Published online Aug 28, 2007. doi: 10.3748/wjg.v13.i32.4328
Revised: June 10, 2007
Accepted: June 18, 2007
Published online: August 28, 2007
AIM: To investigate the anti-fibrosis effect of the tissue transglutaminase (tTG) specific inhibitor cystamine on liver fibrosis.
METHODS: Sixty-eight male Sprague Dawley rats were divided into three groups: normal control, liver fibrosis control and cystamine-treated group. Liver fibrosis was induced by intraperitoneal injection of carbon tetrachloride (CCl4), and Cystamine was administrated by intraperitoneal injection starting 2 d before the first administration of CCl4. Animals in each group were further divided into 2 subgroups according to two time points of 4 wk and 8 wk after treatment. Hepatic function, pathological evaluation (semi-quantitative scoring system, SSS) and liver hydroxyproline (Hyp) content were examined. Real-time PCR was used to detect the expression of tTG, smooth muscle alpha actin (α-SMA), tissue inhibitor of metalloproteinase 1 (TIMP-1) and collagen-1 mRNA. The expressions of tTG and α-SMA protein were detected by Western Blotting.
RESULTS: Eight weeks after treatment, the SSS score of liver was significantly less in the cystamine group than that in the fibrosis control group (P < 0.01). The levels of alanine aminotransferase (ALT) and total bile acid (TBA) at the 4 wk and 8 wk time points were decreased in the cystamine group compared with those in fibrosis controls (P < 0.01). Liver hydroxyproline content at the 4 wk and 8 wk time points showed a substantial reduction in the cystamine group compared to fibrosis controls (P < 0.01). The expression of tTG, α-SMA, collagen-1, TIMP-1 mRNA and tTG, as well as α-SMA protein was downregulated in the cystamine group compared to fibrosis controls.
CONCLUSION: Cystamine can ameliorate CCl4 induced liver fibrosis and protect hepatic function. The possible mechanism is related to the reduced synthesis of the extracellular matrix (ECM) caused by the inhibition of hepatic stellate cell activation and decreased expression of TIMP-1.
-
Citation: Qiu JF, Zhang ZQ, Chen W, Wu ZY. Cystamine ameliorates liver fibrosis induced by carbon tetrachloride
via inhibition of tissue transglutaminase. World J Gastroenterol 2007; 13(32): 4328-4332 - URL: https://www.wjgnet.com/1007-9327/full/v13/i32/4328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i32.4328
Liver fibrosis is characterized by increased synthesis and decreased degradation of the extracellular matrix (ECM)[1]. Decreased ECM degradation results partly from irreversible cross-linking of the ECM and partly from increased expression of tissue inhibitor of metalloproteinase (TIMP) by hepatic stellate cells (HSCs). In the liver, both parenchymal and non-parenchymal cells produce tissue transglutaminase (tTG), and it appears to be released into the extracellular space. tTG is a member of the large transglutaminase (TG) family, which are involved in numerous biochemical processes. tTG not only displays cross-linking activity, but also functions as a GTPase, and it has been shown to be involved in a variety of fibrotic diseases including scleroderma, experimental renal fibrosis and human hepatic fibrosis[2,3]. Indeed, tTG expression is upregulated when there is any injuring process. Studies have demonstrated that liver cell death secondary to ethanol administration occurs by apoptosis, not by liver cell necrosis[4,5], and tTG may be a factor in apoptosis induced by ethanol. Several reports suggest that tTGase activity was increased associated with transforming growth factor beta (TGF-β) and ECM production and α-SMA-positive HSC number also increased after CCl4 intoxication in rats[6,7]. These findings have raised the hypothesis that tTG may contribute to the development of liver fibrosis and inhibition of tTG may be effective in treatment of liver fibrosis. In this study, we investigated whether tTG plays a role in the progression of rat hepatic fibrogenesis induced by carbon tetrachloride (CCl4) via administration of the tTG specific inhibitor cystamine.
Sixty-eight Sprague Dawley rats weighing 300-350 g (Animal Center, Chinese Academy of Sciences, Shanghai) were studied. The animals were housed in an environmentally controlled vivarium with timed light control (12 h light-dark cycle) and allowed free access to a standard pellet diet and water. The animals were divided into three groups: normal control (N), fibrosis control (F) and cystamine treatment (C). Rats in each group were subdivided into two groups according to two time points of 4 wk and 8 wk as follows: N4 (n = 10), N8 (n = 10); F4 (n = 13), F8 (n = 8), C4 (n = 14), C8 (n = 13).
Cystamine (tTG specific inhibitor) was purchased from Sigma Co, USA. Mouse monoclonal tTG antibody was purchased from Stratek Scientific, UK (No: CUB 7402), and labelled streptavidin-biotin (LSAB) immunohistochemistry kits and mouse monoclonal α-SMA antibody were from Dako Co, USA. The Beckman Coulter LX 20 automatic biochemical analytic instrument and real time PCR amplifier (ABI Prism 7700 Sequencer Detector, Applied Biosystems, Co) were products from the USA.
Normal control rats were injected intraperitoneally with peanut oil (0.2 mL/100 g) twice a week. Liver fibrosis control rats were induced by intraperitoneal injection of 50% CCl4 solution (CCl4:oil = 1:1) at a dose of 0.2 mL/100 g twice a week for 8 wk. One subgroup of rats that had cystamine treatment for the first 4 wk were given cystamine 2 d before the first injection of CCl4 at a dose of 112 mg/kg per day as in a previous study[8]. The other subgroup of rats that had cystamine treatment for the second 4 wk (8 wk subgroup) were administrated cystamine after injection of CCl4 for 4 wk at the same dose. Rats in each group were sacrificed 3 d after the final administration according to each time point. Blood collected from the aorta was centrifuged and then the serum was stored at -80°C for hepatic function examination. Liver was removed and fixed in 10% neutral formalin for pathological examination and other assays.
The levels of aspartate aminotransferase (ALT), total bilirubin (TB), and total bile acid (TBA) were tested with Beckman Coulter LX 20 (USA).
The hydroxyproline kit obtained from Nanjing Jiancheng Biological Engineering Research Institute was used to analyze liver hydroxyproline content (μg/g).
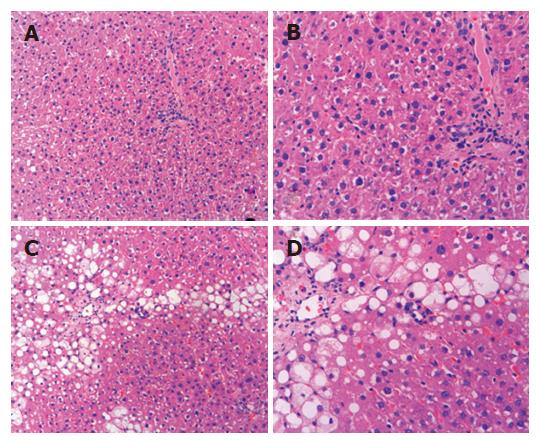
Tissues from each liver were fixed in 10% formalin and embedded in paraffin, and then cut into 5 μm pieces and mounted on slides. The samples were stained with hematoxylin and eosin (HE) for histopathological examination and a semi-quantitative scoring system (SSS)[9] was adopted for evaluation of the degree of liver fibrosis.
Taqman real-time polymerase chain reaction (PCR) was used to determine the relative expression of messenger RNAs (mRNAs) for tTG, α-SMA, TIMP-1 and collagen-1 in total RNA extracted from snap-frozen liver by the acid-phenol method. All primers were designed by using the Primer Expression 2.0 software (PE Applied Biosystems). Primers synthesized by Shanghai Shenggong Corp. were as follows: (1) tTG, sense: 5'-gtatgatgcgtccttcgtgt-3', antisense: 5'-cagtttgttcaggtggttgg-3'; (2) α-SMA: sense:5'-TGGGAAATGCCACAGGTT-3', antisense: 5'-CTGCGGTTCTGGGACTTG-3'; (3) TIMP-1: sense: 5'-atattctgtctggatcggc-3', antisense: 5'-gcttcgtcatactcctgttt-3'; (4) collagen-1: sense: 5'-tctccaagaggcagggttc-3', antisense: 5'-ggttagcttcggctca tgc-3'; HPRT, sense: 5'-AAAGCCAAGTACAAAGCCTAAA-3', antisense: 5'-CTGTCTGTCTCACAAGGGAAGT-3'. The conditions of the reaction were as follows: initial steps were 95°C for 10 s, followed by a denaturing step for 5 s at 95°C, and an annealing/extension step at 60°C for 30 s performed in 40 cycles. The PCR products were analyzed directly by electrophoresis on 2.0% agarose gels. Relative concentrations were calculated and normalized according to the expression of the housekeeping gene HPRT.
Total protein was determined with a protein assay (BioRad Inc, California, USA) and electrophoresed on a 4%-12% polyacrylamide gradient gel with 10% sodium dodecyl sulfate (SDS), and subsequently transferred to a nitrocellulose membrane. The membrane was blocked for 1 h in 1% to 5% nonfat dry milk in Tween Trisbuffered saline (TTBS). Western blot analysis of liver tissue was performed by using a monoclonal anti-α-SMA and anti- tTG. Each antibody was used at a concentration of 1:1000. Membranes were incubated overnight with the primary antibody or with nonimmune immunoglobulin G (as a negative control) in TTBS, after which the secondary horseradish-peroxidase-conjugated antibodies were applied in TTBS containing 0.1% nonfat dry milk for 1 h. Reactive bands were identified by using enhanced chemiluminescence (ECL; Amersham, Bucks, UK) and autoradiography according to the manufacturer's instructions.
All data are presented as mean ± SD. Statistical comparison among groups was made by one-way analysis of variance (ANOVA) using SPSS12.0 statistical software (Lead Technologies, Inc, USA). A P value less than 0.05 was considered statistically significant.
Eight weeks after treatment, the liver appeared normal in control rats. The score of SSS evaluation for hepatic fibrosis in cystamine treatment group (4.20 ± 0.81) was significantly less than that in fibrosis control rats (6.46 ± 0.68) (P < 0.01, Figure 1).
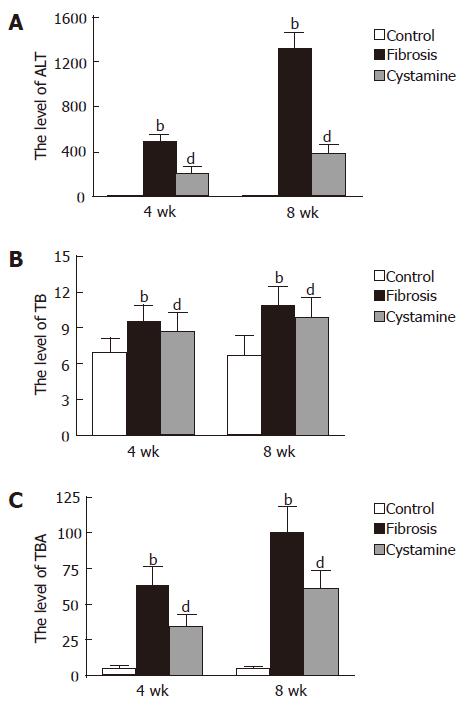
In 4 wk and 8 wk time point, the levels of ALT, TBA and TB in fibrosis control rats were much greater than those in normal control rats (P < 0.01); The levels of ALT and TBA of cystamine-treated rats were significantly less than those in fibrosis controls (P < 0.01); however, there was no significant difference in the levels of TB in the two time point compared with fibrosis controls (Figure 2).
The levels of hydroxyproline (Hyp) in fibrosis controls at 4 wk (77.71 ± 12.47 μg/g) and 8 wk (130.58 ± 25.80 μg/g) were higher than those in normal controls (4 wk, 53.88 ± 10.56 μg/g; 8 wk, 54.67 ± 11.77 μg/g, P < 0.01). The levels of Hyp in cystamine-treated rats at 4 wk (60.64 ± 8.94 μg/g) and 8 wk (69.87 ± 13.48 μg/g) were significantly less than those in fibrosis controls (P < 0.01).
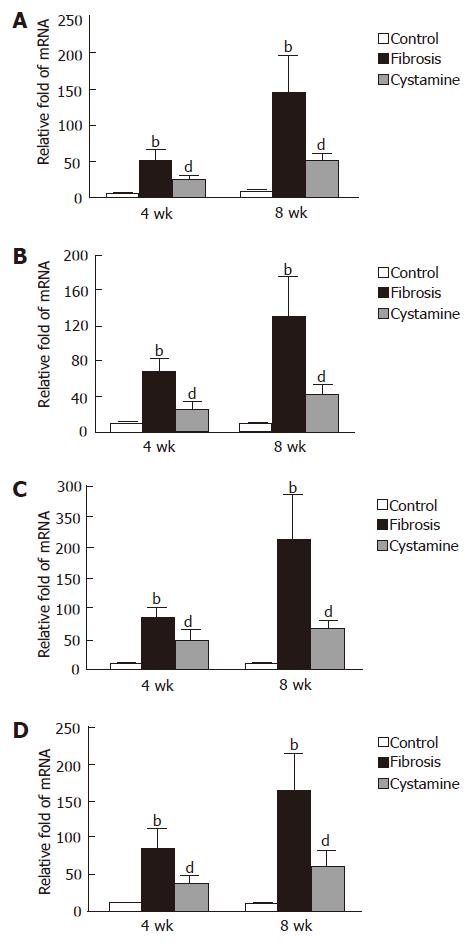
The expression of tTG, α-SMA, collagen-1 and TIMP-1 mRNA was upregulated in fibrosis control rats at each time point compared to normal control rats; however, after administration of cystamine, the expression of tTG, α-SMA, collagen-1 and TIMP-1 mRNA was significantly downregulated (P < 0.01, Figure 3).
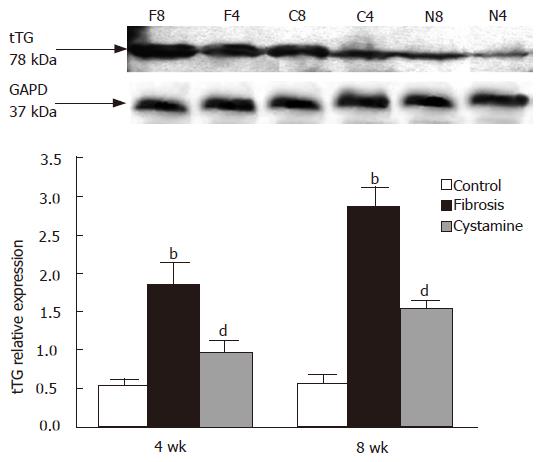
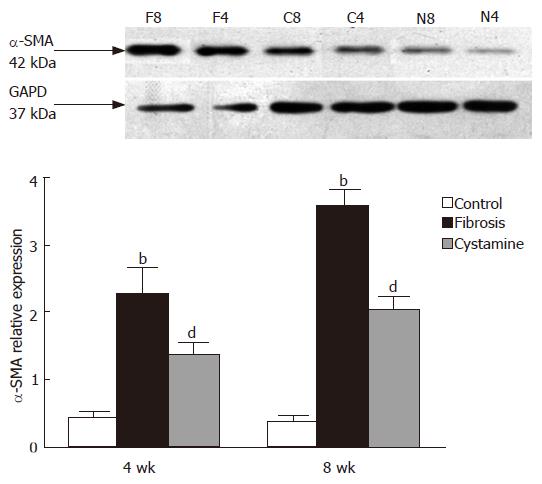
The expression of tTG protein in the liver was decreased after treatment of cystamine. The relative expression difference in each group is shown in Figure 4 after image analysis. The expression of α-SMA protein was also decreased after administration of cystamine (Figure 5).
Transglutaminases (TGs) are a large family of enzymes that catalyze a calcium-dependent acryl transfer reaction between the γ-carboxamide group of a polypeptide bound lysine residue to form an ε-(γ-glutamyl) lysine isopeptide bond or to form a (γ-glutamyl) polyamine bond through catalyzing the incorporation of a polyamine into a polypeptide-bound glutamine. Known as “natural biological glues”[10], TGs can result in covalent and irreversible cross-links between or within proteins and are thus involved in a variety of biochemical processes. Tissue transglutaminase (tTG), one member of the TGs, is widely expressed in endothelial, mesangial, and smooth muscle cells and fibrotic tissue, and localized in the cytosol, plasma membrane, and the nucleus of cells, as well as the extracellular space. tTG is a multifunctional enzyme that has been implicated in several physiological processes and pathological conditions such as maintaining membrane integrity, cell adhesion, cell death or differentiation, signal transduction and tissue remodeling[11]. Recent studies suggest that tTG may contribute to hepatic injury and fibrosis[12,13].
Schnabel and colleagues found a significant upregulation of total tTG at the mRNA and protein level in activated HSCs and ECM during transdifferentiation of quiescent HSCs to collagen producing myofibroblasts (MFB)[14]. This finding indicates that tTG plays an important role in the process of HSC activation, and hence, in the mechanism of liver fibrogenesis since the activated HSCs are not only the major source of ECM, but also can continuously upregulate the expression of TIMP-1, which blocks matrix metalloproteinase (MMP) activity and promotes survival of activated HSCs from apoptosis[15,16]. In addition, tTG mediated covalent binding of latent TGF-binding protein 1 (LTBP-1) to the ECM is a prerequisite for the activation of TGF-β, which is stored in its inactive form in the ECM[6,17]. The transdifferentiation of HSCs to ECM-producing MFB is a crucial event in hepatic fibrogenesis, which is stimulated by TGF-β[7].
It is well established that tTG exerts important effects on the progression of liver fibrogenesis through its ECM cross-linking activity. Issa and colleagues[18] confirmed that livers harvested from rats treated for 6 wk with CCl4 (representing reversible fibrosis) showed the histologic appearance of early macronodular cirrhosis uniformly negative for cross-linking. Over a 15 to 28 d recovery period, the livers showed a dramatic remodeling of the fibrotic matrix with a return to near-normal histology. However, livers from rats treated with CCl4 for 12 wk showed a persistent fibrosis with clear evidence of cross-linking even during 366 d of recovery. The data above suggest that tTG mediated matrix cross-linking may be a major factor determining the speed and extent of matrix degradation in hepatic fibrosis.
However, there is still controversy about the role of tTG in chronic liver injury and fibrosis. Nardacci and colleagues[19] suggest that tTG is a protective factor against liver fibrosis, they observed that there are greater mortality and more severe hepatic fibrosis after injection of CCl4 in tTG knock-out mice than in wild-type mice. Thus, more studies are needed to elucidate the importance of tTG during hepatic fibrosis.
In the present study, we have established a liver injury and fibrosis rat model induced by intraperitoneal injection of CCl4. Two cohorts of rats according to two different stages were administrated the tTG specific inhibitor cystamine for the purpose of observing the role of tTG in the development of liver fibrogenesis. The first stage was when rats were injected with CCl4 for a total of 4 wk, and cystamine treatment was started one day before the first injection of CCl4. This was carried out in order to investigate the effects of cystamine on the activation of HSCs and hepatic function during the early period of liver injury. The second stage was when rats were given cystamine intraperitoneously after 4 wk of CCl4 treatment for 4 wk to observe the effects of cystamine on activated HSCs and the progression of hepatic fibrosis. Our results showed that cystamine significantly downregulated the expression of tTG mRNA and protein, inhibited the activation of HSCs associated with a diminution of α-SMA, collagen-1 and TIMP-1 mRNA expression, as well as α-SMA protein, and there was a decrease in hydroxyproline content and dramatic improvement of liver pathologic evaluation. Furthermore, after administration of cystamine, the hepatic function of cystamine-treated rats was robustly improved. The results of this study strongly suggest that cystamine was beneficial in treatment of liver injury and fibrosis. The possible mechanism might be related to the inhibition of HSC activation, decreased expression of TIMP-1, reduced synthesis of ECM, and protection of hepatic cells against injury.
In this study, we analyzed the changes in histology, the expression of tTG mRNA and protein, the activation of HSCs and the hydroxyproline content, as well as hepatic function after administration of cystamine in CCl4 induced fibrosis. In the current study, we did not undertake experiments for detection of in situ HSC apoptosis and ECM cross-linking, and therefore, to some extent this affects the interpretation of our observations. However, based on our results we confirmed that cystamine might ameliorate CCl4 induced liver fibrosis via inhibition of tTG. We are currently carrying out experiments to investigate the molecular mechanism of HSC apoptosis and ECM cross-linking in advanced fibrosis (12 wk of CCl4 treatment) through tTG RNA interfere to further study the role of tTG in the progression of hepatic fibrosis.
In summary, tTG plays an important role in the development of hepatic fibrosis. Cystamine, a specific inhibitor of tTG, can attenuate CCl4-induced liver fibrosis and protect hepatic function via inhibiting activation of HSCs and downregulating expression of TIMP-1 which result in the reduction of the synthesis of ECM. Better understanding of the role of tTG in pathogenesis of liver fibrosis could facilitate the development of new therapeutic strategies.
S- Editor Liu Y L- Editor McGowan D E- Editor Yin DH
| 1. | Sarem M, Znaidak R, Macías M, Rey R. Hepatic stellate cells: it's role in normal and pathological conditions. Gastroenterol Hepatol. 2006;29:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Johnson TS, Griffin M, Thomas GL, Skill J, Cox A, Yang B, Nicholas B, Birckbichler PJ, Muchaneta-Kubara C, Meguid El Nahas A. The role of transglutaminase in the rat subtotal nephrectomy model of renal fibrosis. J Clin Invest. 1997;99:2950-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Grenard P, Bresson-Hadni S, El Alaoui S, Chevallier M, Vuitton DA, Ricard-Blum S. Transglutaminase-mediated cross-linking is involved in the stabilization of extracellular matrix in human liver fibrosis. J Hepatol. 2001;35:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Lamb RG, Koch JC, Snyder JW, Huband SM, Bush SR. An in vitro model of ethanol-dependent liver cell injury. Hepatology. 1994;19:174-182. [PubMed] |
| 5. | Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol. 1997;136:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 294] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 330] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942-8950. [PubMed] |
| 9. | Zeng MD, Wang TL, Wang BE. Consensus on evaluation of the diagnosis and efficacy of hepatic fibrosis. Zhonghua GanZangBing ZaZhi. 2002;10:327-328. [PubMed] |
| 10. | Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368:377-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 780] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 11. | Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1133] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Wu J, Zern MA. Tissue transglutaminase, a key enzyme involved in liver diseases. Hepatol Res. 2004;29:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Mirza A, Liu SL, Frizell E, Zhu J, Maddukuri S, Martinez J, Davies P, Schwarting R, Norton P, Zern MA. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am J Physiol. 1997;272:G281-G288. [PubMed] |
| 14. | Schnabel C, Sawitza I, Tag CG, Lahme B, Gressner AM, Breitkopf K. Expression of cytosolic and membrane associated tissue transglutaminase in rat hepatic stellate cells and its upregulation during transdifferentiation to myofibroblasts in culture. Hepatol Res. 2004;28:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Nunes I, Shapiro RL, Rifkin DB. Characterization of latent TGF-beta activation by murine peritoneal macrophages. J Immunol. 1995;155:1450-1459. [PubMed] |
| 18. | Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Nardacci R, Lo Iacono O, Ciccosanti F, Falasca L, Addesso M, Amendola A, Antonucci G, Craxì A, Fimia GM, Iadevaia V. Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol. 2003;162:1293-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |