (TNF) antibody are chosen for moderate-to-severe psoriasis, with recent studies highlighting the immune system, and particularly the TNF/NF-kB/interleukin (IL)-23-Th17 axis, as a pathogenic hub of psoriasis vulgaris[1]. These studies thus significantly and directly contributed to the development of a new treatment strategy for psoriasis.
Elevated serum levels of epidermal growth factor (EGF) and excessive expression of EGF receptor (EGFR) in affected skin have also been reported in patients with psoriasis vulgaris[3,4], suggesting pathological keratinocyte proliferation through the EGFR signaling pathway. However, to our knowledge, studies on EGF signaling biology and the potential therapeutic effect of anti-EGFR antibody are scarce in the psoriasis literature[5-8]. In this case report, we describe a patient with advanced colon cancer and concomitant psoriasis vulgaris, who received anti-EGFR antibody monotherapy using cetuximab and panitumumub.
A 55-year-old male patient was referred to our department for the treatment of colon cancer. He was diagnosed with psoriasis vulgaris at 27 years of age, and since then has received topical therapy with corticosteroids and activated vitamin D3 analogue, narrow-band ultraviolet B (NB-UVB) phototherapy, immunosuppressive drug therapy, and oral etretinate, resulting in partial and temporary relief of the symptoms. At the age of 54 years, he received anti-TNF antibody therapy and subsequently, showed marked improvement in his skin lesions. However, during the treatment, sigmoid colon cancer with multiple liver and lung metastases was identified.
On presentation to our clinic, he underwent laparoscope-assisted sigmoidectomy for the prevention of colonic obstruction and drug therapy with CapeOX (capecitabine, 4200 mg/d, days 1-14 and oxaliplatin 250 mg/d, 3-wk intervals) in combination with bevacizumab (anti-vascular endothelial growth factor antibody, 700 mg/d, 3-wk intervals) and IRIS (S-1, 120 mg/d, days 1-14 and irinotecan, 240 mg/d, 2-wk intervals) as the first- and second-line treatments, respectively. During these treatments, the skin lesion of psoriasis showed neither significant worsening nor improvement.
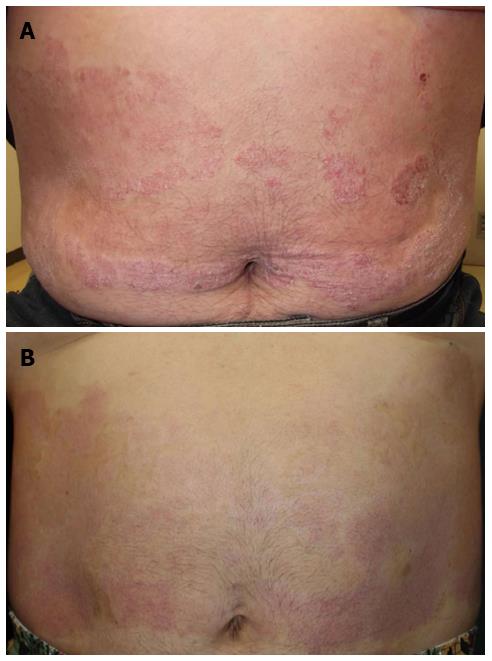
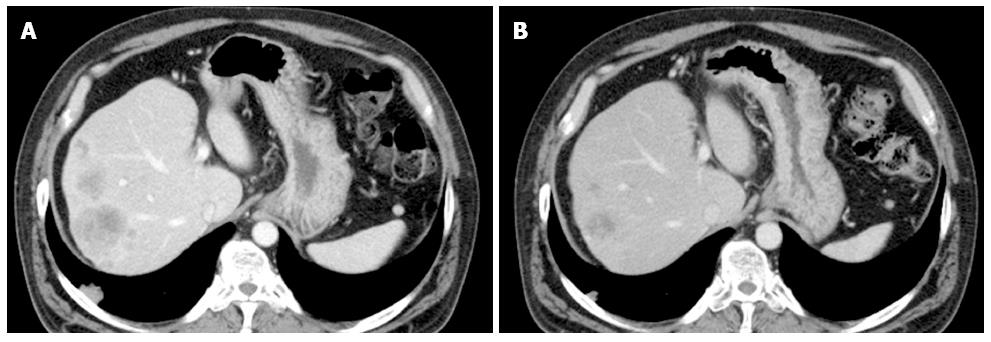
At 29 mo after the initial diagnosis, the patient commenced third-line treatment with cetuximab antibody (a human-and-mouse chimeric anti-EGFR monoclonal antibody, 800 mg on day 1, 500 mg on day 8, and once a week thereafter). Notably, seven days after the first administration of cetuximab, the psoriasis showed significant improvement (Figure 1). Subsequently, an allergic reaction necessitated replacement of the cetuximab with panitumumab (a fully human anti-EGFR monoclonal antibody, 600 mg/d, 2-wk intervals); however, the skin lesion improvement was maintained. The third-line treatment was continued for six months and the best response was partial response (Figure 2). The patient showed grade1 folliculitis on his face as an adverse effect of cetuximab or panitumumab, although it did not require treatment. The psoriasis symptoms worsened after discontinuation of the anti-EGFR monotherapy, and the patients died five months later.
EGFR is expressed in epithelial cells including keratinocytes and hair follicles, and plays a critical role in the development and function of normal skin[9,10]. In addition, skin lesions of psoriasis patient have revealed EGFR overexpression[3,4,11], suggesting sustained keratinocyte viability due to EGFR-mediated hyperstimulation as an underlying mechanism in the development of psoriatic skin lesions. In support of this hypothesis, EGFR tyrosine kinase inhibitor used for the treatment of lung cancer induced some improvement in psoriasis skin lesions[12], while Trivin et al[5] reported the regression of psoriasis after combination therapy with cetuximub and 5-fluorouracil/folinic acid for metastatic colon cancer. Although this report could not eliminate the possible effect of 5-fluorouracil, Neyns et al[6] later reported marked improvement of psoriasis during cetuximub monotherapy for the treatment of metastatic colon cancer.
Contrarily and surprisingly, Mas-Vidal et al[8] reported a case of psoriasis developing 12 d after the initiation of cetuximub treatment and resolving with discontinuation of treatment, and accordingly, they suggested a causal association between cetuximub and psoriasis. Similarly, Zorzou et al[13] reported the unexpected exacerbation of psoriasis after EGFR tyrosine kinase inhibitor treatment in patients with squamous cell lung cancer. Although a mechanism for the development and exacerbation of psoriasis with these treatments remains unclear[8], thresholding of EGFR signal blockade could induce alterations in the skin immune system and/or to the activation of alternative signaling pathways for psoriatic keratinocyte proliferation in selected patients. In any case, these inconsistent reports necessitate further studies and patient data collection focusing on the link between psoriasis vulgaris and EGFR.
Anti-EGFR antibody is effective in treating KRAS wild-type advanced colorectal cancer and various kinds of solid tumors. However, dermatological toxicities such as papulopustular rash occur in 70%-90% of patients, with the consequent physical and psychosocial discomfort potentially resulting in discontinuation of treatment[14]. Therefore, anti-EGFR antibodies are not generally used in patients with concomitant chronic skin disease, despite the lack of definitive evidence suggesting adverse skin effects. The case reported herein now presents important data and supports the previous experience that the use of cetuximab and/or panitumumab for colorectal cancer is not necessarily contraindicated in patients with psoriasis vulgaris[6], and indeed, suggests that such therapy could be beneficial for the treatment of psoriasis vulgaris. Importantly, the currently prevalent treatment with anti-EGFR antibodies is unlikely to ‘‘cure’’ psoriasis. Therefore, the case presented by Trivin et al[5] is quite attractive in that complete remission of psoriasis was sustained for more than 6 months after discontinuation of the treatment.
In conclusion, we experienced a patient with psoriasis vulgaris whose symptoms lessened during treatment with anti-EGFR antibody monotherapy for metastatic colon cancer. We hope that this case report serves to further clarify the pathogenesis of psoriasis vulgaris. In addition, patients with concomitant skin disease should still be considered for anti-EGFR antibody therapy.
A 55-year-old male patient with long-standing psoriasis vulgaris was referred to our department for the treatment of colon cancer.
Metastatic colon cancer and psoriasis vulgaris.
Computed tomography revealed metastatic colon cancer, and dermatological inspection showed typical skin lesions of psoriasis vulgaris.
Postoperative pathological examination revealed typical sigmoid colon cancer (not described in the manuscript).
Anti-epidermal growth factor receptor (EGFR) antibody was effective against both metastatic colon cancer and psoriasis vulgaris.
The effect of anti-EGFR antibody on psoriasis vulgaris is not well understood.
For the treatment of metastatic colon cancer, anti-EGFR antibody therapy should be still considered in patients with psoriasis vulgaris and/or chronic dermatological diseases.
This report describes a quite rare case of colon cancer and concomitant psoriasis vulgaris successfully treated with anti-EGFR antibody.
| 1. | Johnson-Huang LM, Lowes MA, Krueger JG. Putting together the psoriasis puzzle: an update on developing targeted therapies. Dis Model Mech. 2012;5:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, Wolff K. Fitzpatrick’s Dermatology in General Medicine Eighth Edition. Chapter 18 psoriasis. New York: McGraw-Hill companies 2012; 197-237. |
| 3. | King LE, Gates RE, Stoscheck CM, Nanney LB. The EGF/TGF alpha receptor in skin. J Invest Dermatol. 1990;94:164S-170S. [PubMed] [DOI] [Full Text] |
| 4. | Anderson KS, Petersson S, Wong J, Shubbar E, Lokko NN, Carlström M, Enerbäck C. Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. Br J Dermatol. 2010;163:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Trivin F, Boucher E, Raoul JL. Complete sustained regression of extensive psoriasis with cetuximab combination chemotherapy. Acta Oncol. 2004;43:592-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Neyns B, Meert V, Vandenbroucke F. Cetuximab treatment in a patient with metastatic colorectal cancer and psoriasis. Curr Oncol. 2008;15:196-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Nishizawa A, Satoh T, Yokozeki H. Erythrodermic psoriasis improved by panitumumab, but not bevacizumab. Acta Derm Venereol. 2012;92:360-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Mas-Vidal A, Coto-Segura P, Galache-Osuna C, Santos-Juanes J. Psoriasis induced by cetuximab: a paradoxical adverse effect. Australas J Dermatol. 2011;52:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 750] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 10. | Pastore S, Lulli D, Girolomoni G. Epidermal growth factor receptor signalling in keratinocyte biology: implications for skin toxicity of tyrosine kinase inhibitors. Arch Toxicol. 2014;88:1189-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Nanney LB, Stoscheck CM, Magid M, King LE. Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986;86:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 229] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Overbeck TR, Griesinger F. Two cases of psoriasis responding to erlotinib: time to revisiting inhibition of epidermal growth factor receptor in psoriasis therapy? Dermatology. 2012;225:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Zorzou MP, Stratigos A, Efstathiou E, Bamias A. Exacerbation of psoriasis after treatment with an EGFR tyrosine kinase inhibitor. Acta Derm Venereol. 2004;84:308-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Baas JM, Krens LL, Guchelaar HJ, Ouwerkerk J, de Jong FA, Lavrijsen AP, Gelderblom H. Recommendations on management of EGFR inhibitor-induced skin toxicity: a systematic review. Cancer Treat Rev. 2012;38:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gao F, Ranieri G S- Editor: Qi Y L- Editor: A E- Editor: Wang CH