Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4479
Revised: February 18, 2011
Accepted: February 25, 2011
Published online: October 28, 2011
AIM: To investigate the expression and localization of paxillin in rat pancreas during development.
METHODS: Pancreata from Sprague Dawley rat fetuses, embryos, young animals, and adult animals were used in this study. Expression levels of paxillin in pancreata of different development stages were detected by reverse transcription polymerase chain reaction and Western blotting. To identify the cell location of paxillin in the developing rat pancreas, immunohistochemistry and double-immunofluorescent staining were performed using antibodies for specific cell markers and paxillin, respectively.
RESULTS: The highest paxillin mRNA level was detected at E15.5 (embryo day 15.5) following a decrease in the later developmental periods (P < 0.05 vs E18.5, P0 and adult, respectively), and a progressively increased paxillin protein expression through the transition from E15.5 to adult was detected. The paxillin positive staining was mainly localized in rat islets of Langerhans at each stage tested during pancreas development.
CONCLUSION: The dynamic expression of paxillin in rat pancreas from different stages indicates that paxillin might be involved in some aspects of pancreatic development.
- Citation: Guo J, Liu LJ, Yuan L, Wang N, De W. Expression and localization of paxillin in rat pancreas during development. World J Gastroenterol 2011; 17(40): 4479-4487
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4479
Development of the endocrine and exocrine pancreas involves a complex process of cell differentiation that ultimately gives rise to four distinct hormone producing cell types (α, β, δ, PP) and two kinds of enzyme secreting cells (acinar and ductal cells)[1]. Organogenesis of the pancreas is a highly coordinated process. Morphologic development of the mouse pancreas first appears at E9.5. At approximately E16 the islet progenitor cells leave the contiguous epithelium, migrate through the adjacent extracellular matrix (ECM) into the surrounding mesenchyme, and aggregate to form the islets of Langerhans[2,3]. The islets are not fully formed until shortly before birth in E18-E19, and undergo further remodeling and maturation for 2-3 wk after birth[4]. Thus, the developing pancreas presents a challenge for developmental biologists because of the complex morphogenetic processes underlying the development of this organ.
The factors that control pancreatic organogenesis and tissue maintenance remain unclear. Of particular interest are the ECM and their receptors, integrins, which exert a profound role during development controlling morphogenetic decisions and maintaining homeostasis during adulthood. Progression in islet cell development is accompanied by, and dependent upon, cell adhesion viaβ1 integrin and its respective α-subunits. The β1 family of integrins play critical roles in islet cell architecture, development, integrity and function[5].
Paxillin interacts directly with several focal adhesion proteins including vinculin, talin, and integrin β1[6,7]. A principal function for paxillin is in the integration and dissemination of signals from integrins and growth factor receptors to provide efficient cellular migration[8]. Paxillin is an important mediator of signal cross-talk in the complex multistep process of net cellular movement through its phosphorylation and multipotent associations[9-12], and functions as an adaptor protein coordinating the activities of many focal adhesion proteins. Thus, paxillin is in a position to play a role in the integration and regulation of adhesion and signaling, yet little is known regarding its function during embryogenesis[13]. Given that it mediates integrin signal transduction, it might be expected that paxillin may be involved in numerous aspects of cell behavior and development in the pancreas. To the best of our knowledge, no study has investigated the relationship between paxillin expression and pancreas development, and the expression of paxillin during pancreatic development in rats is poorly understood. Knowledge of the regional and temporal expression of paxillin will be useful in understanding its potential role in pancreatic development. Therefore, we examined the expression of paxillin in rat pancreas during development.
Sprague-Dawley (SD) rats were purchased from the Animal Center of Nanjing Medical University (Nanjing, China). SD rats (2:1, male:female) were mated overnight. At noon the next day, if a vaginal plug was discovered, it was considered as Day 0.5 of gestation (E0.5). Embryos were removed at E12.5, E15.5 and E18.5 from the uterus of pregnant rats, which were sacrificed by cervical dislocation. Pancreata from E15.5 and E18.5 rat embryos were isolated according to their specific vacuolated morphology, as previously described[14], under a stereomicroscope. Rat pancreata at postnatal (P) days 0, 7, 14, 21 and from adults, were directly isolated by the unaided eye. All experiments were conducted in accordance with the Chinese Law for Animal Protection and were approved by the local animal care committee. Five rats were used at each age stage. Dissected tissues were immediately rinsed 3 times with phosphate buffered saline (PBS) to remove serum proteins, and fixed with 4% paraformaldehyde in PBS overnight for histology, or frozen in liquid nitrogen for RNA and protein isolation.
Pancreata from E15.5, E18.5, P0, P14, P21, and nonpregnant adult rats were fixed with 4% paraformaldehyde in PBS overnight and embedded in paraffin. Pancreata were cut into 5-μm sections and mounted on gelatin/chrome alum-coated glass slides. Following deparaffinization, the presence of paxillin and insulin was determined immunohistochemically. To expose antigenic sites for paxillin/insulin, dewaxed sections were heated four times to 95 °C in a 600 W microwave oven maintained for 5 min
and allowed to cool for 20 min. Endogenous peroxidase activity was then eliminated by incubation with 0.5% (v/v) hydrogen peroxide solution in absolute methanol for 15 min at 20 °C. Non-specific protein binding was eliminated by incubation with 10% non-fat dry milk in PBS for 1 h at 20 °C. Sections were then incubated with a polyclonal antibody (sc-7336; Santa Cruz Biotechnology) against paxillin or insulin (sc-9168; Santa Cruz Biotechnology) at a dilution of 1:200 and 1:500, respectively, for 18 h at 4 °C. Incubation for 1 h with horseradish peroxidase conjugated secondary antibody (1:500 dilutions) at room temperature followed. The antigen-antibody complex was then visualized by incubating the sections with 3, 3’-diaminobenzidine solution in the dark for
3 min. Sections counterstained with hematoxylin were dehydrated, and coverslipped. Images were taken at a magnification of × 400. Controls were processed by omitting the primary antibody in the immunolabeling procedure.
The paraffin sections were deparaffinized in xylene and rehydrated in graded ethanol and distilled water. The non-specific binding sites were blocked in 1% bovine serum albumin for 30 min. For paxillin and amylase or glucagon double immunofluorescence, the goat anti- paxillin primary polyclonal antibody was applied and revealed using fluorescein isothiocyanate-labeled rabbit anti-goat IgG (1:400, sc-2777, Santa Cruz Biotechnology). Mouse anti-amylase primary polyclonal antibody (1:500, sc-45667; Santa Cruz Biotechnology) or mouse anti-glucagon primary polyclonal antibody (1:1000, G2654; Sigma Aldrich) was then applied and revealed by cy3-labeled anti-mouse IgG (1:400, AP192C; Chemicon International, Inc. Temecula, CA, United States). Sections were placed in gel mounted aqueous mounting medium (G0918; Sigma, St. Louis, United States) with a cover glass, and examined under an Olympus BX51 Research Microscope (Olympus Optical, Tokyo, Japan). To rule out cross-reactivity in this staining system, the controls used were: first, single staining with the alternative secondary antibody and second, staining in the absence of primary antibody. In neither case was staining detectable. Images were taken at a magnification of × 400.
The pancreata was homogenized in a detergent lysis buffer containing 8 mol/L urea, 2% CHAPS, 40 mmol/L Tris, 65 mmol/L DTT and 2% IPG buffer. The lysate was then centrifuged at 15 000 g for 1 h at 4 °C. The total protein concentration of each sample was analyzed using a modified Bradford assay. All samples were stored at -80 °C prior to the electrophoresis.
An equal amount of protein sample (40 μg) from each time point was resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA). After transfer, the membrane was blocked with 5% fat-free milk in Tris-buffered saline and 0.05% Tween 20 overnight at 4 °C. Primary antibodies were incubated with the membrane as described above and detected by peroxidase-linked rabbit anti-goat conjugates (Santa Cruz Biotechnology). Densitometric quantification of bands at subsaturating levels was performed using the Syngenetool gel analysis software (Syngene, Cambridge, United Kingdom). Loading controls of presumably constantly expressed proteins such as β-tubulin were used; however, their variability and increase in development precluded their use[5]. For negative controls, the primary antibody was omitted.
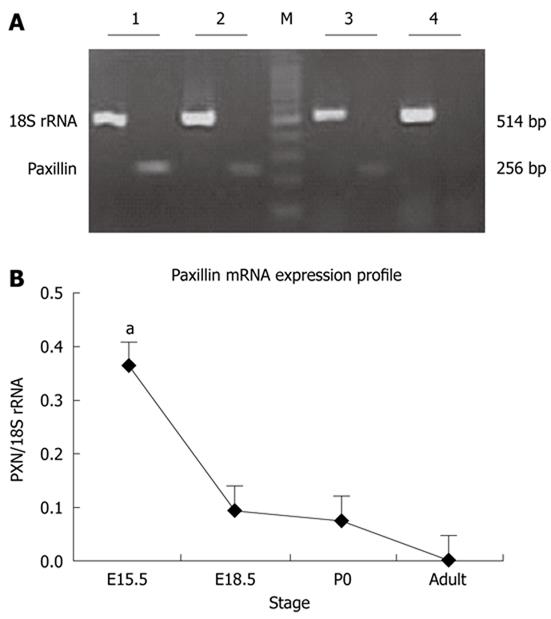
Total RNA was extracted from the pancreata at each time point with TRIZOL reagent (Invitrogen Life Technologies, Burlington, Ontario, Canada), according to the manufacturer’s instructions. The quality of the RNA was verified by agarose gel electrophoresis using ethidium bromide staining. For each polymerase chain reaction (PCR), 2 μg DNA-free total RNA with oligo (deoxythymidine) primers and reverse transcriptase were used. PCR was performed in 25-μL reactions containing 25 ng of cDNA, 0.2 nmol of each primer pair, and 0.3 μL of Taq DNA polymerase. PCR was carried out in a T-gradient Biometra PCR thermal cycler (Montreal Biotech Inc., Kirkland, Quebec, Canada) to determine the annealing temperature for paxillin primers. The primer pairs used were as follows: paxillin, forward: 5'-GGAGCAGAACGACAAGCC-3', reverse: 5'-GCACAGAGCCCAGGAGA-3' (256 bp); 18S rRNA, forward: 5'-ACGAACCAGAGCGAAAGC-3', reverse: 5'-GGACATCTAAGGGCATCACAG-3' (514 bp). PCR conditions were as follows: 2 min at 94 °C for hot start, followed by up to 35 cycles of 94 °C for 30 s, 53.4 °C for 30 s, and 72 °C for 45 s, with a final extension of 5 min at 72 °C. To estimate the linear range of the nested reactions, we analyzed the PCR products at 10, 15, 20, 25, 30, and 35 cycles. The amplified products were analyzed on 1% agarose gels and visualized by ethidium bromide staining. The data were normalized by 18S rRNA.
Total RNA samples were used to generate cRNA probes by two rounds of transcription. A poly (dT) primer (with its 5’ end carrying T7 promoter sequence) was used to synthesize cDNA from total RNA. The cDNA were used to amplify cRNA using T7 polymerase. The cRNA product from this first round amplification was then used to generate more cDNA by random priming, with the 3’ end carrying a T7 promoter sequence. This cDNA was used to transcribe biotinylated cRNA, which was used to hybridize to the RAE 230A microarrays produced by Affymetrix.
Analysis of the experimental data was performed using PD Quest 7.0 software and the paired Student t-test. P < 0.05 was considered statistically significant. Data are presented as the mean ± SD.
The mRNA expression levels of paxillin in the whole pancreas of the developing rat were examined through Affymetrix oligonucleotide microarray (RAE230A) (Table 1)
| Gene symbol | Probeset ID | E12.5 | E15.5 | E18.5 | P0 | Adult | |||||
| Integrinβ1 | 1368819_at | 748.3 | P | 3277.2 | P | 3847.5 | P | 1055.6 | P | 427.5 | P |
| Integrinβ4 | 1368612_at | 58.2 | A | 295.3 | P | 542.6 | P | 149.4 | P | 115.5 | P |
| Integrinβ5 | 1370801_at | 25.4 | A | 49.5 | P | 56.3 | P | 5.2 | A | 2.8 | A |
| Vinculin | 1375538_at | 168.7 | P | 1532.5 | P | 1790.7 | P | 141.9 | P | 23.1 | P |
| ILK | 1387777_at | 634.5 | P | 1388.5 | P | 1449.5 | P | 583.3 | P | 122.8 | P |
| Clathrin | 1398842_at | 1040.0 | P | 2918.2 | P | 3682.7 | P | 1306.9 | P | 429.0 | P |
| Paxillin | 1371664_at | 298.8 | P | 997.5 | P | 847.0 | P | 285.3 | P | 62.4 | P |
| Actopaxin | 1370266_at | 57.7 | A | 425.6 | P | 212.5 | P | 42.5 | P | 10.2 | A |
and reverse transcription-PCR (Figure 1). As shown in Figure 1, the highest paxillin mRNA expression level was observed at E15.5, followed by decreased mRNA level of paxillin from E18.5 to adult. At the same time, mRNA expression of genes related to paxillin was also detected by Affymetrix oligonucleotide microarray (RAE230A), such as actopaxin, ILK, clathrin, vinculin, and Integrinβ1, as shown in Table 1. Absolute signal values of a selection of genes expressed in E12.5, E15.5, E18.5, P0 and adult rat pancreata were given. The highest expression level of these genes was almost detected at E15.5 (paxillin and actopaxin) or E18.5 (Integrinβ1, Integrinβ4, Integrinβ5, vinculin, ILK and clathrin). Expression profile of these mRNAs was similar to paxillin.
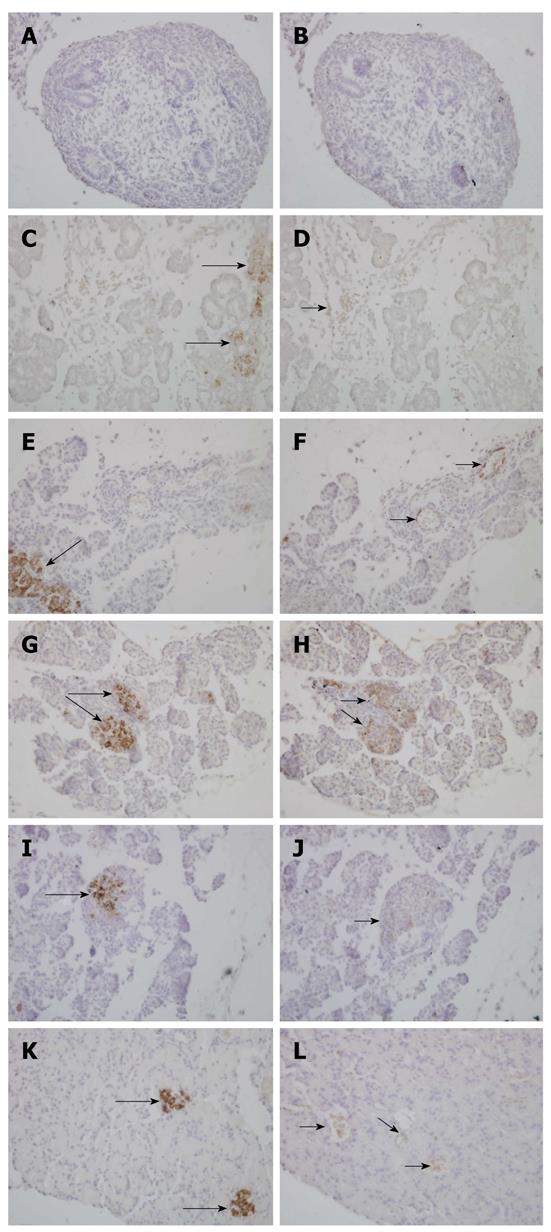
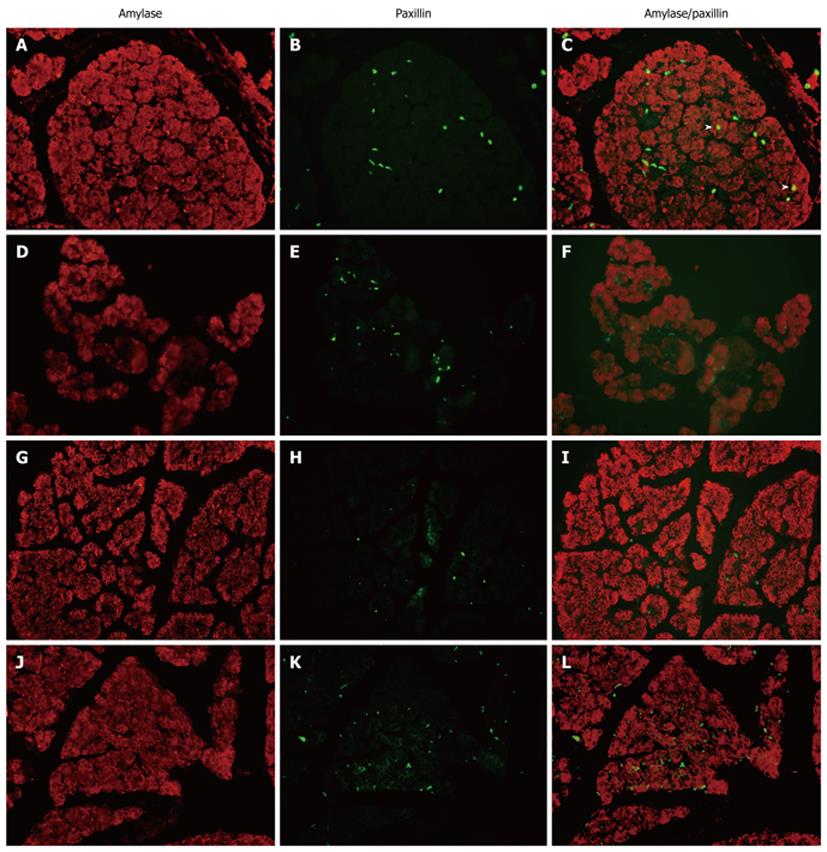
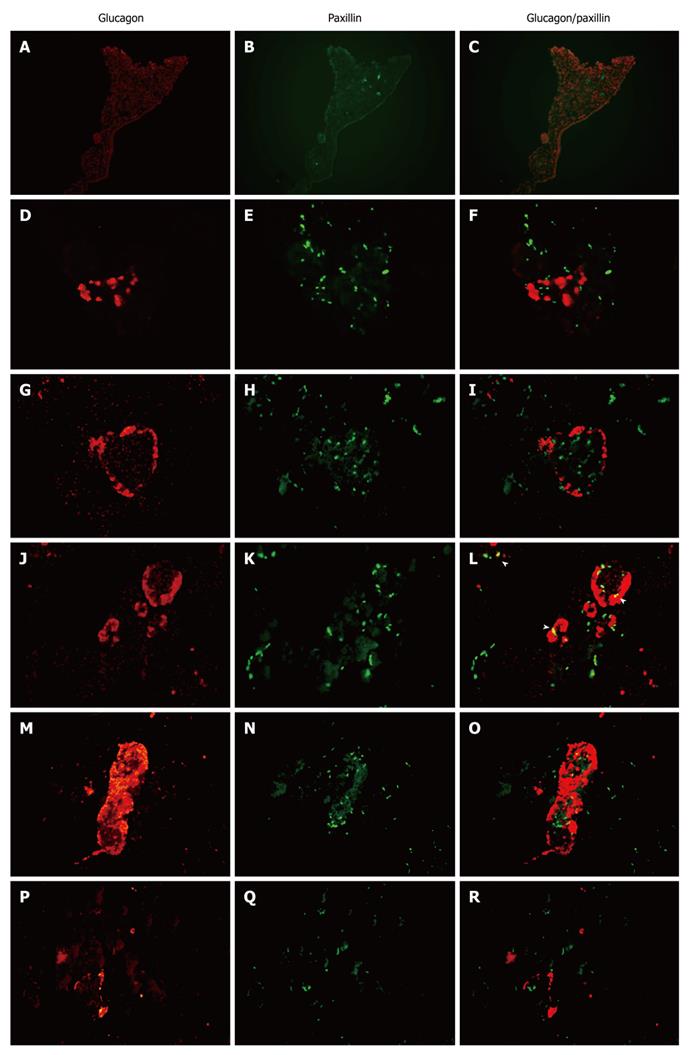
To investigate the spatiotemporal expression of paxillin within the rat pancreas through fetal to postnatal life, we examined paxillin expression through immunohistochemistry (Figure 2). We found that paxillin maintained expression in the pancreas from E18.5 to adult, and at P14 and P21 paxillin was mainly localized in rat islets of Langerhans. Furthermore, double immunofluorescence was used to detect expression of paxillin and amylase (Figure 3) or glucagon (Figure 4). As shown in Figure 3, although little co-expression of paxillin and amylase was detected, paxillin could be detected in the exocrine portion during development as has been reported previously[15]. As shown in Figure 4, although little co-expression of paxillin and glucagon was detected, paxillin positive cells could be found in the center of islets of Langerhans from E18.5. To the best of our knowledge, there have been no reports concerning the expression of paxillin in rat islets of Langerhans.
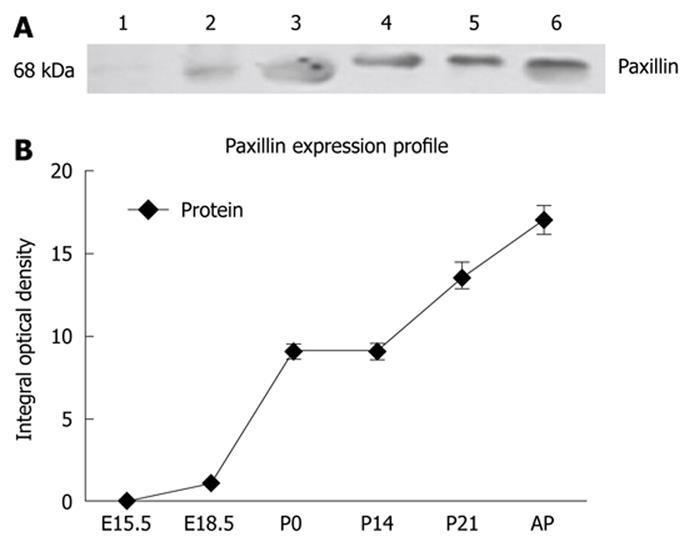
Examining the abundance of paxillin at the protein level during rat pancreatic development, from embryonic to postnatal life in whole pancreata by Western blotting analysis (Figure 5), revealed that there exist two isoforms of paxillin protein at 68 KD in pancreas during the developmental process, which was in agreement with previous reports[13]. During embryonic and neonatal development phases, only the smaller isoform was detected, while the larger isoform was detected only after birth. Interestingly, in the adult pancreas, the two isoforms could both be identified. Paxillin began to be expressed at E15.5, and significantly increased after birth, with a 10 fold increase at birth. The differential expression pattern of two isoforms of paxillin in the developmental process suggested their distinct physiological function[7].
During organogenesis, specialized cell types are generated from progenitor cell populations and are precisely organized into the elaborate structure of the adult organ[16,17]. Biochemical and ultrastructural studies delineated three distinct phases of pancreatic development in rats and mice. The first is during early organogenesis and is known as the “primary transitional phase” in which cytodifferentiation into exocrine or endocrine cells is minimal (approximately from E10 to E12/12.5 in rat). This is followed by the “proto-differentiated state” (from E12.5 to E15.5), in which epithelial cell proliferation is high, leading to rapid growth and lobulation, while cytodifferentiation is poor. During late organogenesis, a third phase or “secondary transitional phase” (from E15.5 to E18.5) is detected in which a dramatic increase in the number of endocrine and exocrine cells with high levels of insulin and exocrine enzymes is observed. During the secondary transition, endocrine cell migration and adhesion, and primary islet formation are key events. At approximately E16 the islet progenitor cells leave the contiguous epithelium, migrate through the adjacent ECM into the surrounding mesenchyme, and aggregate to form the islets of Langerhans. The islets are not fully formed until shortly before birth in E18-E19 and undergo further remodeling and maturation for 2-3 wk after birth[4]. Although some studies have addressed the function of single gene, such as integrins in islet formation, much less is known about the multifactor network that guides islet formation during embryogenesis[18-20]. The present study demonstrates that pancreatic development is accompanied by a specific spatiotemporal pattern of protein and mRNA expression of paxillin, which is a mediator in integrin signaling, suggesting a role for paxillin during pancreatic development.
The protein and mRNA expression of paxillin in the whole pancreas determines a specific temporal pattern of expression from embryonic development to postnatal development. High mRNA expression, along with low protein level of paxillin, was observed in the embryonic pancreas (Figures 1 and 5). Subsequently, into postnatal life, decreased paxillin mRNA expression was paralleled to increased protein levels in postnatal life. What is more, genes related to paxillin, such as ILK, clathrin, vinculin and integrin β1, shared the same expression pattern with paxillin. The highest expression level of these genes was almost detected at E15.5 or E18.5. In the present study, a dramatic increase of paxillin protein expression in the neonatal pancreas, compared with embryonic period, was detected. From the 18th day of gestation until birth, active cellular reorganization takes place to form the classic cellular features of the islet. After birth, apoptosis of a great amount of islet cells take place. From P14 to P21, islet cell proliferation increased dramatically and islet structure is remodeled. Cell adhesion and migration are necessary to islet structure remodeling. Immunohistochemical staining of paxillin demonstrated its expression within rat islets at P14 and P21. To the best of our knowledge, there have been no reports concerning the expression of paxillin in islets of Langerhans. Given the pivotal role of paxillin in cell adhesion and migration, the specific high expression of paxillin protein after birth indicates a potential role in islet structure remodeling.
The expression of paxillin at both the mRNA and protein level indicates that paxillin may be critical in mediating the biological functions of the developing pancreas, especially in maintaining islet structure and function. Regardless, these studies demonstrate that paxillin is expressed at the protein and mRNA level throughout development, indicating potential molecular signals mediated by paxillin that may control various aspects of pancreatic function and development.
In higher eukaryotes, paxillin exists as multiple isoforms (α, β, γ and δ)[6]. Paxillin α is the principal, ubiquitously expressed isoform, whereas the β- and γ-isoforms exhibit restricted expression. The β- and γ-isoforms contain a 34- and 48-amino acid insertion, respectively, between amino acids 277/278. However, there have been reports suggesting the lack of a murine γ-isoform[21,22]. Our study revealed that there exist two isoforms at 68 KD of paxillin protein in the pancreas during development, with different expression profiles. During the embryonic and neonatal development phases, only the smaller isoform was detected, while the larger isoform was detected only after birth. Interestingly, in the adult pancreas, the two isoforms could both be identified. It has been documented in a previous study that different paxillin isoforms show a distinct expression profile in whole embryos during development in mice, which suggested distinct physiological roles for each isoform[7]. Thus, the differential expression of paxillin isoforms in the pancreas during development may indicate their distinct roles in pancreas development. Of course, further studies are needed to verify our hypothesis.
The morphogenesis of embryos depends both on interactions between cells and their surrounding ECM via integrin complexes, and direct cell-cell borders perceived as discrete adhesion systems that may interact with each other during morphogenesis[5,23-26]. The integrin family of transmembrane receptors physically connects the actin cytoskeleton of the cells to the ECM at focal adhesions, and thus, mediates migration and adhesion in many cells. The importance of focal adhesion in early development is well illustrated by mouse embryos deficient in fibronectin or focal adhesion components, including focal adhesion kinase (FAK), paxillin, or integrins. These embryos die early in development (days 5-10) with mesodermal defects. Data from previous studies support roles for paxillin and FAK in the organization of actin around tight junctions or adherens junctions; functions that would help stabilize or remodel cell-cell borders during the migration of this epithelial sheet[9,12].
In summary, our present study has, for the first time, demonstrated that paxillin is localized to the islets of Langerhans in developing rat pancreata via immunohistochemistry and immunofluorescence. Given the important roles of paxillin in intercellular adhesion and cell migration, it may play important roles in pancreas islet formation, which is a process of cell-cell adhesion and cell migration, especially in islet structure and functional maintenance. Further investigations are needed to verify our hypothesis.
Special thanks to Dr. Ying-Bing Ge for his technical skills. Our gratitude also goes to Dr. Zheng-Xian Tao for his comments and criticisms.
Knowledge of the regional and temporal expression of paxillin will be useful in understanding its potential role in pancreatic development. However, no study has investigated the relationship between paxillin expression and pancreas development, and the expression of paxillin during pancreatic development in rats is poorly understood.
The authors examined the expression of paxillin in rat pancreas during development.
The study, for the first time, demonstrated that paxillin is localized to the islets of Langerhans in developing rat pancreata via immunohistochemistry and immunofluorescence. Given the important roles of paxillin in intercellular adhesion and cell migration, it may play important roles in pancreas islet formation, which is a process of cell-cell adhesion and cell migration, especially in islet structure and functional maintenance.
This is an interesting and fairly well performed study.
Peer reviewers: Parviz M Pour, Professor of Pathology, Member of UNMC/Eppley Cancer Center, 98168 Nebraska Med Center, Omaha, NE 68198-680, United States; Raffaele Pezzilli, MD, Department of Internal Medicine and Gastroenterology, Sant’Orsola-Malpighi Hospital, Via Massarenti, 9, Bologna 40138, Italy; Mukaddes Esrefoglu, Dr., Professor, Department of Histology and Embryology, Inonu University, 44280 Malatya, Turkey
S- Editor Tian L L- Editor Rutherford A E- Editor Zheng XM
| 1. | Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Osman NM, Kagohashi Y, Udagawa J, Otani H. Alpha1,4-N-acetylglucosaminyltransferase encoding gene EXTL3 expression pattern in mouse adult and developing tissues with special attention to the pancreas. Anat Embryol (Berl). 2003;207:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 290] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Yashpal NK, Li J, Wheeler MB, Wang R. Expression of {beta}1 integrin receptors during rat pancreas development--sites and dynamics. Endocrinology. 2005;146:1798-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 504] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Mazaki Y, Uchida H, Hino O, Hashimoto S, Sabe H. Paxillin isoforms in mouse. Lack of the gamma isoform and developmentally specific beta isoform expression. J Biol Chem. 1998;273:22435-22441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Mazaki Y, Hashimoto S, Sabe H. Monocyte cells and cancer cells express novel paxillin isoforms with different binding properties to focal adhesion proteins. J Biol Chem. 1997;272:7437-7444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065-3081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Sorenson CM, Sheibani N. Focal adhesion kinase, paxillin, and bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn. 1999;215:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231-E236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 647] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 12. | Schaller MD. FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J Cell Biol. 2004;166:157-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Shi J, Ni XF, Chen Y. Patterning biomolecules with a water-soluble release and protection interlayer. Langmuir. 2007;23:11377-11380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Andreolotti AG, Bragado MJ, Tapia JA, Jensen RT, Garcia-Marin LJ. Cholecystokinin rapidly stimulates CrkII function in vivo in rat pancreatic acini. Formation of CrkII-protein complexes. Eur J Biochem. 2003;270:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Peters J, Jürgensen A, Klöppel G. Ontogeny, differentiation and growth of the endocrine pancreas. Virchows Arch. 2000;436:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Mfopou JK, Willems E, Leyns L, Bouwens L. Expression of regulatory genes for pancreas development during murine embryonic stem cell differentiation. Int J Dev Biol. 2005;49:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development. 2004;131:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Min BH, Jeong SY, Kang SW, Crabo BG, Foster DN, Chun BG, Bendayan M, Park IS. Transient expression of clusterin (sulfated glycoprotein-2) during development of rat pancreas. J Endocrinol. 1998;158:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Bukharova T, Weijer G, Bosgraaf L, Dormann D, van Haastert PJ, Weijer CJ. Paxillin is required for cell-substrate adhesion, cell sorting and slug migration during Dictyostelium development. J Cell Sci. 2005;118:4295-4310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Tumbarello DA, Brown MC, Hetey SE, Turner CE. Regulation of paxillin family members during epithelial-mesenchymal transformation: a putative role for paxillin delta. J Cell Sci. 2005;118:4849-4863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes. 2000;49:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Cirulli V, Beattie GM, Klier G, Ellisman M, Ricordi C, Quaranta V, Frasier F, Ishii JK, Hayek A, Salomon DR. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150:1445-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Bertelli E, Bendayan M. Association between endocrine pancreas and ductal system. More than an epiphenomenon of endocrine differentiation and development? J Histochem Cytochem. 2005;53:1071-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Wang R, Li J, Lyte K, Yashpal NK, Fellows F, Goodyer CG. Role for beta1 integrin and its associated alpha3, alpha5, and alpha6 subunits in development of the human fetal pancreas. Diabetes. 2005;54:2080-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |