Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4488
Revised: February 12, 2011
Accepted: February 19, 2011
Published online: October 28, 2011
AIM: To analyse αV integrin expression induced by gastrin in pancreatic cancer models.
METHODS: αV integrin mRNA expression in human pancreatic cancer cells was analysed using a “cancer genes” array and confirmed by real-time reverse transcription-polymerase chain reaction (PCR). Western blotting and semi-quantitative immunohistochemistry were used to examine protein levels in human pancreatic cancer cell lines and pancreatic tissues, respectively. The role of αV integrin on gastrin-induced cell adhesion was examined using blocking anti-αV integrin monoclonal antibodies. Adherent cells were quantified by staining with crystal violet.
RESULTS: Using a “cancer genes” array we identified αV integrin as a new gastrin target gene in human pancreatic cancer cells. A quantitative real-time PCR approach was used to confirm αV integrin gene expression. We also demonstrate that Src family kinases and the PI 3-kinase, two signalling pathways specifically activated by the CCK-2 receptor (CCK2R), are involved in gastrin-mediated αV integrin expression. In contrast, inhibition of the ERK pathway was without any effect on αV integrin expression induced by gastrin. Our results also show that gastrin modulates cell adhesion viaαV integrins. Indeed, in vitro adhesion assays performed on fibronectin show that gastrin significantly increases adhesion of pancreatic cancer cells. The use of blocking anti-αV integrin monoclonal antibodies completely reversed the increase in cell-substrate adhesion induced by gastrin. In addition, we showed in vivo that the targeted CCK2R expression in the pancreas of Elas-CCK2 mice, leads to the overexpression of αV integrin. This process may contribute to pancreatic tumour development observed in these transgenic animals.
CONCLUSION: αV integrin is a new gastrin target in pancreatic cancer models and contributes to gastrin effects on cell adhesion.
- Citation: Cayrol C, Bertrand C, Kowalski-Chauvel A, Daulhac L, Cohen-Jonathan-Moyal E, Ferrand A, Seva C. αV integrin: A new gastrin target in human pancreatic cancer cells. World J Gastroenterol 2011; 17(40): 4488-4495
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4488
Pancreatic cancer has a poor prognosis with a 5-year survival rate < 5%. Despite intensive efforts to improve therapy, treatment remains unsatisfactory and most patients die within months as a result of rapid local spread of tumour or metastatic dissemination[1]. This poor prognosis is mainly due to the propensity of this tumour to invade the adjacent structures and metastasize to distant organs early in the course of this disease; however, the molecular basis for these characteristics of pancreatic cancer is incompletely understood. A better understanding of the genes involved in tumour growth and migration may allow development of novel treatment strategies to rapidly tackle this disease.
Several lines of evidence support the role of gastrin, a digestive peptide hormone and its G protein-coupled receptor (CCK2R) in pancreatic cancer development. Gastrin and its receptor are up-regulated in human pancreatic adenocarcinoma as well as in preneoplastic lesions[2,3]. A splice variant of the CCK2R has recently been identified, which has constitutive activity and is exclusively expressed in certain human colon and pancreatic cancers[4-6]. In addition, we have reported in Elas-CCK2 transgenic mice, expressing functional human CCK2R in pancreatic exocrine cells, an increased pancreatic growth, an acinar to ductal trans-differentiation, postulated to be a preneoplastic step in pancreatic carcinogenesis and the development of tumours[7,8].
Besides proliferation, gastrin has been shown to modulate cell adhesion and migration. We and others have recently demonstrated in vitro that prolonged activation of the CCK2R by gastrin induces stress fibre formation, alters cell morphology, increases loss of cell-cell adhesion, as well as motility of epithelial cells[9-12]. We have also shown the loss of intercellular adhesion in acini of Elas-CCK2 mice before tumour formation[13].
Several signalling pathways activated by the CCK2R have been implicated in the proliferative effects or cell migration induced by gastrin. They include: MAP-kinases[14,15], the phosphatidylinositol 3-kinase and the JAK2/STAT3 pathway[16,17]. In addition, Src family tyrosine kinases and p125FAK have also been shown to play a crucial role in these biological effects of gastrin[18].
In gastric epithelial cells, several target genes of the CCK2R have already been identified. They include genes involved in gastric acid secretion[19], early response genes, c-Fos[20], c-Jun and c-Myc[21,22] and other growth-related genes such as cyclin D1[23], Reg-1[24], or the HB-EGF[25]. In addition, in the same cellular model, gastrin also regulates the expression of genes associated with cell migration and invasion such as the MMP9 gene, a matrix metalloproteinase[26]. In several cellular models such as gastric and colonic cancer cells, intestinal epithelial cells or fibroblasts transfected with the CCK2R, gastrin has also been shown to enhance cyclooxygenase-2 gene expression, known to play an important role in inflammation processes and carcinogenesis[27-29].
In contrast, to our knowledge, very few gastrin-regulated genes have been identified in pancreatic models expressing the CCK2R. Recently, we showed that Reg proteins are targets of CCK2R activation and are induced during the early steps of carcinogenesis in Elas-CCK2 mouse pancreas[30]. In addition, we also identified β1 integrin as a gastrin-regulated gene in human pancreatic cancer cells and demonstrated its involvement in modulation of cell adhesion by the CCK2R[31].
In this study, we identified αV integrin, another member of the large integrin family, as a new gastrin target in the human pancreatic cancer cell line, Panc-1. Integrins which mediate cell adhesion play an important role in cell migration, survival and differentiation. Here we show in vitro that αV integrin is involved in the modulation of cell adhesion by the CCK2R. In addition, we demonstrate in vivo that the targeted CCK2R expression in the pancreas of Elas-CCK2 mice, which present preneoplastic lesions and develop pancreatic tumours, leads to αV integrin expression.
The human pancreatic cancer cell line, Panc-1 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, United States). The cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% FCS at 37 °C in a humidified atmosphere containing 5% CO2. In all experiments, cells were serum-starved for 18 h prior to gastrin stimulation. Human gastrin 2-17ds (Bachem, Switzerland) was used in all experiments.
Total RNA was isolated from Panc-1 cells treated with or without gastrin as indicated using the RNeasy RNA Isolation Kit (Qiagen, Valencia, CA, United States). After pretreating RNA with 10 units DNase (Invitrogen, Carlsbad, CA, United States), cDNA was produced from 1 μg of total RNA using the Superscript First-Strand Synthesis System for reverse transcription-polymerase chain reaction (PCR) (Invitrogen, Carlsbad, CA, United States).
A specific “Cancer array” (96 genes) from SuperArray (Bioscience Corporation, Beverly, MA, United States) was used in this study. Total RNA was isolated from Panc-1 cells as described above. Reverse transcription of cellular RNA was carried out with the RT-Labeling kit (SuperArray, Bioscience Corporation, Beverly, MA, United States) according to the manufacturer’s instructions. The biotinylated probes from gastrin-stimulated cells and unstimulated cells were hybridized overnight to separate membranes at 60 °C, washed with SSC/SDS solutions, incubated with the avidin-alkaline phosphatase conjugate and exposed to a chemiluminescent substrate. Analysis of the images and quantitation of the spots in both membranes were performed by the ScanAlyze 2.5 software, and normalization of the values and comparison of the intensities was achieved by the GE ArrayAnalyzer 1.3 (SuperArray, Bioscience Corporation, Beverly, MA, United States) software.
αV integrin expression was determined via real-time PCR, using fluorescent SYBR green dye (Applied Biosystems, Framingham, MA, United States) to allow semi quantitative analysis of gene expression levels. Amplification was conducted using ABI-Stepone + Detection System (Applied Biosystems, Framingham, MA, United States). Relative fold changes were determined using the 2-ΔΔCT method, in which 18S gene was used for normalization.
Primers used (18S: forward-CGCAGCTAGGAATAATGGAATAGG, reverse-CATGGCCTCAGTTCCGAAA; αV integrin: forward-TGCCCAGCGCGTCTTC, reverse-TGGGTGGTGTTTGCTTTGG).
Western blotting analyses were performed on lysates from Panc-1 cells stimulated or not with gastrin. Fractions, containing identical levels of proteins, were separated by SDS-PAGE and analyzed by Western blotting with the indicated antibodies. The immunoreactivity was visualized with an enhanced chemiluminescence system (Pierce, IL, United States). Anti-αV integrin antibodies were from Chemicon (Temecula, CA, United States).
Cell adhesion assays were carried out in 96-well plates using 105 cells/cm2 in a final volume of 100 μL/well of serum-free medium. Wells were coated overnight at 4 °C with fibronectin diluted at 5 μg/mL in phosphate buffered solution (PBS) then washed twice with 100 μL of PBS and blocked with 1% bovine serum albumin (BSA)-PBS for 30 min at room temperature before addition of the cell suspension. The cells were incubated for 2 h at 37 °C with or without gastrin. Adherent cells were fixed with 50 μL of 96% ethanol for 10 min, stained with 50 μL of 0.1% crystal violet, rinsed extensively with water and dried at room temperature. Stained cells were solubilised with 50 μL of 0.2% Triton X-100 and quantified by measuring the absorbance at 570 nm. For adhesion inhibition experiments, cells were pretreated for 30 min at 37 °C with or without 5 μg/mL function-blocking antibodies directed against αV integrin and treated or not with gastrin for 2 h.
Homozygous Elas-CCK2 mice used in this study have been described previously[8]. Homozygous Elas-CCK2 mice in a B6SJLF1 background 3 at least 6-mo old and 3 corresponding control littermates were used. Mice were reared in a routine animal facility of the I2MR and maintained on a 12:12 h light-dark cycle. All the experiments were performed during the daytime. All procedures were approved by the I2MR Animal Facility Care Committee.
Mice were killed by decapitation, the pancreas was excised, fixed and embedded in paraffin using standard techniques. Immunohistochemistry was performed as previously described[16] using anti-αV integrin antibodies (Chemicon, Temecula, United States). Sections were incubated with the appropriate secondary and tertiary peroxidase-labelled antisera (DAKO, Glostrup, Denmark) at room temperature, exposed to a solution of diaminobenzidine. All dilutions and washes were performed with phosphate-buffered saline, pH 7.4, containing 0.1% bovine serum albumin.
All results are presented as mean ± SE. Statistical significance was calculated using unpaired Student’s t test. Values of P < 0.05 were considered statistically significant. All analyses were performed using “GraphPad Prism” software.
Gastrin increasesαv integrin expression in Panc-1 cells
In order to identify new gastrin-regulated genes, a human cancer array of 96 genes was probed with samples from either control Panc-1 cells or cells treated with gastrin for 24 h.
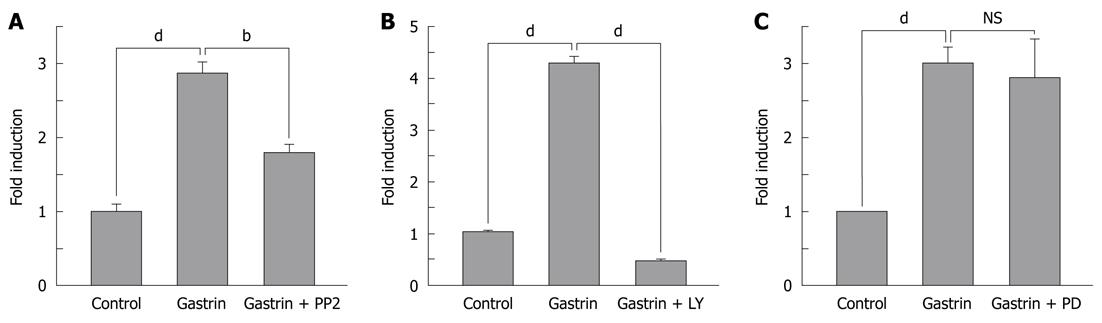
Among the genes positively modulated by the CCK2R in these experiments, we observed a significant increase in the expression of αV integrin (Figure 1A). A quantitative real-time PCR approach was used to confirm and quantify the αV integrin gene expression. In response to gastrin, the increase in αV integrin gene expression was time-dependent. A significant effect due to gastrin was detectable 3 h after treatment. At 24 h, we observed a 5-fold increase in the expression of αV integrin in response to gastrin (Figure 1B).
In addition, we also confirmed the increase in protein levels of αV integrin in gastrin-stimulated cells using Western blotting analysis (Figure 1C and D).
Signalling pathways involved inαv integrin expression stimulated by gastrin
As mentioned in the Introduction, gastrin exerts its trophic effects and modulates cell adhesion through a variety of intracellular pathways depending on the cellular model. We previously identified the signalling pathways specifically activated by the CCK2R in Panc-1 cells[31]. They include the ERK pathway, the PI3K/AKT pathway and the activation of Src-kinases.
To determine the cellular mechanism by which gastrin increased αV integrin gene expression, we examined gastrin-regulated αV integrin gene expression in Panc-1 by quantitative real-time PCR in the absence or presence of different specific inhibitors, LY294002, PP2, or PD098059 which block the PI 3-kinase pathway, Src family kinases and the ERK pathway, respectively. When cells were pre-incubated with PP2, the response to gastrin was decreased by 60% and totally blocked in cells pre-treated with LY294002 (Figure 2A and B), whereas the inhibitors alone did not significantly affect basal αV integrin expression (PP2: 1.09 ± 0.2 fold induction, LY204002: 0.93 ± 0.35 fold induction). These results indicate that Src family kinases and the PI 3-kinase pathway mediate gastrin-increased αV integrin gene expression in Panc-1 cells. In contrast, the inhibitor of the ERK pathway was without any effect (Figure 2C).
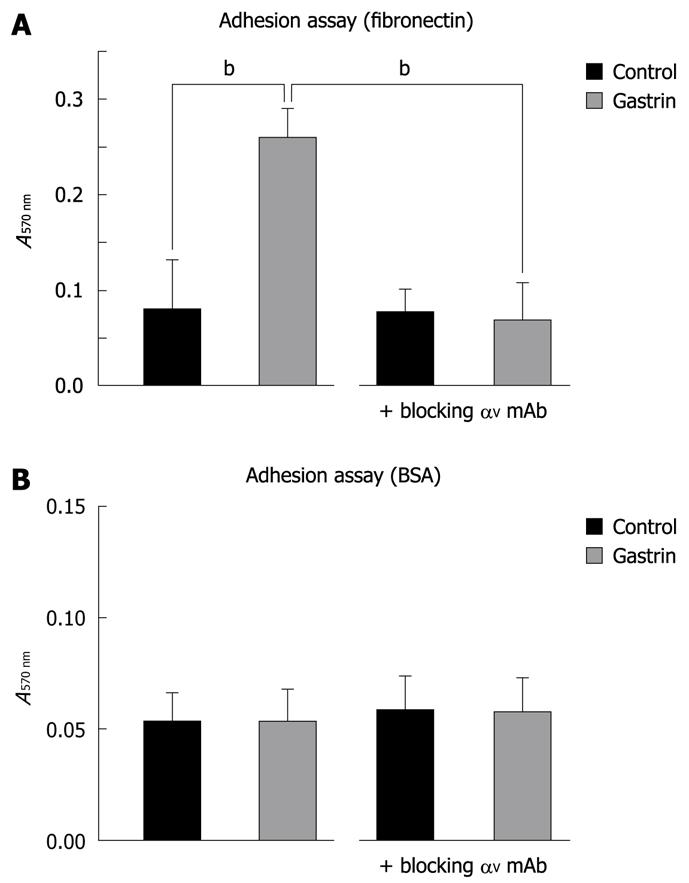
In this study, we have identified αV integrin as a new gastrin target in Panc-1 cells. Integrins act as adhesion receptors linking the extracellular matrix (ECM) to the cytoskeleton. For many cell types, integrin-mediated adhesion is required for cell growth and cell survival. In the second part of this study, we investigated whether gastrin had an effect on Panc-1 cell adhesion. In a cell adhesion assay using fibronectin-coated wells, we showed that gastrin induced a significant increase in Panc-1 cell adhesion (Figure 3A). As expected in BSA only controls, we did not observe any effect of gastrin on cell adhesion (Figure 3B).
To determine the role of αV integrin in gastrin-enhanced Panc-1 cell adhesion, we used blocking anti-αV integrin monoclonal antibodies. When added 30 min prior to gastrin stimulation, the antibodies significantly decreased gastrin-stimulated Panc-1 cell adhesion (Figure 3A). This confirmed that αV integrin plays an important role in Panc-1 cell adhesion stimulated by gastrin.
Immunohistochemical staining ofαv integrin in the pancreas of Elas-CCK2 mice
We recently described that Elas-CCK2 mice express human CCK2R in acini. These mice exhibited an increased pancreatic growth, an acinar to ductal trans-differentiation, postulated to be a preneoplastic step in pancreatic carcinogenesis, and developed tumors[8].
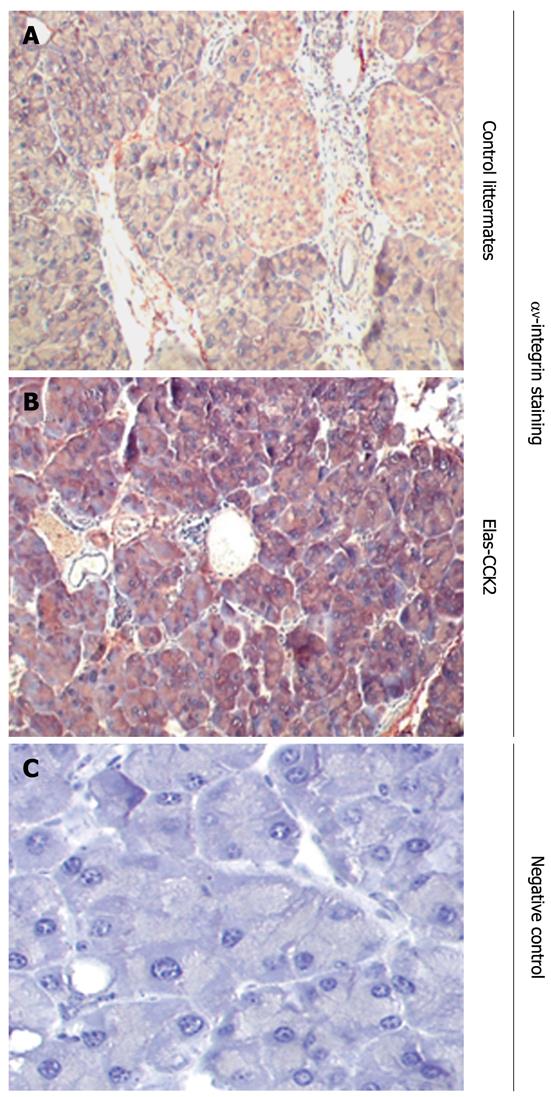
Thus, to analyse in vivo the relevance of αV integrin expression in correlation to CCK2R expression, we analysed αV integrin overexpression in pancreatic tissue sections from Elas-CCK2 mice and control littermates using immunohistochemistry methods. As shown in Figure 4, tissues derived from Elas-CCK2 mice showed an upregulation of αV integrin (Figure 4B) as compared to control mice (Figure 4A).
Several lines of evidence suggest that gastrin and CCK2R could contribute to pancreatic carcinogenesis by modulating processes such as proliferation, cell adhesion or migration. In the current study, we identified αV integrin as a new gastrin-regulated gene in human pancreatic cancer cells and demonstrated its involvement in modulation of cell adhesion by gastrin.
Integrins, a large family of cell-surface receptors, act as the bridge between ECM proteins and cytoskeletal proteins[32]. They are crucial for cell migration but also modulate signal transduction cascades implicated in cell survival or proliferation. Several studies have demonstrated that integrins played a key role in the malignant behaviour of neoplastic cells and were important mediators of tumour invasion and metastasis formation through interactions with ECM proteins[33-35]. Alterations in integrin expression have been correlated with aggressive growth and metastatic capacity of several tumours[36-40]. In addition, several integrin subunits are upregulated in pancreatic carcinoma, in particular the fibronectin receptor β1 and β3 integrins, two subunits known to interact with αV integrin[41-43]. We previously identified β1 integrin as a gastrin target in pancreatic cancer[31]. Here, we show that gastrin increases the expression of another member of the integrin family, αV integrin, at the mRNA and protein level in a human pancreatic tumour cell line. In addition, the use of blocking anti-αV integrin monoclonal antibodies completely reversed the increase in cell-substrate adhesion induced by gastrin. Previously we showed an inhibitory effect of anti-β1 integrin antibodies on gastrin-induced cell adhesion, suggesting that the heterodimer αVβ1 might be important in gastrin signalling. However, since the β3 subunit is also overexpressed in pancreatic adenocarcinomas and can interact with αV subunit, it might be important to analyse, using anti-β3 integrin, whether it also contributes to gastrin-induced cell adhesion.
In gastric cells, the regulation by gastrin of numerous genes, including genes involved in gastric acid secretion[19], early response genes[20] or genes associated with cell migration[26], involves the activation of the ERK1/2 pathway. In other cellular models such as colon cancer cells, the PI-3-kinase pathway is also involved in the regulation of gastrin target genes. To our knowledge, very little is known about gene regulation by gastrin in pancreatic tumour models. In this study, we demonstrated in pancreatic cancer cells that Src family kinases and the PI-3-kinase pathway play a crucial role in the expression of αV integrin modulated by gastrin.
The present study and previously published studies by our group demonstrate that gastrin affects cell adhesion and migration by different complementary mechanisms. First, gastrin modulates cell-cell adhesion by inducing a dissociation of the E-cadherin-catenin-complex leading to cytoskeleton reorganization and cell invasion. Here, we show that gastrin also modulates cell-substrate adhesion via the αV integrin.
Another important finding of this study is that the expression of a G protein-coupled receptor, namely the CCK2R, targeted in mouse pancreatic acinar tissue, leads to the over-expression of αV integrin. These transgenic mice display an increased growth of the pancreas and develop preneoplastic lesions then pancreatic tumours presenting a ductal phenotype similar to that observed in human pancreatic tumours.
We thank Dr. André F (Marseille, France) for blocking anti-αV integrin antibodies. We thank Dr. Dufresne M (Toulouse, France) for providing us with pancreatic tissue sections from Elas-CCK2 mice and control mice.
Pancreatic cancer has a poor prognosis with a 5-year survival rate < 5%. Despite intensive efforts to improve therapy, treatment remains unsatisfactory and most patients die within months as a result of rapid local spread of tumour or metastatic dissemination. A better understanding of the genes involved in tumour growth and migration may allow the development of novel treatment strategies to rapidly tackle this disease.
Integrins play a key role in the malignant behaviour of neoplastic cells and are important mediators of tumour growth invasion and metastasis. Several publications support the role of gastrin, a peptide hormone, in pancreatic cancer development. However, the mechanism by which gastrin regulates integrin signalling in pancreatic cancer has not been addressed. In this study, the authors show that regulation of αV integrin by gastrin may contribute to pancreatic tumour development.
This is the first study to report that αV integrin is a gastrin target in human pancreatic cancer cells. Furthermore, we identified the signalling pathways involved in gastrin-mediated αV integrin expression. Another important finding of this study is that the expression of a G protein-coupled receptor, namely the CCK2R, targeted in mouse pancreatic acinar tissue, leads to the over-expression of αV integrin. These transgenic mice display an increased growth of the pancreas and develop preneoplastic lesions then pancreatic tumours presenting a ductal phenotype similar to that observed in human pancreatic tumours.
A better understanding of the genes involved in tumour growth and migration may allow the development of novel treatment strategies for patients with pancreatic cancer.
Integrins, a large family of cell-surface receptors, act as the bridge between extracellular matrix proteins and cytoskeletal proteins. They are crucial for cell migration but also modulate signal transduction cascades implicated in cell survival or proliferation.
This is a very well written and clearly laid out manuscript. The authors appear to have carried out the experiments to a high standard and the data are convincing. There are one or two experimental controls that are not included however if the authors can include these or comment on the fact that their inclusion would strengthen their observations.
Peer reviewers: Catherine Greene, PhD, Senior Lecturer, Department of Medicine, Royal College of Surgeons in Ireland, Education and Research Centre, Beaumont Hospital, Dublin 9, Ireland; Dae-Yeul Yu, PhD, Professor, Aging Research Center, Korea Research Institute of Bioscience and Biotechnology, 111 Gwahangno, Yuseong-gu, 305-806 Daejeon, South Korea
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Chua YJ, Cunningham D. Adjuvant treatment for resectable pancreatic cancer. J Clin Oncol. 2005;23:4532-4537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Caplin M, Savage K, Khan K, Brett B, Rode J, Varro A, Dhillon A. Expression and processing of gastrin in pancreatic adenocarcinoma. Br J Surg. 2000;87:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Goetze JP, Nielsen FC, Burcharth F, Rehfeld JF. Closing the gastrin loop in pancreatic carcinoma: coexpression of gastrin and its receptor in solid human pancreatic adenocarcinoma. Cancer. 2000;88:2487-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Hellmich MR, Rui XL, Hellmich HL, Fleming RY, Evers BM, Townsend CM. Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J Biol Chem. 2000;275:32122-32128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Ding WQ, Kuntz SM, Miller LJ. A misspliced form of the cholecystokinin-B/gastrin receptor in pancreatic carcinoma: role of reduced sellular U2AF35 and a suboptimal 3'-splicing site leading to retention of the fourth intron. Cancer Res. 2002;62:947-952. [PubMed] |
| 6. | Schmitz F, Otte JM, Stechele HU, Reimann B, Banasiewicz T, Fölsch UR, Schmidt WE, Herzig KH. CCK-B/gastrin receptors in human colorectal cancer. Eur J Clin Invest. 2001;31:812-820. [PubMed] [DOI] [Full Text] |
| 7. | Clerc P, Saillan-Barreau C, Desbois C, Pradayrol L, Fourmy D, Dufresne M. Transgenic mice expressing cholecystokinin 2 receptors in the pancreas. Pharmacol Toxicol. 2002;91:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Clerc P, Leung-Theung-Long S, Wang TC, Dockray GJ, Bouisson M, Delisle MB, Vaysse N, Pradayrol L, Fourmy D, Dufresne M. Expression of CCK2 receptors in the murine pancreas: proliferation, transdifferentiation of acinar cells, and neoplasia. Gastroenterology. 2002;122:428-437. [PubMed] [DOI] [Full Text] |
| 9. | Bierkamp C, Kowalski-Chauvel A, Dehez S, Fourmy D, Pradayrol L, Seva C. Gastrin mediated cholecystokinin-2 receptor activation induces loss of cell adhesion and scattering in epithelial MDCK cells. Oncogene. 2002;21:7656-7670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ferrand A, Kowalski-Chauvel A, Bertrand C, Pradayrol L, Fourmy D, Dufresne M, Seva C. Involvement of JAK2 upstream of the PI 3-kinase in cell-cell adhesion regulation by gastrin. Exp Cell Res. 2004;301:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Noble F, Roques BP. Phenotypes of mice with invalidation of cholecystokinin (CCK(1) or CCK(2)) receptors. Neuropeptides. 2002;36:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Taniguchi T, Takaishi K, Murayama T, Ito M, Iwata N, Chihara K, Sasaki T, Takai Y, Matsui T. Cholecystokinin-B/gastrin receptors mediate rapid formation of actin stress fibers. Oncogene. 1996;12:1357-1360. [PubMed] |
| 13. | Bierkamp C, Bonhoure S, Mathieu A, Clerc P, Fourmy D, Pradayrol L, Seva C, Dufresne M. Expression of cholecystokinin-2/gastrin receptor in the murine pancreas modulates cell adhesion and cell differentiation in vivo. Am J Pathol. 2004;165:2135-2145. [PubMed] |
| 14. | Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657-20663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Todisco A, Takeuchi Y, Urumov A, Yamada J, Stepan VM, Yamada T. Molecular mechanisms for the growth factor action of gastrin. Am J Physiol. 1997;273:G891-G898. [PubMed] |
| 16. | Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710-10715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates tyrosine phosphorylation of insulin receptor substrate 1 and its association with Grb2 and the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26356-26361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates the formation of a p60Src/p125FAK complex upstream of the phosphatidylinositol 3-kinase signaling pathway. FEBS Lett. 1999;445:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Höcker M, Zhang Z, Merchant JL, Wang TC. Gastrin regulates the human histidine decarboxylase promoter through an AP-1-dependent mechanism. Am J Physiol. 1997;272:G822-G830. [PubMed] |
| 20. | Stepan VM, Tatewaki M, Matsushima M, Dickinson CJ, del Valle J, Todisco A. Gastrin induces c-fos gene transcription via multiple signaling pathways. Am J Physiol. 1999;276:G415-G424. [PubMed] |
| 21. | Taniguchi T, Matsui T, Ito M, Murayama T, Tsukamoto T, Katakami Y, Chiba T, Chihara K. Cholecystokinin-B/gastrin receptor signaling pathway involves tyrosine phosphorylations of p125FAK and p42MAP. Oncogene. 1994;9:861-867. [PubMed] |
| 22. | Wang JY, Wang H, Johnson LR. Gastrin stimulates expression of protooncogene c-myc through a process involving polyamines in IEC-6 cells. Am J Physiol. 1995;269:C1474-C1481. [PubMed] |
| 23. | Zhukova E, Sinnett-Smith J, Wong H, Chiu T, Rozengurt E. CCK(B)/gastrin receptor mediates synergistic stimulation of DNA synthesis and cyclin D1, D3, and E expression in Swiss 3T3 cells. J Cell Physiol. 2001;189:291-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Fukui H, Kinoshita Y, Maekawa T, Okada A, Waki S, Hassan S, Okamoto H, Chiba T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology. 1998;115:1483-1493. [PubMed] [DOI] [Full Text] |
| 25. | Sinclair NF, Ai W, Raychowdhury R, Bi M, Wang TC, Koh TJ, McLaughlin JT. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004;286:G992-G999. [PubMed] [DOI] [Full Text] |
| 26. | Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873-879. [PubMed] |
| 27. | Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM. Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem. 2002;277:48755-48763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Slice LW, Hodikian R, Zhukova E. Gastrin and EGF synergistically induce cyclooxygenase-2 expression in Swiss 3T3 fibroblasts that express the CCK2 receptor. J Cell Physiol. 2003;196:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Gigoux V, Clerc P, Sanchez D, Coll MG, Corominola H, Leung-Theung-Long S, Pénicaud L, Gomis R, Seva C, Fourmy D. Reg genes are CCK2 receptor targets in ElasCCK2 mice pancreas. Regul Pept. 2008;146:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Cayrol C, Clerc P, Bertrand C, Gigoux V, Portolan G, Fourmy D, Dufresne M, Seva C. Cholecystokinin-2 receptor modulates cell adhesion through beta 1-integrin in human pancreatic cancer cells. Oncogene. 2006;25:4421-4428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [PubMed] |
| 33. | Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Vogelmann R, Kreuser ED, Adler G, Lutz MP. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer. 1999;80:791-795. [PubMed] |
| 37. | Sawai H, Funahashi H, Yamamoto M, Okada Y, Hayakawa T, Tanaka M, Takeyama H, Manabe T. Interleukin-1alpha enhances integrin alpha(6)beta(1) expression and metastatic capability of human pancreatic cancer. Oncology. 2003;65:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Ahmed N, Riley C, Oliva K, Rice G, Quinn M. Ascites induces modulation of alpha6beta1 integrin and urokinase plasminogen activator receptor expression and associated functions in ovarian carcinoma. Br J Cancer. 2005;92:1475-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Mayoral R, Fernández-Martínez A, Boscá L, Martín-Sanz P. Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis. 2005;26:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered 'CYR61-alphavbeta3 integrin loop' regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;24:761-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Linder S, Castaños-Velez E, von Rosen A, Biberfeld P. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1321-1327. [PubMed] |
| 42. | Löhr M, Trautmann B, Göttler M, Peters S, Zauner I, Maier A, Klöppel G, Liebe S, Kreuser ED. Expression and function of receptors for extracellular matrix proteins in human ductal adenocarcinomas of the pancreas. Pancreas. 1996;12:248-259. [PubMed] [DOI] [Full Text] |
| 43. | Shimoyama S, Gansauge F, Gansauge S, Oohara T, Beger HG. Altered expression of extracellular matrix molecules and their receptors in chronic pancreatitis and pancreatic adenocarcinoma in comparison with normal pancreas. Int J Pancreatol. 1995;18:227-234. [PubMed] |