Published online Aug 28, 2007. doi: 10.3748/wjg.v13.i32.4321
Revised: March 23, 2007
Accepted: March 28, 2007
Published online: August 28, 2007
AIM: To explore the syndrome differentiation in traditional Chinese medicine (TCM) and gene protein expression in gastric carcinoma.
METHODS: Preoperative data of gastric cancer cases were collected from the General Surgery Department and classified according to the criteria for syndrome differentiation in TCM. E-cadherin (E-cad) and ICAM-1 gene protein expressions were detected in postoperative specimens from these cases by the immunohistochemical EnVision two-step method.
RESULTS: The E-cad positive expression rate was 90% in 100 cases of gastric carcinoma. The difference in E-cad expression was significant between the different syndrome differentiation types in TCM (P < 0.01). Further group-group comparison showed that there was a significant difference in E-cad expression between the stagnation of phlegm-damp type and the deficiency in both qi and blood and the deficiency-cold of stomach and spleen types, where E-cad expression was high. There was no significant difference between the internal obstruction of stagnant toxin type and the in-coordination between liver and stomach type, where E-cad expression was relatively low. The ICAM-1 positive expression rate was 58%, and there was no statistically significant difference between the two groups (χ2= 8.999, P > 0.05).
CONCLUSION: E-cad expression is relatively low in the internal obstruction of stagnant toxin type and the in-coordination between liver and stomach type, where tumor development and metastasis may be associated with low E-cad expression, or with low homogeneous adhesiveness between tumor cells.
- Citation: Sun DZ, Xu L, Wei PK, Liu L, He J. Syndrome differentiation in traditional Chinese medicine and E-cadherin/ICAM-1 gene protein expression in gastric carcinoma. World J Gastroenterol 2007; 13(32): 4321-4327
- URL: https://www.wjgnet.com/1007-9327/full/v13/i32/4321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i32.4321
Guided by the “yin-yang and five-element theories” and supported by the “viscera-state and meridian doctrines”, traditional Chinese medicine (TCM) elucidates the kinetic laws of human birth, aging, disease and death, and treats illnesses in the light of “syndrome differentiation”. Nevertheless, TCM is unable to explain various human physiopathogical phenomena. Most of its theories and doctrines are abstracted and deduced from direct experience and perception. These theories and doctrines have been playing an undeniable role, but they restrain the advance in TCM science. With the rapid advance in modern science and technology, people have recognized microscopic changes in human organs at cell and molecular levels. However, the potential correlation between TCM theories and microscopic changes has been rarely studied. Syndrome differentiation in TCM, a crucial step in clinical practice of TCM, is a comprehensive analysis of external manifestations of the human body based on TCM theories, and reflects physiopathological changes in the human body (from the TCM viewpoint). Treatment based on syndrome differentiation is the core of TCM therapy for tumors. Traditional Chinese herbal medicines play a unique role in treating tumors, especially in improving the quality of life and prolonging survival of tumor patients. This study was to explore the syndrome differentiation in TCM and E-cadherin (E-cad) and ICAM-1 expression in gastric carcinoma.
All data were obtained from patients admitted to Changzheng Hospital between November 2002 and January 2005, who were diagnosed with or highly suspected of having gastric carcinoma and scheduled to receive surgical operation on the next day. A total of 500 patients were enrolled in the study, including those who were diagnosed with gastric carcinoma by gastroscopic biopsy and scheduled to receive surgical operation, as well as those who refused to undergo preoperative gastroscopic biopsy, but were highly suspected of having gastric carcinoma by B-ultrasound and CT scan and scheduled to undergo surgical resection.
The following patients were excluded from the study, including (1) those who were highly suspected of having gastric carcinoma preoperatively which were excluded from malignant tumors by pathologic study of surgically resected specimens, (2) those who failed to undergo surgical operation on the next day because of various reasons (unable to decide whether there was tumor infiltration or lymph node metastasis), (3) those with non gastric carcinoma, including mesothelioma, gastric lymphoma, gastric metastatic tumor and tumors disseminating from other parts of the body. A total of 81 cases were excluded from the study, including 36 cases of non gastric carcinoma, 21 cases of benign tumor, 6 cases of mesothelioma, 2 cases of tumor spreading from colonal carcinoma and 1 case of pancreatic cancer, 1 case of hamartoma, 32 cases of tumor with unknown pathology and 13 cases who did not receive surgical operation.
Finally, a total of 419 cases were included in the present study, including 148 cases of in-coordination between liver and stomach type (IBLS), 84 cases of stagnation of phlegm-damp type (SPD), 74 cases of internal obstruction of stagnant toxin type (IOST), 46 cases of deficiency-cold of stomach and spleen type (DCSS), 29 cases of yin impairment due to stomach heat type (YISH), and 38 cases of deficiency in both qi and blood type (DOQB). The details are shown in Table 1. A total of 100 cases were selected randomly for immunohistochemical analysis.
| Items | Syndrome differentiation | All | P | ||||||
| IBLS | SPD | IOST | DCSS | YISH | DOQB | ||||
| (n) | 148 | 84 | 74 | 46 | 29 | 38 | 419 | ||
| Sex | M | 99 | 64 | 57 | 34 | 11 | 21 | 286 | 0.001 |
| W | 49 | 20 | 17 | 12 | 18 | 17 | 133 | ||
| Age (yr) | <40 | 11 | 9 | 7 | 3 | 2 | 2 | 34 | 0.894 |
| ≥ 40 | 137 | 75 | 67 | 43 | 27 | 36 | 385 | ||
| PC | Squamous carcinoma | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| adenoma | 122 | 70 | 64 | 39 | 26 | 36 | 357 | 0.277 | |
| Mucoid adenoma | 10 | 3 | 7 | 4 | 2 | 2 | 28 | ||
| Signet-ring | 15 | 11 | 3 | 3 | 1 | 0 | 33 | ||
| AS | I (I-II) | 2 | 2 | 1 | 0 | 1 | 1 | 7 | 0.899 |
| II (II-III) | 75 | 41 | 38 | 31 | 16 | 20 | 221 | ||
| III (III-IV) | 70 | 38 | 34 | 14 | 12 | 16 | 184 | ||
| IV | 1 | 3 | 1 | 1 | 0 | 1 | 7 | ||
| In | 1 | 21 | 5 | 6 | 2 | 2 | 2 | 38 | 0.298 |
| 2 | 32 | 24 | 21 | 11 | 6 | 4 | 98 | ||
| 3 | 51 | 22 | 29 | 20 | 9 | 15 | 146 | ||
| 4 | 44 | 33 | 18 | 13 | 12 | 17 | 137 | ||
| LNM | No | 50 | 30 | 28 | 16 | 9 | 13 | 146 | 0.989 |
| Yes | 98 | 54 | 46 | 30 | 20 | 25 | 273 | ||
| DM | 0 | 116 | 64 | 58 | 37 | 19 | 31 | 325 | 0.676 |
| 1 | 32 | 20 | 16 | 9 | 10 | 7 | 94 | ||
| CS | I | 42 | 20 | 18 | 10 | 4 | 6 | 100 | 0.216 |
| II | 18 | 13 | 16 | 7 | 6 | 4 | 64 | ||
| IIIa-IIIb | 48 | 22 | 22 | 20 | 10 | 13 | 135 | ||
| IV | 40 | 29 | 18 | 9 | 9 | 15 | 120 | ||
| TS | Lump | 25 | 16 | 18 | 11 | 7 | 8 | 85 | 0.278 |
| Ulcer | 19 | 15 | 12 | 4 | 7 | 7 | 64 | ||
| Ulcerative lump | 82 | 44 | 39 | 30 | 13 | 21 | 229 | ||
| LB stomach | 4 | 1 | 0 | 1 | 1 | 2 | 9 | ||
| Local mucous lesion | 18 | 8 | 5 | 0 | 1 | 0 | 32 | ||
| TL | Fundal cardia | 7 | 8 | 9 | 5 | 4 | 4 | 37 | 0.452 |
| Body | 57 | 34 | 33 | 24 | 11 | 18 | 177 | ||
| Antrum/pylorus | 79 | 38 | 31 | 15 | 12 | 16 | 191 | ||
| Multiple | 5 | 4 | 1 | 2 | 2 | 0 | 14 | ||
Gastric carcinoma was divided into six types according to the six-type differentiation method set by Beijing Coordination Group of the First National Symposium on Gastric Carcinoma (1978). Syndrome differentiation criteria included (1) IBLS with symptoms of stomach fullness, intermittent dull pain which may radiating two flanks, hiccup and vomiting, deep or thin pulse, pink tongue, and thin or light yellow tongue fur; (2) DCSS with symptoms of gastric dull pain which can be relieved by press and warmth, eating in the morning and vomiting in the evening or vice versa, pallor, cold extremities and mental tiredness, loose stool and edema, pale and enlarged tongue, white and moist tongue fur, and deep and slow pulse; (3) IOST with symptoms of gastric stabbing pain, epigastric fullness and rigidity, hemoptysis and melena, dry and scaly skin, dark purplish tongue, deep and thin pulse; (4) YISH with symptoms of intragastric burning heat, oral dryness and thirst, gastric discomfort with acid rereflux, postprandial gastric pain, feverish sensation in chest, palms and soles, dry stool, anorexia, thin and wiry pulse, red tongue with little fur or yellow fur with little saliva; (5) SPD with symptoms of chest suffocation and diaphragm fullness, sallow complexion and edema, vomiting and profuse sputum, gastric fullness and loose stool, subcutaneous nodules, pink tongue, and greasy tongue fur; and (6) DOQB with symptoms of generalized hypodynamia, palpitation and shortness of breath, dizziness and halo vision, dim complexion, vexation of deficiency type and insomnia, spontaneous perspiration and night sweating, deficiency in both yin and yang, as well as deep, thin and weak pulse, pale tongue with thin fur.
All tumor specimens were obtained were from the General Surgery Department and paraffin-embedded in the Pathology Department of Changzheng Hospital.
Mouse anti human E-cad protein primary antibody (a concentrated type: 0 mL, 1:50, serial No. M0536) was purchased form Fuzhou Maixin Biotechnology Development Co., Ltd (Fuzhou, China). Mouse anti human ICAM-1 protein primary antibody (a concentrated type: 0 mL, 1:50, serial No. M0146) was purchased from Shanghai Changdao Biotechnology Co., Ltd (Shanghai, China). PBS buffer solution was prepared for washing before use. EDTA buffer solution was purchased from DAKO. Hematoxylin and DAB color test kit were products of Fuzhou Maixin Biotechnology Development Co., Ltd. Other reagents included 10% neutral formalin, xylene and 3% hydrogen peroxide methanol solution.
Main experimental instruments included paraffin section machine (LEICA 2135, Germany), automatic tissue processing machine (SHANDON, UK), electronic balance (MP-110-I, Shanghai No. 2 Balance Instrumentation Factory, Shanghai, China), biological photomicroscope (OLYMPUS, Japan), ZH-3 paraffin section freezing table (Shenyang Zong Heng Hi-technology Co., Ltd, Shenyang, China), water-jacketed electrothermostatic incubator (PYX-DHS-40 × 50, Shanghai Yue Jin No. 1 Medical Instruments Factory, Shanghai, China), processing microshaker (75-2A, Shanghai Medical Analytic Instrumentation Factory), and drying box (Shanghai Xin Li Instruments Manufacturer).
Human gastric tumor specimens fixed in 10% neutral formalin were dehydrated with ethanol, hyalinized with xylene, paraffin-embedded, sliced into 4-μm sections, deparaffined with xylene, gradient hydrated with alcohol, washed with distilled water, and soaked in 3% hydrogen peroxide methanol solution to block endogenous hydrogen peroxidase. Antigen repair was done with 0.01mol citric acid buffer solution (pH 6.0) in a hyperbaric pot. The specimens were then added into non-immune bovine serum, incubated at 37°C for 20 min, added into 50 μL concentrated primary antibody E-cad and ICAM-1 (1:50), incubated in a 4°C refrigerator overnight, added into 50 μL EnVision, and incubated in the thermostatic incubator at 37°C for 2 h. The sections were added into 50 μL DAB, and hematoxylin for 2 min. Alcohol was differentiated with 1% hydrochloric acid, washed with fresh water, stepwise dehydrated with alcohol, hyalinized with xylene, and blocked with neutral resin.
The sections were color developed with DAB. The brown color refers to a positive result. Positive cells were confirmed as long as the positive product was definite, even though the distribution of positive staining may not be homogenous and density may be different. Any 5 positive color stains in the high power field was set as the criterion for judgment: light brown (+), yellow brown (++) and dark brown (+++).
The density of color reaction and the number of expressing cells were used to judge the value. The density of immunohistochemical stain was classified as (-), (+), (++) and (+++), which were assigned as 0, 1, 2 and 3, respectively. The number of expressing cells of 0, 1%-25%, 25%-50%, 50%-75% and 75%-100% was assigned as 0, 1, 2, 3 and 4, respectively[1]. The multiplication of the two values was used as the result.
Abnormal distribution data were tested with Kruskal-Wallis H test using SAS statistic software (6.02). Intra- and inter-group comparison was analyzed and tested with Nemenyi test.
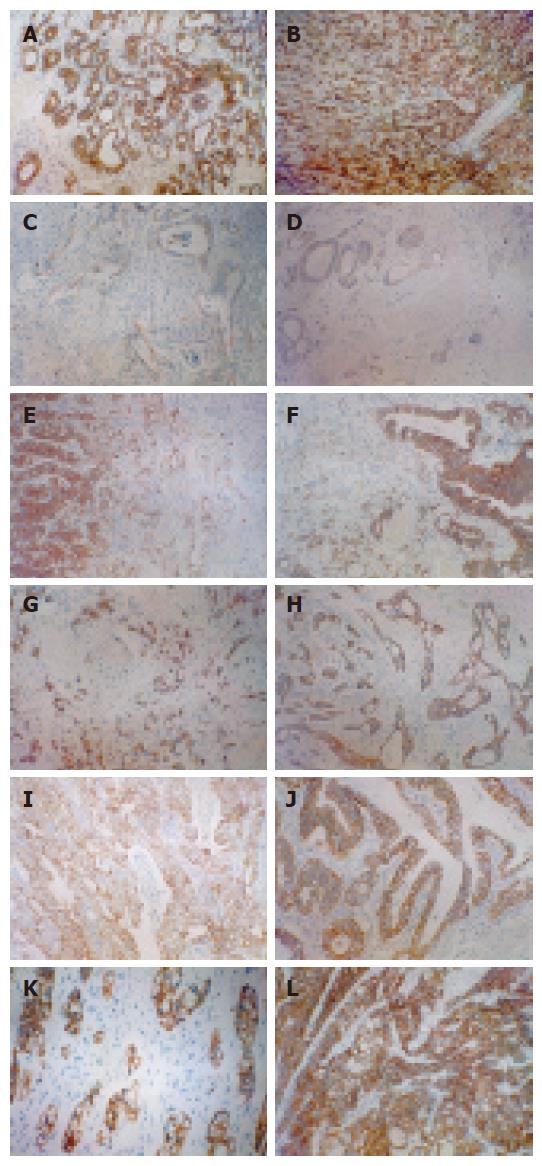
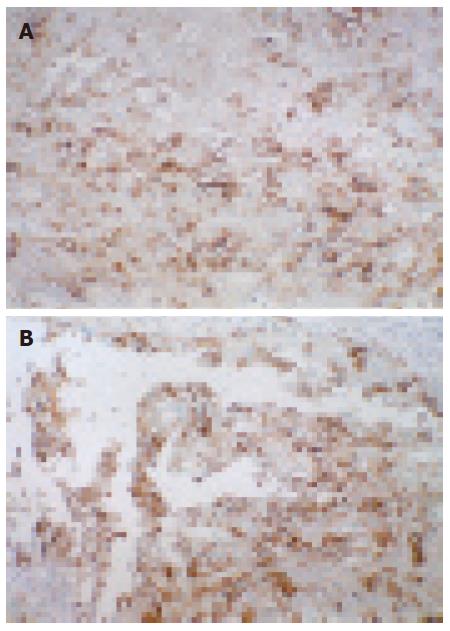
E-cad immunohistochemical expression in each group is shown in Figure 1A-L, ICAM-1 immunohistochemical expression is shown in Figure 2A and B. The E-cad immunohistochemical expression values in different syndrome differentiation types of gastric carcinoma are listed in Table 2.
| SD in TCM | n | E-cad expression value | ||||||||||||||||
| SPD (1) | 17 | 6 | 6 | 9 | 6 | 0 | 4 | 6 | 6 | 6 | 9 | 6 | 3 | 6 | 8 | 9 | 6 | 3 |
| YISH (2) | 16 | 2 | 6 | 6 | 0 | 4 | 8 | 2 | 2 | 4 | 2 | 8 | 6 | 4 | 12 | 4 | 1 | |
| IOST (3) | 16 | 0 | 0 | 6 | 12 | 6 | 0 | 3 | 0 | 3 | 3 | 2 | 0 | 8 | 1 | 4 | 4 | |
| DOQB (4) | 17 | 6 | 4 | 6 | 8 | 8 | 6 | 2 | 12 | 9 | 6 | 4 | 4 | 4 | 0 | 6 | 8 | 9 |
| DCSS (5) | 17 | 6 | 6 | 4 | 12 | 4 | 6 | 8 | 9 | 6 | 8 | 2 | 4 | 1 | 12 | 2 | 4 | 4 |
| IBLS (6) | 17 | 0 | 12 | 2 | 0 | 2 | 3 | 2 | 1 | 6 | 4 | 1 | 6 | 2 | 6 | 2 | 4 | 0 |
The E-cad positive expression rate was 90% in 100 cases of gastric carcinoma. There was a significant difference in E-cad gene protein expression between different syndrome differentiation types in TCM (P < 0.01).
Further group-group comparison was conducted to deduce differences in location between syndrome differentiation types and the results were analyzed by Nemenyi test (Table 3). There was no significant different between SPD and DOQB and DCSS, and E-cad expression was relatively lower in IOST and IBLS. The difference was significant in other group-group comparisons (P < 0.01).
| Comparison groups | χ2 | P | Comparison groups | χ2 | P |
| 1, 2 | 88.434 | < 0.01 | 2, 6 | 60.018 | < 0.01 |
| 1, 3 | 264.674 | < 0.01 | 3, 4 | 265.648 | < 0.01 |
| 1, 4 | 0.001 | > 0.05 | 3, 5 | 193.162 | < 0.01 |
| 1, 5 | 6.021 | > 0.05 | 3, 6 | 0.697 | > 0.05 |
| 1, 6 | 303.01 | < 0.01 | 4, 5 | 5.936 | > 0.05 |
| 2, 3 | 46.373 | < 0.01 | 4, 6 | 302.395 | < 0.01 |
| 2, 4 | 88.107 | < 0.01 | 5, 6 | 223.599 | < 0.01 |
| 2, 5 | 48.806 | < 0.01 |
The ICAM positive expression rate was 58% in 100 cases of gastric carcinoma. There was no significant difference in ICAM-1 expression between different TCMSD types (P > 0.05) (Table 4).
| SD type | n | ICAM-1 | ||||||||||||||||
| SPD | 17 | 0 | 0 | 0 | 1 | 1 | 1 | 6 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 |
| YISH | 16 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 | 1 | 2 | 1 | 2 | 0 | 1 | 1 | |
| IOST | 16 | 2 | 0 | 4 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 4 | 1 | 4 | 1 | |
| DOQB | 17 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 6 | 1 |
| DCSS | 17 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| IBLS | 17 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 2 | 1 | 0 | 1 | 0 | 0 |
The significance of syndrome differentiation in TCM was stated as early as in Tang Dynasty as “ The same internal disease may be represented by different external symptoms and signs, and different internal diseases may have the same external manifestations and vice versa. Therefore, sufficiency or deficiency in five-zang organs and six-fu organs, luxuriance or exsiccation and patency or obstruction of blood vessels is not exactly what is observed by the eyes. They should be judged by differential diagnosis”. TCM pays special attention to the integrity and holism of the human body and its interrelationship with nature. The component parts of the human body are inseparable and are functionally coordinative and mutually beneficial while affecting each other pathologically. At the same time, TCM adheres to the basic principle of treatment based on differentiation of symptoms and signs, treats the same disease by different methods and different diseases by the same method, and advocates individualized treatment, which vividly reflects the essence of TCM treatment.
Modern biologic research has entered an era of integrating various research technologies and methods to tackle difficult biological problems at biomolecular level as a whole, which is exemplified by studies in the new scientific fields of genomics and proteomics. According to the central dogma of molecular biology and life, the task of molecular biology is to study life phenomena and nature of diseases, governing and controlling the development and progression of phenotype (protein-cell-organism) process. Life phenomena, diseases and syndrome differentiation in TCM can obtain the most essential answers from DNA or gene research.
Tumor metastasis may occur in all gastric carcinoma differentiation types, including deficiency syndrome, blood stagnation syndrome and phlegm syndrome, but it is of greater significance to know which differentiation type has a higher metastasis rate. Therefore, to study differences in gene expression between different gastric carcinoma differentiation types and the differentiation nature of gastric carcinoma at gene level and judge the metastatic tendency of different differentiation types are of great significance. This is also the reason why we used E-cad and ICAM-1 expression to study syndrome differentiation of gastric carcinoma in TCM.
Cadherins, a group of calcium dependent trans-membrane proteins, are members of the cell-cell adhesion molecule family essential for close intercellular connection[2], which mainly mediate adhesion reaction of homologous cells. Cadherins are divided into several subtypes, among which E-cad is a carcinoma inhibitory gene most closely related to tumor invasion and metastasis. Experiments showed that transfection of E-cad to tumor cells could reverse their invasive behavior[3], and use of anti-E-cad antibody could block cell-cell connection, thus enabling carcinoma cells to acquire high invasiveness. Introduction of E-cad antisense RNA into tumor cell strain highly expressing calcium adhesion protein may significantly increase its metastatic ability, thus further confirming that E-cad inhibits tumor metastasis [3]. E-cad gene mutation due to loss of cell-cell adhesion during the morula stage leads to fatal impairment to the embryo[4,5], indicating that cadherins play an essential role in the process of histolodifferentiation. Furthermore, E-cad gene has been accepted as a cancer inhibitory gene[6,7]. When E-cad is down-regulated or dysfunctional, adhesion between homogenous cells is lost. For normal tissues, this means that they are unable to develop normally, and for epithelial tumors, this means that they have the tendency to grow invasively and are likely to exfoliate from the primary focus and metastasize to local lymph nodes and distant locations. Down-regulation or dysfunction of E-cad may be an early event in the development and progression of gastric carcinoma[8]. According to Berx et al[9], the type of E-cad mutation in defuse gastric carcinoma is a shear-induced in-frame deletion. Recent studies show that down-regulation of E-cad expression in malignant tumor tissue is associated with the degree of differentiation[10-12]. Cai JC et al[13] reported that the E-cad positive expression rate was only 10% in gastric carcinoma group and 100% in control group, indicating that E-cad is lost in gastric carcinoma. It was reported that the E-cad positive rate is 36.96% in gastric carcinoma, and there is a decreasing tendency in malignant tumors. It was also reported that E-cad expression was significantly lower in poorly differentiated[13], especially in metastatic foci, where E-cad expression is reduced or even disappeared[14,15]. In the present study, the E-cad positive expression rate in 100 cases of gastric carcinoma was 90% and E-cad expression was significantly different in different differentiation syndrome types(P < 0.01)and E-cad expression was relatively low in the IOST and IBLS types, suggesting that tumor metastasis in them may be associated with E-cad.
ICAM-1 CD54, a single-chain transmemebrane glycoprotein encoded by No. 19 chromosome gene, is a member of the adhesion molecule immunoglobin superfamily (IgSF), which has 5 extracellular immu-noglobulin-like sites and a short cytoplasmic trailer structure, with a molecular weight varying with the cell type from 76 000 KD to 114 000 KD. This difference mainly depends on the degree of glycosylation of the molecule. Intercellular adhesion and identification are the necessary processes in immunological surveillance. Antigen dependence and/or non-dependence between target cells and lymphocytes are the initial step of tumor immunological reaction. It was reported that ICAM-1 on the surface of cancer cell or of presenting cell is a kind of co-stimulation molecules[16], enabling T cell receptor mediated cancer cells to combine with T lymphocytes more steadily. ICAM expression on the surface of cancer cells can enhance its susceptibility to lymphocyte-mediated cytotoxicity. Decreased ICAM expression often leads to lymph node metastasis, suggesting that ICAM-1 gene transfection can inhibit lymph node metastasis. To investigate whether ICAM-1 gene could treat peritoneal metastasis, Tanaka et al[17] transfected the ICAM-1 cDNA to the gastric carcinoma cell strain OCUM-2MD3 (cancer cells having a high peritoneal metastasis rate), and found that ICAM-1 was significantly expressed on cell surface. The survival of mice peritoneally transplanted with gene transfected 2MD3/ICAM-1 was significantly higher than that of animals transplanted with 2MD3 only, suggesting that ICAM-1 gene transfection therapy can effectively prevent peritoneal metastasis of gastric carcinoma. In the present study, the ICAM-1 positive expression rate was 58%, and there was no significant difference in ICAM-1 expression between the syndrome differentiation types (P > 0.05), demonstrating that the difference in tumor recurrence and metastasis between syndrome differentiation types is not due to ICAM-1 change.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2396] [Cited by in RCA: 2431] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 3. | Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Löchner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1107] [Cited by in RCA: 1166] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263-8267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 688] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci USA. 1995;92:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 369] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Becker KF, Keller G, Hoefler H. The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol. 2000;9:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lee JH, Koh JT, Shin BA, Ahn KY, Roh JH, Kim YJ, Kim KK. Comparative study of angiostatic and anti-invasive gene expressions as prognostic factors in gastric cancer. Int J Oncol. 2001;18:355-361. [PubMed] |
| 8. | Yu J, Ebert MP, Miehlke S, Rost H, Lendeckel U, Leodolter A, Stolte M, Bayerdörffer E, Malfertheiner P. alpha-catenin expression is decreased in human gastric cancers and in the gastric mucosa of first degree relatives. Gut. 2000;46:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Berx G, Becker KF, Höfler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690-1695. [PubMed] |
| 11. | Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Karatzas G. E-cadherin expression as a differentiation marker in gastric cancer. Hepatogastroenterology. 1998;45:2437-2442. [PubMed] |
| 12. | Guzmán P, Araya J, Villaseca M, Roa I, Melo A, Muñoz S, Roa J. [Immunohistochemical expression of the E-cadherin-catenin complex in gastric cancer]. Rev Med Chil. 2006;134:1002-1009. [PubMed] |
| 13. | Cai JC, Jiang SJ, Chen ZP. [Relationship between E-cadherin and growth pattern of gastric cancer and degree of cell differentiation]. Zhonghua Bing Li Xue Za Zhi. 1994;23:132-134. [PubMed] |
| 14. | Schuhmacher C, Becker KF, Reich U, Schenk U, Mueller J, Siewert JR, Höfler H. Rapid detection of mutated E-cadherin in peritoneal lavage specimens from patients with diffuse-type gastric carcinoma. Diagn Mol Pathol. 1999;8:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Nabeshima K, Inoue T, Shimao Y, Kataoka H, Koono M. Cohort migration of carcinoma cells: differentiated colorectal carcinoma cells move as coherent cell clusters or sheets. Histol Histopathol. 1999;14:1183-1197. [PubMed] |
| 16. | Bertolino P, Schrage A, Bowen DG, Klugewitz K, Ghani S, Eulenburg K, Holz L, Hogg N, McCaughan GW, Hamann A. Early intrahepatic antigen-specific retention of naïve CD8+ T cells is predominantly ICAM-1/LFA-1 dependent in mice. Hepatology. 2005;42:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Tanaka H, Yashiro M, Sunami T, Ohira M, Hirakawa-Y S Chung K. Lipid-mediated gene transfection of intercellular adhesion molecule-1 suppresses the peritoneal metastasis of gastric carcinoma. Int J Mol Med. 2002;10:613-617. [PubMed] |