INTRODUCTION
Our modern lifestyle has drastically changed the chemistry of the environment in which we live. There is ample documentation of increased toxicity in our food, air and water. In addition, chronic illnesses often are connected to the production of toxic chemicals that inflame and cause oxidative stress. Moreover, many medications are metabolized in the liver, and in effect, can function as toxins. Unfortunately, many of us promote an increase of our own toxin levels with excessive alcohol intake, smoking and over counter drug use[1]. Drug detoxification is an important function of the liver. It is a complex process that occurs in the endoplasmic reticulum of the hepatocyte, which involves several phases. Its main role in detoxification involves neutralizing harmful compounds, such as drugs, pesticides, hormones and bacterial toxins from the intestines.
The seaweeds are considered the most nutritious plants on earth. Their nutritive values greatly exceed those found in other food sources that humans can readily utilize. From the time immemorial the macroscopic marine algae have been closely associated with human life and are being commonly used in numerous ways as a source of food/feed and medicine. In folk medicine, seaweeds have been used for a variety of remedial purposes, such as in eczema, gallstone, renal trouble, scabies, psoriasis, asthma, arteriosclerosis, heart disease, ulcers and cancer. The cell walls of marine algae characteristically contain polysaccharides, which are not found in land plants and which may have specific functions in ionic regulation and wide spectrum of pharmacological importance. Sulphated polysaccharide (e.g. fucoidan) is structurally unique that is found only in the cell walls of several types of brown seaweed. Brown algae substantially differ from algae of other divisions and terrestrial plants in their composition[2,3]. A perusal of literature study has shown that the brown algae contain essential minerals, vitamins, free amino acids, mannitol, glucitols, sulphated polysaccharides and phlorotannins. These compounds in brown algae are found to have wide spectrum of biological properties[4-7].
Based on the previous studies regarding free radical scavenging property of Sargassum polycystum (S. polycystum) extract against acetaminophen-induced toxic hepatitis in rats, the present study was attempted to assess the defensive nature of S. polycystum (Brown alga) extract against acetaminophen-induced alterations in drug metabolizing microsomal enzyme system, TNF-α during toxic hepatitis and cellular structural integrity during toxic hepatitis.
MATERIALS AND METHODS
Sample collection and extraction
S. polycystum was collected from Gulf of Mannar, Rameswaram, India. The species authentication was done by Prof. V. Krishnamurthy (Krishnamurthy Institute of Algology, Chennai, India). The seaweed sample was washed in seawater and then fresh water to remove the epiphytes and other contamination. The coarsely powdered seaweed material was extracted with ethanol in cold for a period of 72 h with occasional shaking. The crude extract was filtered, concentrated on a water bath, and then dried in vacuum. The resulting dried powder was used for the animal experimentation. The extract was subjected to thin layer chromatography (TLC) and phytochemical analysis, which showed positive result for the presence of terpenoids and flavonoids[8].
Animals
Male Wistar strain albino rats, weighing about 100-130, were procured from Fredrick Institute for Plant Protection and Toxicology, Padappai, Chennai, India. The animals were housed in cages under proper environmental conditions and fed with a commercial pelleted diet (M/s Hindustan Foods Ltd, Bangalore, India). The animals had free access to water throughout the experimental period.
Experimental protocol
The experimental animals were divided into four groups, each group comprising six animals. Group I consisted of normal control rats fed with standard diet. Group II rats were intoxicated with acetaminophen (800 mg/kg body weight, intraperitoneally in saline solution in a boiling water bath and used after cooling at 37°C). Group III rats were orally pre-treated with S. polycystum extract alone (200 mg/kg body weight for 21 d). Group IV rats were orally pre-treated with S. polycystum extract (200 mg/kg body weight for 21 d) prior to acetaminophen induction (800 mg/kg body weight, intraperitoneally).
At the end of the experimental period the animals were deprived of standard diet for 20 h and then anesthetized with diethyl ether, followed by cervical decapitation. Blood collected without anticoagulant for serum. The liver was excised and immersed in ice-cold 0.01 mol/L tris (hydroxymethyl) aminomethane (Tris)-1.15 mol/L KCL buffer (pH 7.4). After rinsing twice in buffer, the liver was weighed and homogenized with a Teflon potter-Elvehjem homogenizer in ice-cold measured volumes of Tris-KCL buffer. The homogenate was then centrifuged at 9000 g for 15 min at 0°C. The microsomal pellet was then layered with 1 mL of Tris-KCl buffer and recentrifuged for an hour at 105 000 g at 0°C.
Microsomal enzyme profile
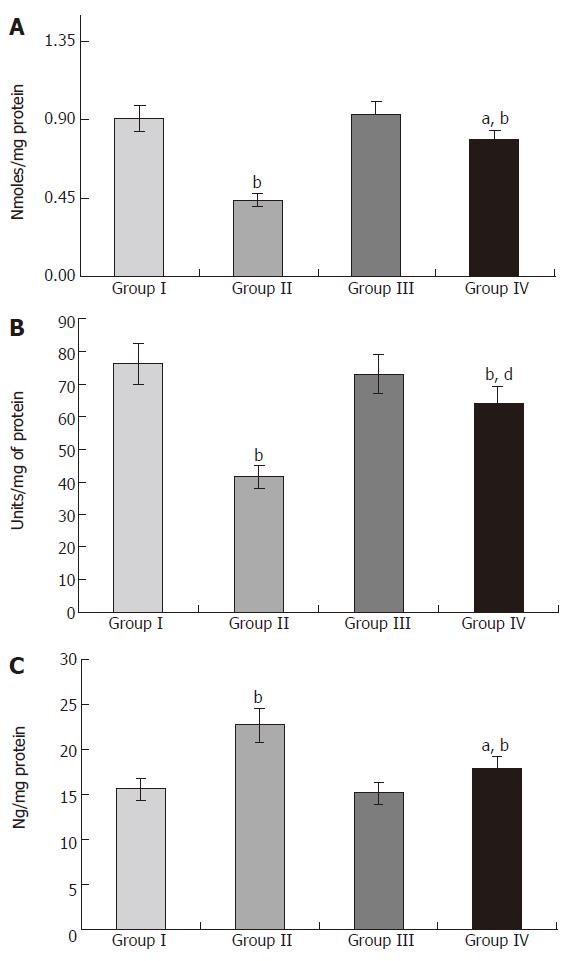
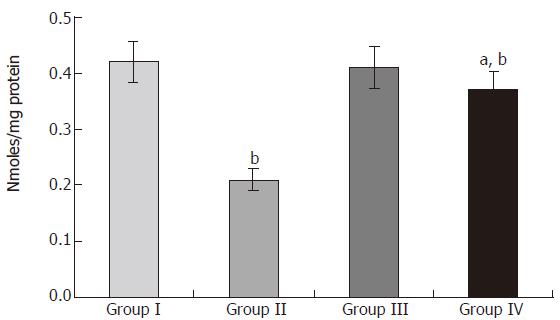
The microsomal pellets were layered and stored frozen prior to performing enzyme assays. Cytochrome p450 and b5 assay was performed as previously described by Omura and Sato[9]. NADPH-cytochrome p450 reductase assay performed as previously described by Phillips and Langdon[10].
Tumor necrosis factor-α (TNF-α)
One hundred microgram of the protein sample in carbonate buffer (pH 9.4) was coated on the 96-well polyvinyl chloride ELISA plates and kept overnight at 4°C. The coated wells were washed twice with phosphate-buffered saline (pH 7.4) containing 0.5 mL/L Tween 20, blocked with 10 g/L bovine serum albumin (BSA) in phosphate-buffered saline (100 μL/well) and incubated at 37°C for 1 h. The coated wells were washed thrice with phosphate-buffered saline containing 0.5 mL/L Tween 20. Then 50 μL of anti-TNF-α was added and incubated at 37°C for 1 h or overnight at 4°C. After incubation, the ELISA plates were washed as above and 50 μL of anti-mouse goat anti-rabbit-IgG-HRP (1:5000) diluted with phosphate-buffered saline was added and incubated for 1 h. The wells were washed with PBS-Tween 20 thrice and with PBS thrice. Fifty microliter of substrate solution (1 μL of H2O2, 0.5 mg OPD in 1 mL citrate-phosphate buffer, pH 5.0) was added. Fifty microliter of 1.5 mol/L H2SO4 was added after 30 min to stop the reaction. The intensity of the color was recorded on an ELISA reader at 490 nm[11].
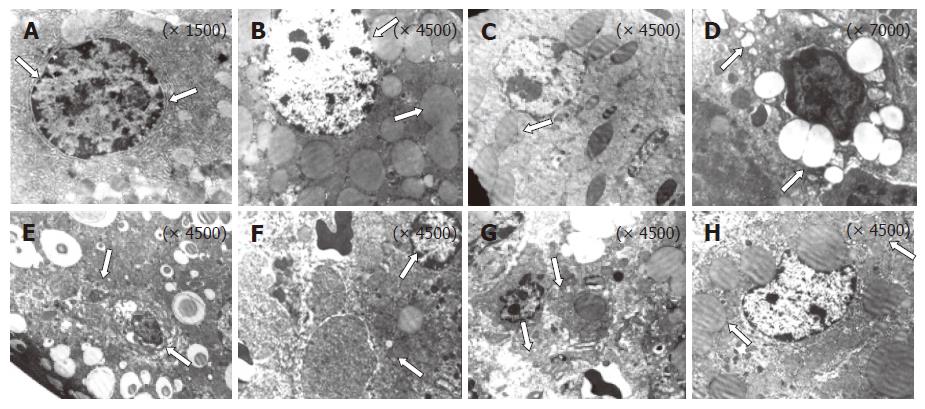
Transmission electron microscopy
A portion of the liver tissue was instantaneously immersed in 25 g/L of glutaraldehyde solution, buffered with 0.1 mol/L sodium cacodylate (pH 7.4). The specimen was then placed in the buffer fixative medium, followed by washing with sodium cacodylate and fixation in 20 g/L
osmium tetroxide buffered with 0.1 mol/L sodium cacodylate. After dehydration in a graded series of alcohol and propylene oxide, the tissues were transferred to the propylene oxide: ethanol mixture (1:1) and embedded in resin. The specimens were mounted on epoxy resin blocks and left in the oven at 65°C for 72 h. Thin sections were cut with an ultramicrotome, stained with uranyl acetate and lead citrate, and then examined under an EM-9A electron microscope.
Statistical analysis
Values were expressed as mean ± SD for six rats in each group and significance of the differences between mean values were determined by one-way analysis of variance (ANOVA), followed by the Duncan test for multiple comparison. A P value less than 0.05 was considered statistically significant.
DISCUSSION
Puntarulo and Cederbaum[12] have found a close parallelism between formation of malondialdehyde and loss of microsomal enzyme activities. Cyt P450 and NADPH cytochrome P450 reductase are not rapidly organized in the microsomal membrane and they possess lateral mobility, which largely depends on fluidity of the membrane[13]. Peroxidation of membrane lipids has been shown to repress the membrane fluidity[14]. Therefore, the observed impairment in the activities of xenobiotic metabolizing enzymes in acetaminophen-intoxicated rats could account for the alteration in membrane fluidity.
In the liver, exogenous and endogenous compounds are metabolized by the cytochrome P450-dependent monoxygenase system. The enzymes involved in this process are located in the endoplasmic reticulum of the liver, and their activities are dependent on many environmental factors[15,16]. When considering biotrans-formation processes, it is important to analyze them together with other cellular processes taking place in the hepatocyte. Impairment of these processes may disturb cellular nutritional status, thus affecting the detoxication reactions[17-19]. This enzyme system, therefore, provides our primary defense against xenobiotics and is a major determinant in the therapeutic efficacy of pharmacological agents[20].
The catalytic cycle of cytochrome P450 (Cyt p450) is complex and involves two single electron transfer process. Electrons are relayed from NADPH to Cyt P450 via the flavoprotein called NADPH Cyt P450 reductase[21]. Cyt b5 is a small hemoprotein that influences the rate of some Cyt P450 catalyzed reactions[22]. The altered levels of Cyt P450 and Cyt b5 during acetaminophen administration have also been attributed to the increase in the degradation of heme by acetaminophen-induced heme oxygenase activity. Acetaminophen also affects thiol groups in cytochrome apoproteins and thus prevents heme incorporation[23]. The activity of NADPH Cyt P450 reductase depends upon the concentration of its substrate Cyt P450. Low availability of Cyt P450 might have been responsible for the reduction in the activity of NADPH Cyt P450 reductase. It has also been suggested that, once exposed acutely to xenobiotics such as acetaminophen, hepatic cytochrome P450 can undergo oxidative destruction through its active intermediates and can induce carbon monoxide (CO) overproduction mediated by hemeoxygenase 2 (HO-2), even when the inducible enzyme is not over-expressed[24,25].
The rats pretreated with S. polycystum extract showed appreciable prevention in the acute impairment of microsomal enzyme activities when compared with acetaminophen-intoxicated rats. Thus, it is evident in this study that near normal activity of NADPH Cyt P450 reductase indirectly lends support to the hypothesis that NADPH Cyt P450 reductase may be the locus within the cytochrome P450 electron transport chain, which is most susceptible to peroxidative attack by free radicals[26]. A hypothesis states that bioactive compounds that are able to optimize the microsomal enzyme function can provide protection against the hepatotoxicity only when they are given before the metabolic activation of the hepatotoxin and fail to afford any protection after the generation of reactive metabolites[27]. These observations contemplate that the seaweed extract probably acted against acetaminophen-induced microsomal lipid peroxidation triggered during toxic hepatitis.
Covalent protein modification, and free radical damage due to acetaminophen metabolism have been identified as pathways leading to hepatocyte damage; however, evidence has also been published that TNF-α may participate in the processes causing liver failure, because the administration of anti-TNF-α antibodies to acetaminophen-treated animals ameliorated the enzyme leakage during the early phase of the intoxication[28,29].
Overdoses of acetaminophen depleted glutathione stores, leading to accumulation of NAPQI, mitochondrial dysfunction[30] and the development of acute hepatic necrosis. The depletion of glutathione enhances the expression of TNF-α[31]. TNFQ primes phagocytic NADPH oxidase to the enhanced production of oxygen-free radicals and contributes to liver damage[32]. Acetaminophen caused a moderate sinusoidal perfusion failure (-15%) and infiltration of neutrophils along with activation of nuclear factor-kappaB (NF-κB) and intercellular adhesion molecule-1 and cytokine-induced neutrophil chemoattractant-1 mRNAs.
Reactive oxygen species (ROS) activate NF-κB by releasing the inhibitory subunit from NF-κB complex[33]. Increase in the production of ROS during acetaminophen metabolism leads to activation of NF-κB and increase in the synthesis of TNF-α. The animals pretreated with S. polycystum extract showed considerable inhibition in the elevation of TNF-α, which may be attributed to the inhibition of excessive reactive metabolite by their antioxidant nature.
The electron microscopic observation demonstrated the contribution of multiple factors toward the development of toxic hepatitis, and showed the damage in nuclear membrane with swelling of mitochondria in acetaminophen-intoxicated rats. The increase in lipid peroxides observed in microsomal membrane might be viewed as an additional index of alteration of membrane permeability, suggesting that oxidative modifications of unsaturated lipids are also detectable in vivo after AAP administration. The respiratory enzyme NADH dehydrogenase is located in the mitochondrial membrane[34]. NAPQI, the toxic metabolite of acetaminophen, arylates and oxidizes essential protein sulfhydryls in the mitochondrial respiratory chain, thereby limiting the ability of the mitochondria to meet the energy demand of the cell and affecting cellular energy homeostasis[35], which is consistent with our electron microscopic observation which depicts remarkable morphological alterations of mitochondria. The appearance of lipid droplets and sinusoids, concurrent with the evolution of hepatic necrosis in acetaminophen-induced rats, represents the adaptive response to the disturbance of phospholipid metabolism induced by potentially toxic stimuli. In addition, the acetaminophen-induced secondary lipidosis as reflected by the accentuated lamellated dense bodies is likely to result from the binding of the toxic metabolite with membrane phospholipids. The exact mechanism of the aforementioned relationship is still elusive. The rats pretreated with S. polycsytum considerably prevented the alterations in liver cell structural integrity triggered by acetaminophen, thereby showing that the extract contain bioactive compounds which may have a role in modulating the mitochondrial functional status and lipid metabolizing enzymes[36,37]
In conclusion, the defensive action of the seaweed extract may be due to their anti-hepatotoxic and antioxidant property against the acetaminophen-induced severe impairment in the drug metabolizing microsomal enzyme system and inhibitory action on TNF-α. The extract also possesses promising protection against acetaminophen-induced alterations in liver cell structural integrity during toxic hepatitis. Even though the extract possesses a promising preventive mode of efficacy against acetaminophen toxicity, the mechanism of its action is still elusive. Presently, we have isolated 8 fractions from the S. polycystum extract and their structural identification using nuclear magnetic resonance (NMR) and high performance liquid chromatography (HPLC), and the mechanism of action in liver protection is under way.