Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4470
Revised: June 8, 2011
Accepted: June 15, 2011
Published online: October 28, 2011
AIM: To explore the role of actin-bundling protein, fascin during the progression of pancreatic cancer.
METHODS: The plasmid expressing human fascin-1 was stably transfected into the pancreatic cancer cell line MIA PaCa-2. The proliferation, cell cycle, motility, scattering, invasiveness and organization of the actin filament system in fascin-transfected MIA PaCa-2 cells and control non-transfected cells were determined.
RESULTS: Heterogeneous overexpression of fascin markedly enhanced the motility, scattering, and invasiveness of MIA PaCa-2 cells. However, overexpression of fascin had minimal effect on MIA PaCa-2 cell proliferation and cell cycle. In addition, cell morphology and organization of the actin filament system were distinctly altered in fascin overexpressed cells. When transplanted into BALB/c-nu mice, fascin-transfected pancreatic cancer cells developed solid tumors at a slightly slower rate, but these tumors displayed more aggressive behavior in comparison with control tumors.
CONCLUSION: Fascin promotes pancreatic cancer cell migration, invasion and scattering, thus contributes to the aggressive behavior of pancreatic cancer cells.
- Citation: Xu YF, Yu SN, Lu ZH, Liu JP, Chen J. Fascin promotes the motility and invasiveness of pancreatic cancer cells. World J Gastroenterol 2011; 17(40): 4470-4478
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4470.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4470
Pancreatic cancer is characterized by aggressiveness and early metastasis, and the survival rate for this cancer is among the lowest of all cancer types. In an effort to elucidate additional targets for the detection and therapy for this type of cancer, our lab completed a proteomic analysis of primary pancreatic cancer and normal pancreas samples[1]. We identified 70 proteins that were expressed at least 2-fold higher in pancreatic cancers when compared with normal pancreas samples. Of these proteins, 18 were involved in cytoskeleton regulation, and fascin was one of the identified proteins that had the greatest change between pancreatic cancer and normal pancreas samples. Because cell motility is based on rearrangement of the actin cytoskeleton and this process of rearrangement is governed by multiple actin-binding proteins, we postulated that these proteins may play some role in the invasion and metastasis of pancreatic cancer. Several other studies have previously shown that the actin-bundling protein, fascin, which is specifically expressed in pancreatic cancer when compared with normal pancreas, is closely associated with the status of pancreatic cancer cell differentiation and plays an important role in pancreatic cancer progression[2-5].
Fascin was identified in the 1970s to be a 55-kD globular protein that cross-links F-actin into well-ordered and tightly packed parallel bundles that are concentrated in cell protrusions during cell migration. Fascin is highly expressed in specialized cells that are rich in filopodia, such as neurons, glial cells, mature dendritic cells and actively migrating cells, such as the endothelial cells of microvessels[6,7]. Fascin expression is often absent in normal epithelial cells, such as the epithelia of the bile duct[8], urinary bladder[9], breast[10], colon[11], ovary[12], pancreas[1] and stomach[13]. Fascin expression is upregulated in several human neoplasms, such as breast[10], lung[14], kidney[15], ovary[12], prostate and pancreatic cancers[3,5,16]. Fascin overexpression is often correlated with an invasive tumor phenotype, poor prognosis and decreased disease-free survival.
The role of fascin in the malignant behavior of pancreatic cancer remains unknown. To determine the functional consequences of fascin overexpression in pancreatic cancer cells, we stably transfected a human pancreatic cancer cell line, MIA PaCa-2, with a plasmid containing full-length human fascin cDNA. The proliferation, cell cycle, motility, scattering, invasiveness and organization of the actin filament system were evaluated in fascin-transfected MIA PaCa-2 cells and in non-transfected control cells.
The human pancreatic cancer cell lines, BxPC-3, MIA PaCa-2 and AsPC-1 were obtained from American Type Culture Collection (Rockville, MD, United States), and the PC-1, PC-4 and PC-7 cell lines were established and maintained in our laboratory. The BxPC-3, AsPC-1, PC-1, PC-4 and PC-7 cell lines were cultured in RPMI 1640 (GIBCO, Paisley, United Kingdom) with 10% fetal bovine serum (FBS) (HyClone Laboratories, United States) and penicillin-streptomycin (100 IU/mL-0.1 mg/mL). The MIA PaCa-2 cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) (GIBCO, Paisley, United Kingdom) supplemented with 10% FBS and penicillin-streptomycin (100 IU/mL-0.1 mg/mL). All cells were maintained in a humidified 5% CO2 atmosphere at 37 °C.
The fascin antibody (M3567) was purchased from DAKO (Glostrup, Denmark); the β-actin antibody was purchased from Sigma (MO, United States); the EnVasionTM Detection Kit was purchased from DAKO (Glostrup, Denmark); and the FITC-conjugated secondary antibody was purchased from Boster (WuHan, China).
A pcDNA3 vector containing the full-length human fascin cDNA (pcDNA3-Fascin) was kindly provided by Dr. Josephine C. Adams (Lener Research Institute, Cleveland, Ohio, United States). The insert was cut out with the EcoR I restriction enzyme to acquire the pcDNA3 control vector (pcDNA3-Vector). The sequence was verified by DNA sequencing. MIA PaCa-2 cells were transfected with either pcDNA3-Fascin or pcDNA3-Vector. Approximately 5 × 104 MIA PaCa-2 cells per well were seeded in a 6-well culture plate and were subsequently transfected with 5 μg of plasmid using 10 μL of Lipofectamine 2000 (GIBCO, United States) in 250 μL of Opti-MEM (GIBCO, United States). After 48 h, G418 (GIBCO, United States) was added to the cells for selection at a concentration of 800 μg/mL. After 10 to 14 d, antibiotic-resistant colonies were picked, pooled and maintained in DMEM containing 10% FBS and 400 μg/mL G418.
Cells were rinsed twice with D-Hanks and solubilized with lysis buffer [50 mmol/L Tris (pH 8.0), 1% Nonidet p-40, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 0.5% deoxysodium cholate, 1 × cocktail (Roche, Mannheim, Germany)] for 30 min on ice. The total extract was cleaned by centrifugation at 12 000 r/min for 30 min at 4 °C, and the supernatant was collected. The protein concentration was determined with the Bradford assay (BioRad, CA, United States). A total of 40 μg of total cell extract was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The separated proteins were transferred onto an Immobilon-PVDF membrane (Millipore, Bedford, MA, United States) and were blocked and incubated with the primary antibody overnight at 4 °C. The EnvasionTM Detection Kit with DAB liquid substrate (DAKO, Glostrup, Denmark) was used for protein detection.
Cells were cultured on sterile coverslips and were incubated for 24 h in a humidified 5% CO2 atmosphere at 37 °C. The coverslips containing the cells were then fixed with 4% paraformaldehyde in phosphate buffered solution (PBS) for 10 min, washed with PBS, permeabilized in 0.2% Triton X-100 in PBS for 5 min, washed and then blocked with normal goat-serum for 30 min at room temperature. Cells were then incubated with an appropriate primary antibody for 1 h at 37 °C, and were rinsed 3 times with PBS. For protein detection by immunocytochemistry, an EnvasionTM ChemTM Detection Kit (DAKO, Glostrup, Denmark) was used. The reaction color was developed by incubating sections with DAB liquid substrate. The slides were then washed with water and counterstained with hematoxylin. The slides were then dehydrated and mounted with mounting media. For immunofluorescence, a FITC-conjugated goat anti-mouse IgG secondary antibody was used. After washing, the slides were mounted with glycerol and imaged with an immunofluorescence microscope (Olympus BX51).
For the proliferation assay, 1 × 104 MIA PaCa-2 cells per well were seeded in a 24-well culture plate in DMEM supplemented with 10% FBS. Every 24 h, cells from 3 independent wells were collected by trypsinization and counted using a hemocytometer.
Cell migration was evaluated by the wound healing assay[17]. Fascin-transfected MIA PaCa-2 cells and non-transfected control cells were plated separately into 6-well culture plates and cultured to 70%-80% confluence in DMEM containing 10% FBS. After a 24-h serum starvation period, the monolayer of cells was wounded by manual scratching with a sterile plastic 200 μL micropipette tip, washed with PBS 5 times to remove cell debris, photographed with an inverted tissue culture microscope (Leica LEITZDM IL) and then placed in complete medium in a humidified 5% CO2 atmosphere at 37 °C. After 20 h of incubation, the wells were re-evaluated under the microscope, and the wounded area was re-imaged for comparison.
The ability of the cells to aggregate was tested by hanging drop suspension cultures[18]. Cells were trypsinized with 0.25% trypsin in the presence of 0.01% ethylene diamine tetraacetic acid, washed twice in PBS, and resuspended at 2.5 × 105 cells/mL in DMEM containing 10% FBS. Drops of medium (20 μL in each drop, containing 5000 cells) were pipetted onto the inner surface of a Petri dish lid. The lid was then placed on the Petri dish, and the drops with the cells suspended were left hanging from the lid. To compensate for evaporation, 8 mL of serum-free culture medium was added to the bottom of the Petri dish. After incubation at 37 °C for 12 h, the lid of the Petri dish was inverted and photographed under an inverted tissue culture microscope.
For the invasion assay, the BioCoat Matrigel Invasion Chamber (Becton Dickinson Bioscience, United States) was used according to the manufacturer’s instructions. Briefly, 2.5 × 104 MIA PaCa-2 cells suspended in 500 μL serum-free medium were seeded onto Matrigel-coated filters, and 750 μL of DMEM containing 10% FBS was added as a chemoattractant in the lower portion of the wells. After incubation at 37 °C with 5% CO2 for 24 h, the inserts were removed, and the non-invading cells that remained on the upper surface of the filter were scraped off with cotton swabs. Cells on the bottom surface of the membranes were fixed with ethanol and stained with 0.05% crystal violet. The number of cells invading through the Matrigel membrane was counted. Data are presented as the average of triplicate determinants.
For the cell cycle analysis, a minimum of 1 × 106 cells were harvested and fixed in 70% ethanol at 4 °C. After 12 h, cells were centrifuged (1000 g, 7 min, 4 °C), resuspended in PBS containing 0.05 mg/mL RNase A (Sigma, United States) and then incubated at room temperature for 30 min. After the cells were washed, they were stained with 10 μg/mL propidium iodide, filtered through a 60 μm mesh, and analyzed by flow cytometry (Elite Epics ESP, Coulter, United States). A total of 10 000 cells were analyzed with MODFIT software.
All procedures involving mice were approved by the College Committee on Use and Care of Animals at the Peking Union Medical College and conformed to the relevant regulatory standards. Four-week-old male athymic nude (BALB/c-nu) mice (Vitalriver, Beijing) were housed in specific pathogen-free conditions. To generate tumor xenografts, 5 × 106 tumor cells suspended in 0.1 mL of medium were inoculated subcutaneously in the right flank of the mice. Animals were inspected every 3 d. When the tumors from fascin-overexpressing MIA PaCa-2 cells (named MIA PaCa-2 Fascin) or vector control MIA PaCa-2 cells (named MIA PaCa-2-Vector) developed to a visible size, the mice were euthanized, the tumors were collected, cut into 1 mm3 pieces and then implanted subcutaneously in the right flank of BALB/c-nu mice. A total of 6 mice were used in each group. Animals were inspected and the tumors were measured every 3 d. Mice were humanely euthanized when they were overwhelmed by tumor burden. All tumors and major organs were fixed in formalin and embedded in paraffin. Histopathological analysis was performed following routine hematoxylin and eosin (HE) staining on tissue sections. The tumor volume was calculated on the basis of the following formula: volume = (π/6) LWH (L = length, W = width, H = height).
The Student t-test and the Fisher exact probability test were used for statistical analysis; a P value of less than 0.05 was considered significant.
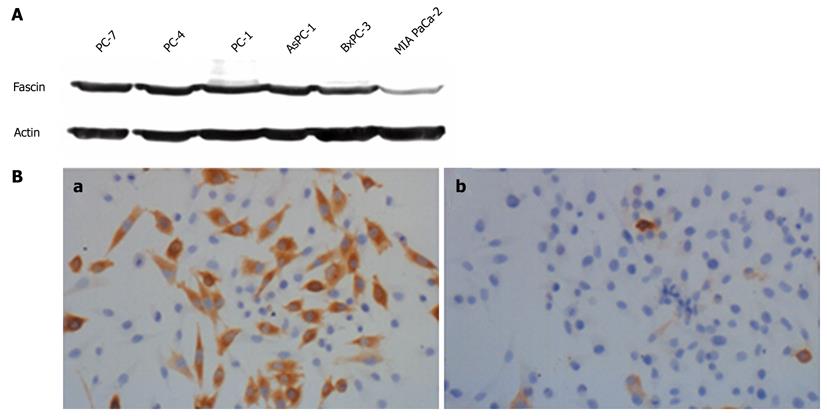
Western blotting analysis was performed to investigate the expression of fascin in different human pancreatic cancer cell lines. Fascin protein was present at different expression levels in all of the tested pancreatic cancer cell lines. BxPC-3, AsPC-1, PC-1, PC-4 and PC-7 expressed fascin at a high level, whereas MIA PaCa-2 expressed fascin at a very low level (Figure 1A). Because MIA PaCa-2 cells endogenously express fascin at low levels, we chose this cell line to examine the effect of heterogeneous fascin expression on the biological properties of pancreatic cancer cells.
MIA PaCa-2 cells were transfected with either pcDNA3-Fascin or the pcDNA3-Vector and stable clones were selected by G418 treatment. MIA PaCa-2 Fascin cells and MIA PaCa-2 Vector cells were used for further analysis (Figure 1B).
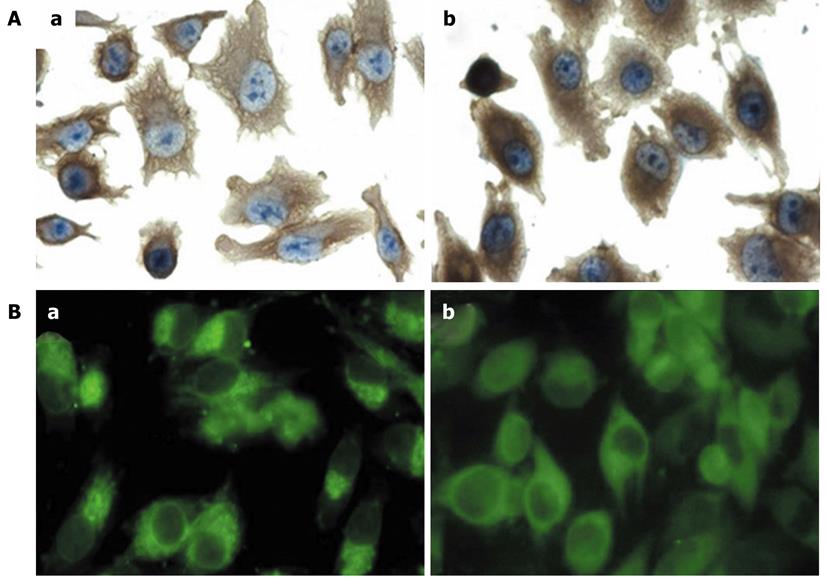
There was an increase in membrane protrusions in the MIA PaCa-2 Fascin cells when compared with the control MIA PaCa-2 Vector cells. Morphologically, the MIA PaCa-2 Vector cells were more rounded and had fewer projections, whereas MIA PaCa-2 Fascin cells were polarized with elongated membrane projections. In MIA PaCa-2 Fascin cells, actin filaments were distributed as bundles in the cytoplasm that protruded into membrane projections, whereas the actin filaments in MIA PaCa-2 Vector cells were distributed in a diffuse manner (Figure 2A).
This result was also visualized via immunofluorescence as an accumulation of actin filaments in a polarized manner in MIA PaCa-2 Fascin cells and as a diffuse distribution in MIA PaCa-2 Vector cells (Figure 2B).
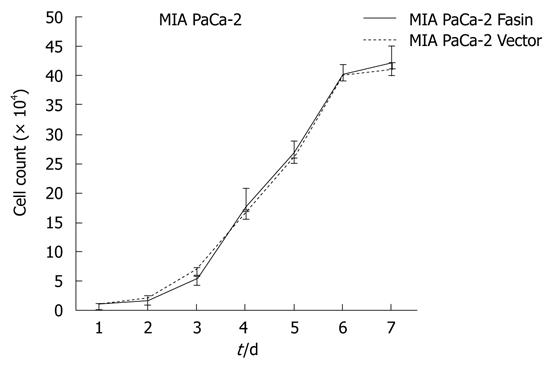
The growth curves of MIA PaCa-2 Fascin and MIA PaCa-2 Vector cells showed no significant difference between the two groups (Figure 3). Therefore, the heterogeneous expression of fascin does not seem to affect pancreatic cancer cell growth rate in vitro.
As shown in Table 1, fascin transfection induced an increase in G1 phase without a significant decrease in G2/M and S phases.
| G1 (%)a | S (%) | G2 (%) | |
| MiaPaCa-2-Fascin | 74.67 ± 3.89 | 17.1 ± 4.16 | 8.23 ± 0.81 |
| MiaPaCa-2-Vector | 66 ± 3.01 | 22.83 ± 4.55 | 11.17 ± 1.66 |
When transplanted into nude mice, both the MIA PaCa-2 Fascin and MIA PaCa-2 Vector cells developed solid tumor masses. The mean tumor volume from MIA PaCa-2 Fascin and MIA PaCa-2 Vector cells was 2.86 ± 2.24 cm3 and 3.08 ± 1.16 cm3, respectively. Tumors from fascin-transfected cells grew at a slightly slower rate in comparison with control tumors, but this difference was not significant (P = 0.8439). These results are in agreement with our in vitro experiments.
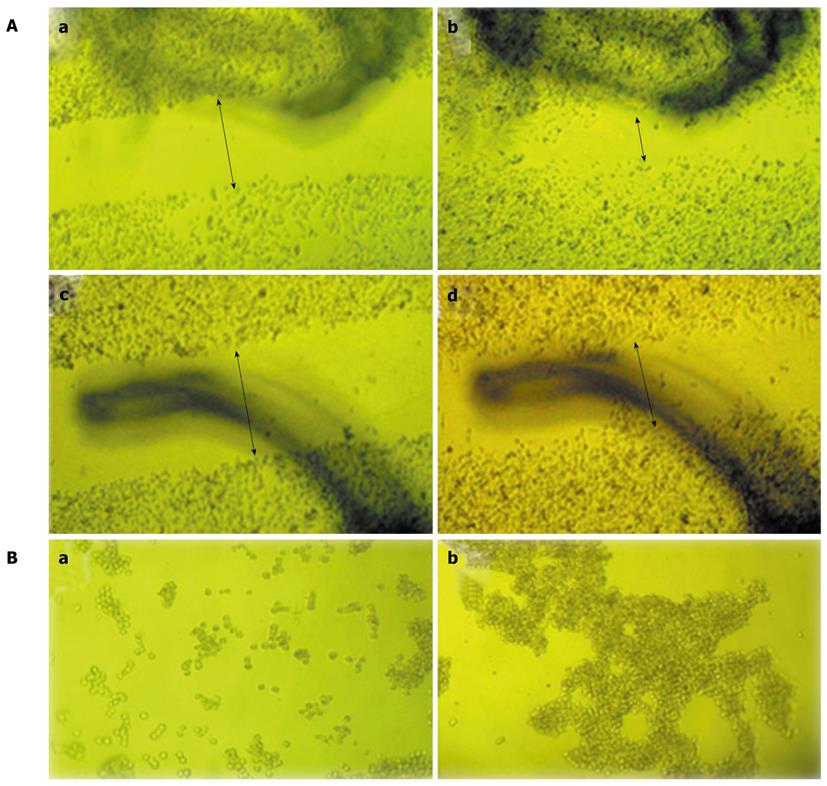
To investigate the effects of fascin on cell migration, in vitro wound healing assays were performed. After wounds were made for 20 h, the MIA PaCa-2 Fascin and MIA PaCa-2 Vector cells exhibited a cell reorientation response along the wounded edge margin and migrated into the wound area. MIA PaCa-2 Fascin cells repopulated the open space more efficiently than did MIA PaCa-2 Vector cells (Figure 4A).
Cell aggregation is an important factor that may critically affect tumor cell metastasis. We tested this using a hanging drop cell aggregation assay. Our results showed that the heterogeneous expression of fascin resulted in a reduction in aggregation when compared with vector control cells (Figure 4B).
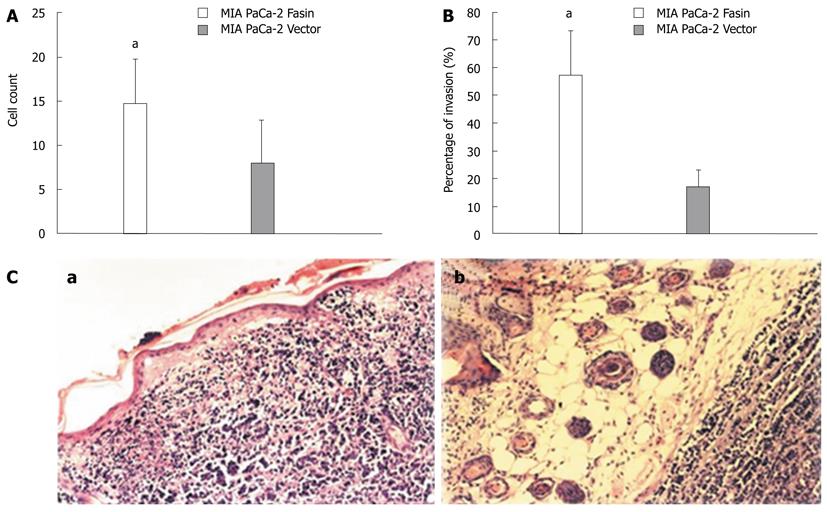
To determine whether fascin promotes pancreatic cancer cell invasion, an in vitro invasion assay was performed using a Matrigel Invasion Chamber. Overexpression of fascin dramatically increased the cell invasive properties of the MIA PaCa-2 cells when compared with control MIA PaCa-2 cells (Figure 5A).
When transplanted into nude mice, the tumors developed from fascin-overexpressing MIA PaCa-2 Fascin cells grew in a more aggressive pattern, as 4 out of 6 of these tumors showed skin invasion, whereas only 1 of the control tumors exhibited skin invasion (Figure 5B and 5C).
We detected fascin expression in 6 pancreatic cancer cell lines (BxPC-3, AsPC-1, MIA PaCa-2 and 3 cell lines established by our laboratory: PC-1, PC-4 and PC-7). All of the cell lines expressed fascin at a relatively high level, except MIA PaCa-2. This finding may indicate that fascin overexpression is a common event in pancreatic cancer, but the pathogenic effects of fascin are different among these cell lines. To elucidate the function of fascin in pancreatic cancer cells, we introduced a fascin-expression vector into MIA PaCa-2 cells and found that heterogeneous expression of fascin resulted in an increase in cell invasiveness and motility with a decrease in cell aggregation. The proliferation and cell cycle distribution of pancreatic cancer cells was not obviously affected by fascin overexpression. To our knowledge, this is the first study to ascertain the function of fascin by means of heterogeneous overexpression in pancreatic cancer cells.
Pancreatic cancer progresses rapidly and demonstrates strong invasion and early metastatic properties with poor prognosis. The characteristics of tumor progression, cell motility and invasiveness, result from a rearrangement of the cytoskeletal microfilaments that is modulated by several types of actin cross-linking proteins[19]. Among these molecules, fascin is implicated in the organization and persistence of filopodia, which plays an important role in cell-matrix adhesion, cell interactions and cell migration[20,21]. Additional evidence has shown fascin-overexpressing tumors to have increased invasive properties. In breast cancer cells, overexpression of c-erbB2 resulted in an increase in fascin expression and tumor cell motility[22]. Also in gastric carcinoma, the outer edges of the tumors tended to have the most intense fascin staining in an immunohistochemical assay[13]. Jawhari et al[23] found that de novo expression of fascin in well-differentiated colon cancer cells increased cell migration through collagen type I- or IV-coated filters, and the cells showed a significant increase in dynamic membrane activity. In addition, in esophageal squamous cell carcinoma the down-regulation of endogenous fascin by RNA interference resulted in a dramatic decrease in cell invasiveness[24]. In this study, we found that heterogeneous overexpression of fascin in pancreatic cancer cells resulted in an increase in cell motility and invasiveness. In fascin-overexpressing cells, there were more membrane protrusions, and the actin filaments were arranged as bundles in the cytoplasm which protruded into the membrane projections. In contrast, the control cells were rounded with diffusely distributed actin filaments and fewer projections. Thus, it seems likely that the rearrangement of the actin cytoskeleton induced by fascin overexpression in pancreatic cancer cells promoted their motility and invasion, which resulted in a more aggressive phenotype.
Cancer cell adhesion and migration are distinct but related events in the process of cancer progression, and cell dissociation is one of the limiting steps during the course of cancer cell migration. Heterogeneous overexpression of fascin in pancreatic cancer cells resulted in an obvious decrease in cell-cell adhesion, as shown in the aggregation assay. To date, there are few reports on the role of fascin in cell-cell adhesion. Ectopic expression of fascin in rat Con8 cells disrupted the dexamethasone-induced formation of tight junctions and adherent junctions by preventing the recruitment of occludin and β-catenin to the site of cell-cell contact, which suggested that fascin was a negative regulator of cell-cell interactions[25]. Another study demonstrated that fascin competed with E-cadherin for an association with β-catenin in vitro[26], and it is conceivable that fascin plays a role in modulating cell adhesion. In contrast, a study in colon cancer cells did not find an effect of fascin on the E-cadherin-β-catenin association and distribution[27]. Thus, the molecular mechanism of fascin involved in cell-cell adhesion still needs to be further explored.
In this study, the overexpression of fascin had no obvious effect on pancreatic cancer cell proliferation, which is in contrast to Jawhari et al[23], who reported that fascin promoted the proliferation of colon cancer cells. In lung carcinomas, highly fascin-positive tumors had a high Ki-67 index[14]. However, in colorectal adenoma, fascin and ki-67 were inversely correlated[11]. The reasons for these divergent findings are currently unknown and may be related to the differences in growth-regulating signaling pathways. As a poorly differentiated pancreatic cancer cell line with the shortest doubling time compared to the other 11 pancreatic cancer cell lines[28], the MIA PaCa-2 cell line may have growth-regulating signaling pathways which are less dependent on fascin expression.
In summary, our study showed that overexpression of fascin promoted pancreatic cancer cell dissociation, migration and invasion, indicating its usefulness as a pancreatic cancer gene therapy target.
We thank Dr. Josephine C Adams for kindly providing the fascin expression plasmid and thank Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences for its support.
Pancreatic cancer is one of the most devastating human malignancies, with an overall 5 year survival rate of 5% and a median survival time of 6 mo, and the major biological hallmarks of this disease are its early and aggressive local invasion and metastasis. Fascin is associated with cell movement and was identified to show the greatest change between pancreatic cancer and normal pancreas samples.
Fascin expression is often absent in normal epithelial cells, and its expression is upregulated in several human neoplasms. Fascin overexpression is often correlated with an invasive tumor phenotype, poor prognosis and decreased disease-free survival. The role of fascin in the malignant behavior of pancreatic cancer remains unknown. In this study, the authors demonstrate that the overexpression of fascin could be a potential mechanism for migration and invasion in pancreatic cancer.
Recent reports have highlighted the importance of fascin in many types of cancer. This is the first study to verify that fascin is over-expressed in pancreatic cancer cells and that it promotes tumor migration and invasion. Furthermore, our in vitro and in vivo studies would suggest that this protein may be a positive factor of invasion and metastasis in this cancer.
By understanding fascin’s overexpression and whether it induces migration and invasion, the findings of this study may represent a future strategy for therapeutic intervention in the treatment of patients with pancreatic cancer.
The cytoskeletal protein, fascin, is an actin-bundling protein that plays a role in cell matrix adhesion, cell interaction and migration. Its overexpression has been reported in many types of tumors, but its function in pancreatic cancer is still unknown.
The authors explored the role of Fascin during the progression of pancreatic cancer in pancreatic cancer cell lines and a mouse model. The key findings were that fascin promotes cancer cell migration, invasion and scattering leading to a more aggressive phenotype. Fascin overexpression did not result in increased cell cycle and proliferation which is in contrast to other tumor types, i.e., colon cancer. In general, the experiments are well explained and executed.
| 1. | Lu Z, Hu L, Evers S, Chen J, Shen Y. Differential expression profiling of human pancreatic adenocarcinoma and healthy pancreatic tissue. Proteomics. 2004;4:3975-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi H, Inoue T, Eguchi T, Miyasaka Y, Ohuchida K, Mizumoto K, Yamada T, Yamaguchi K, Tanaka M, Tsuneyoshi M. Fascin overexpression in intraductal papillary mucinous neoplasms (adenomas, borderline neoplasms, and carcinomas) of the pancreas, correlated with increased histological grade. Mod Pathol. 2007;20:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Maitra A, Iacobuzio-Donahue C, Rahman A, Sohn TA, Argani P, Meyer R, Yeo CJ, Cameron JL, Goggins M, Kern SE. Immunohistochemical validation of a novel epithelial and a novel stromal marker of pancreatic ductal adenocarcinoma identified by global expression microarrays: sea urchin fascin homolog and heat shock protein 47. Am J Clin Pathol. 2002;118:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Maitra A, Adsay NV, Argani P, Iacobuzio-Donahue C, De Marzo A, Cameron JL, Yeo CJ, Hruban RH. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614-8622. [PubMed] |
| 6. | Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Adams JC. Fascin protrusions in cell interactions. Trends Cardiovasc Med. 2004;14:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Swierczynski SL, Maitra A, Abraham SC, Iacobuzio-Donahue CA, Ashfaq R, Cameron JL, Schulick RD, Yeo CJ, Rahman A, Hinkle DA. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum Pathol. 2004;35:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Tong GX, Yee H, Chiriboga L, Hernandez O, Waisman J. Fascin-1 expression in papillary and invasive urothelial carcinomas of the urinary bladder. Hum Pathol. 2005;36:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, Tubbs RR, Adams JC, Hicks DG. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res. 2005;11:186-192. [PubMed] |
| 11. | Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer. 2006;6:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Wen YH, Yee H, Goswami S, Shukla PS. Fascin expression in serous tumors of ovary correlates with aggressiveness of malignancy. Int J Gynecol Pathol. 2009;28:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M. The prognostic relevance of fascin expression in human gastric carcinoma. Oncology. 2004;67:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Pelosi G, Pastorino U, Pasini F, Maissoneuve P, Fraggetta F, Iannucci A, Sonzogni A, De Manzoni G, Terzi A, Durante E. Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer. 2003;88:537-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Zigeuner R, Droschl N, Tauber V, Rehak P, Langner C. Biologic significance of fascin expression in clear cell renal cell carcinoma: systematic analysis of primary and metastatic tumor tissues using a tissue microarray technique. Urology. 2006;68:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Darnel AD, Behmoaram E, Vollmer RT, Corcos J, Bijian K, Sircar K, Su J, Jiao J, Alaoui-Jamali MA, Bismar TA. Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer. Clin Cancer Res. 2009;15:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC, Huang CJ, Kandaswami C, Lee PP, Lee MT. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cells. Biochem Pharmacol. 2004;67:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Tseng Y, Kole TP, Lee JS, Fedorov E, Almo SC, Schafer BW, Wirtz D. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochem Biophys Res Commun. 2005;334:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Fonovic M, Suyama K, Bogyo M, Scott MP. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325:1250-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol. 2005;37:1787-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW, Yu D, Mooney EE, McCrea PD. C-erbB-2/ HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19:4864-4875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Jawhari AU, Buda A, Jenkins M, Shehzad K, Sarraf C, Noda M, Farthing MJ, Pignatelli M, Adams JC. Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro. Am J Pathol. 2003;162:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Xie JJ, Xu LY, Zhang HH, Cai WJ, Mai RQ, Xie YM, Yang ZM, Niu YD, Shen ZY, Li EM. Role of fascin in the proliferation and invasiveness of esophageal carcinoma cells. Biochem Biophys Res Commun. 2005;337:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Wong V, Ching D, McCrea PD, Firestone GL. Glucocorticoid down-regulation of fascin protein expression is required for the steroid-induced formation of tight junctions and cell-cell interactions in rat mammary epithelial tumor cells. J Biol Chem. 1999;274:5443-5453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD. beta-Catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996;134:1271-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Hahn-Strömberg V, Edvardsson H, Bodin L, Franzén L. Disturbed expression of E-cadherin, beta-catenin and tight junction proteins in colon carcinoma is unrelated to growth pattern and genetic polymorphisms. APMIS. 2008;116:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Sipos B, Möser S, Kalthoff H, Török V, Löhr M, Klöppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 258] [Article Influence: 11.2] [Reference Citation Analysis (5)] |
Peer reviewer: Hendrik-Tobias Arkenau, MD, Sarah Cannon Research United Kingdom, 93 Harley Street, London W1G 6AD, United Kingdom
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN