INTRODUCTION
There is a close relationship between the endocrine system and the liver, in which processes such as hormonal inactivation, synthesis of hormonal binding proteins, and growth factors take place. The insulin-like growth factor (IGF) system is an attractive target of study, since it may be modified by liver diseases and, reciprocally, it might play a relevant role in the progression of some hepatic diseases. This system is comprised of two ligands, IGF-I and IGF-II, their specific receptors, IGF-IR and IGF-IIR, and the IGF-binding proteins (IGFBPs)[1-4]. IGFs are peptides involved in proliferation, differentiation and inhibition of apoptosis of several cell types[5]. IGF-IR has been shown to be capable of mediating both IGF-I and IGF-II signalling, whereas IGF-IIR (mannose 6-phosphate) is only able to decrease the bioavailability of IGF-II[6].
Circulating IGF-I is synthesized primarily by the liver in response to growth hormone, but most tissues express IGF-I, which exerts autocrine and paracrine functions[7]. Within the normal adult liver, IGFs are believed to play relatively little effect, since IGF-II expression is down-regulated and IGF-I, although highly expressed, does not exert its actions due to low IGF-IR expression on hepatocytes[8]. Therefore, liver is the organ with the highest levels of IGF-I expression, while it exhibits almost undetectable levels of IGF-IR mRNA. This pattern of expression may be partially explained by down-regulation of IGF-IR promoted by locally produced IGF-I[9]. In contrast to hepatocytes, it is believed that the presence of IGF-IR in non-parenchymal rat liver cells (Kupffer cells, myofibroblasts and hepatic stellate cells [HSC])[10] and in human HSC[11] makes these cell types susceptible to IGFs mitogenic effects.
The role of IGF-I system in hepatic diseases and in liver regeneration remains uncertain. Although an elevation of IGF-IR expression during progression of preneoplastic hepatic foci to hepatocellular carcinoma (HCC) has been reported[12], studies using cultured HCC cells demonstrated a failure of IGF-I to stimulate mitogenesis[10,13,14]. On the other hand, hepatic over-expression of the IGF-II gene has been observed in animal models of hepatocarcinogenesis[15,16] as well as in human HCV-induced cirrhosis and HCC[17-19]. Increased IGF-IR gene expression was observed in HCC and in human hepatoma cell lines[8,12,20], however, there are few studies evaluating different chronic hepatitis C (CHC) staging and cirrhosis concerning IGF-IR expression[11,18]. Therefore, the present study was designed to semi-quantify the hepatic expression of IGF-IR and IGF-I in patients with CHC, searching for possible correlations with histopathological lesions in each compartment of hepatic acini.
MATERIALS AND METHODS
Patients and tissue samples
A series of 34 patients with CHC was evaluated at the Clinical Hospital of the University of Sao Paulo Medical School. The diagnosis of CHC was based on a positive serological test for HCV by at least a second-generation enzyme-linked immunoabsorbent assay, positive HCV-RNA by polymerase chain reaction (PCR) assay, and compatible liver biopsy. All patients received combined treatment with interferon-α (3 million U three times a week) and ribavirin (1000-1200 mg/d, accordingly to body weight) for 48 wk, since genotyping was not available. Hepatic tissue fragments obtained before treatment were divided in two samples: one was formalin-fixed paraffin-embedded for histopathological and for immunohistochemical studies; the other was collected in sterile containers and frozen in liquid nitrogen for RNA extraction. Additionally, in 10 patients, liver biopsy was performed within one month after the end of treatment. Among the 34 treated patients, 14 (41.1%) patients reached sustained virological response, 16 (47.05%) patients were non-responders and 4 (11.76%) patients relapsed in the follow-up period. Among the 10 patients histologically evaluated in the post-treatment period, 5 (50%) patients were sustained virological responders and 5 (50%) patients were non-responders.
In order to obtain normal hepatic tissue, hepatic fragments were collected from six cadaveric liver donors (8-12 h of cold ischemia) following orthopic transplantation. The organs obtained from the six cadaveric donors were non-diseased liver transplanted in patients presenting HCV-related end-stage liver disease. The transplants were performed at Experimental Surgery Division, Clinical Hospital, University of Sao Paulo Medical School.
This study was approved by the Ethical Committee of the University of Sao Paulo Medical School and informed consent was obtained from all patients.
Histological and immunohistochemical analyses
Formalin-fixed paraffin-embedded samples were sectioned at a thickness of 3 μm, stained by hematoxylin and eosin (H&E), Masson’s trichrome staining and reticulin impregnation, and evaluated by a single pathologist. Histopathological classification was based on criteria defined by the Brazilian Consensus on Chronic Hepatitis[21], which assesses individual histologic variables from zero (normal) to four (maximal degree of lesion): staging of architectural disturbances/fibrosis, necro-inflammatory lesions in portal tract, interface hepatitis and parenchymal lesions. Steatosis was also graded as follows: 0 = no fat deposition in hepatocytes; 1 = up to 25% of hepatocytes; 2 = 25%-50%; 3 = 51%-75%; and 4 = more than 75% hepatocytes presenting fat vacuoles.
Immunohistochemistry for IGF-IR was performed on sections deparaffinized with xylene, dehydrated with graded ethanol, incubated in 3% H2O2 to block endogenous peroxidase activity. To improve the staining pattern, antigen retrieval was obtained by heating in 0.5 mol/L EDTA (pH 8.0). The sections were incubated with Protein Block System and Biotin Blocking System (X0909, DAKOCytomation, USA). The primary antibody, mouse monoclonal IGF-IR (α-subunit) Ab-1 (Clone 24-31) (LAB VISION Corporation, Westinghouse, USA) at a dilution of 1:50 was incubated overnight. Signal amplification was achieved by kit LSAB-HRP (Labelled Streptavidin-Biotin) (DAKOCytomation, USA). Chromogen solution included 3, 3-diaminobenzidine tetrahydrochloride (DAB) (Sigma Chemical Co., USA). A sample from liver with cholangiocarcinoma was considered as a positive control and a normal liver sample as negative control. The staining was graded according to the estimative of fraction of positive cells of the interlobular bile ducts, lymphocytes from portal tracts and lobular areas, Kupffer cells, hepatocytes, endothelial cells and ducts cells from periportal areas (canals of Hering). Scores were predefined as follows: 0 = without staining; 1 = small amount of immuno-positive liver cells; and 2 = large amount of immuno-positive liver cells.
Semi-quantitative RT-PCR
After tissue pulverization with a dismembrator (B. Braun Biotech International, Melsungen, Germany) at liquid nitrogen temperature, total RNA was extracted using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, USA) according to the manufacturer’s instructions. After phenol treatment and drying, RNA was dissolved in RNase-free water and RNA concentration was spectrophotometrically determined. RNA quality was checked on agarose gel electrophoresis. The expressions of IGF-IR and IGF-I in the tissue series were performed by using semi-quantitative RT-PCR. Total RNA (3 μg) was reverse-transcribed into complementary DNA (cDNA) using the SuperScripTM II Reverse Transcriptase and random primers (Invitrogen Life Technologies) according to manufacturer’s recommendations and diluted with ddH2O to a final volume of 20 μL. The optimal number of PCR cycles was determined for each primer set so that the amplification process was conducted during the exponential phase of amplification. Concomitant amplification of the break-point control region (BCR) gene transcript was used as the internal control to stringently control for any variability in RNA degradation and RT efficiency. Reactions were carried out in a 45 μL final reaction volume containing 3 μL of cDNA template, 20 mmol/L Tris-HCl (pH 9.0), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.22 mmol/L deoxynucleotide triphosphates (dNTPs), 2.0 U Taq DNA polymerase (GE Healthcare-Amersham plc, Buckinghamshire, UK) and specific primers for the target genes and for BCR gene. In order to achieve exponential co-amplification for both IGF-IR and IGF-I mRNA expressions in relation to the endogenous internal control (BCR) at the same number of cycles, sense and antisense primer concentrations were appropriately adjusted between 0.22 and 0.44 μmol/L. PCR amplifications for both genes were performed under the following cycling conditions: initial denaturation at 94°C for 2 min 30 s, denaturation at 94°C for 30 s, annealing at specific temperature 57°C for 1 min and extension at 72°C for 1 min 30 s, followed by a final extension at 72°C for 1 min 30 s. The number of cycles to achieve the exponential phase was 40. As a positive control for IGF-IR and IGF-I, human placenta was used. For comparison purpose, normal human liver cDNA was commercially acquired (Human Liver QUICK-Clone cDNA [Clontech Laboratories, Inc., Palo Alto, CA, USA]) and, according to the manufacturer’s instructions, cDNA was generated from Poly A+ RNA from samples obtained from two normal human livers. The PCR products were submitted to electrophoresis through a 20 g/L agarose gel containing ethidium bromide and visualized under ultraviolet light. The band intensities were analysed by Molecular Analyst Software (Bio-Rad Laboratories, Hercules, USA). The expression levels of IGF-IR and IGF-I were normalized by the BCR housekeeping gene expression and the ratios of gene expression (IGF-IR or IGF-I/BCR) were calculated in all samples as arbitrary units of optical density (AU). The primers were designed with the primer3_http://www.cgi v 0.2 program[22] as follows: BCR (377-bp product): 5’-GAG AAG AGG GCG AAC AAG-3’ (sense) and 5’-CTC TGC TTA AAT CCA GTG GC-3’ (antisense); IGF-IR (230-bp product): 5’-ACC CGG AGT ACT TCA GCG CT-3’ (sense) and 5’-CAC AGA AGC TTC GTT GAG AA-3’ (antisense); IGF-I (303-bp product): 5’-TCT TGA AGG TGA AGA TGC ACA CCA-3’ (sense) and 5’-AGC GAG CTG ACT TGG CAG GCT TGA-3’ (antisense).
Statistical analysis
Data were tested for statistical significance using the JMP version 5.1 statistical computer program (SAS Institute Inc. Cary, NC, USA). Since assumptions for a parametric test were not valid (Kolmogorov-Sminov, P < 0.05), all data were evaluated by Kruskall-Wallis analysis of variance and the Mann-Whitney U test as a multiple comparison method. The Spearman test was used to assess the statistical significance of correlations among the different variables tested. Chi-square and Fisher’s exact tests were used to establish statistical relationship between groups regarding histopathological and immunohistochemical findings. P values less than 0.05 were considered statistically significant.
RESULTS
Immunohistochemical analysis of IGF-IR
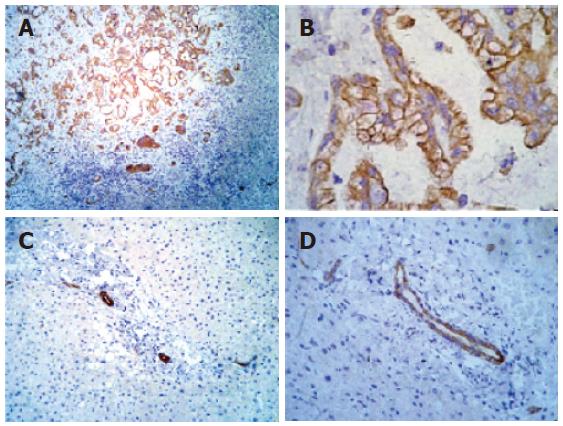
A section of cholangiocarcinoma tissue served as positive control for immunohistochemical analysis of IGF-IR. The positive pattern was observed in cytoplasmic membrane of the neoplastic duct epithelial cells (Figures 1A and 1B). Since positive staining for IGF-IR was detected in ductal cells, they served as internal control, both in normal liver where IGF-IR expression was not detected in hepatocytes (Figures 1C and 1D) and in chronic hepatitis liver samples. In CHC, an expressive increase in IGF-IR immunoreactivity was observed in membrane and in cytoplasm of proliferated bile ducts and in hepatocytes cytoplasm located in all acinar zones (Figures 2A-D). However, nuclear staining was not detected.
Figure 1 Immunohistochemical analysis of IGF-IR in liver tissue.
A: Positive control cholangiocarcinoma tissue showing strongly positive staining for IGF-IR in neoplastic ducts (-100 ×); B: positive control cholangiocarcinoma tissue showing strongly positive staining for IGF-IR in neoplastic ducts (-200 ×); C: normal liver tissue showing IGF-IR immunoreactivity in ductal cells (-100 ×); D: normal liver tissue showing IGF-IR immunoreactivity in ductal cells (-200 ×).
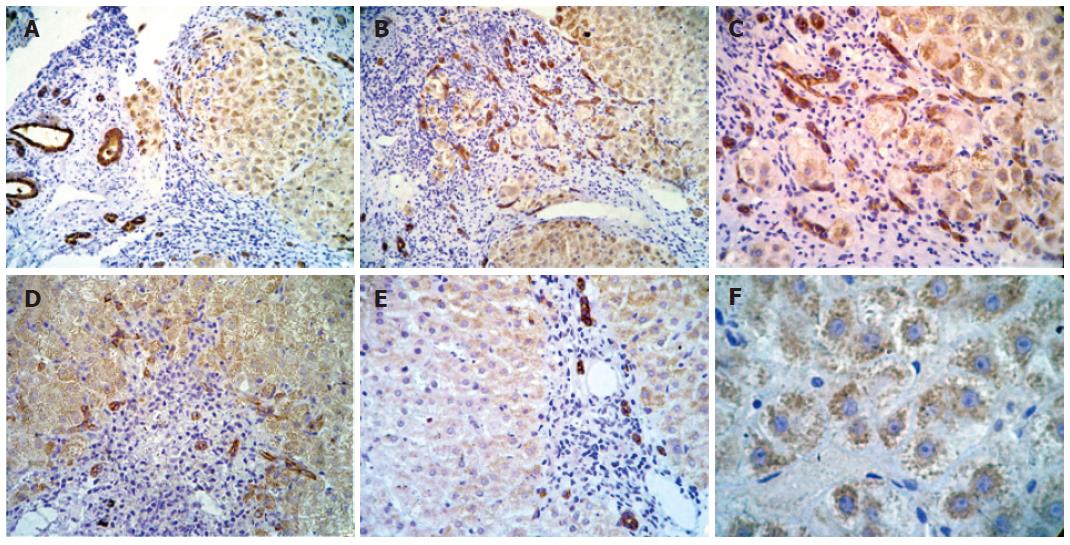
Figure 2 Immunohistochemical analysis of IGF-IR in different samples of liver.
A: Strong positive staining in cytoplasm of hepatocytes in all acinar zones (-100 ×); B: strong positive staining in cytoplasmic membrane and cytoplasm of proliferate bile ducts and in cytoplasm of hepatocytes located in all acinar zones (-100 ×); C: strong positive staining in cytoplasmic membrane and cytoplasm of proliferate bile ducts and in cytoplasm of hepatocytes located in all lobular regions (-200 ×); D: strong positive staining in cytoplasmic membrane and cytoplasm of proliferate bile ducts and in cytoplasm of hepatocytes located in all acinar zones (-100 ×); E: strong positive staining in periportal duct cells (-100x); and F: granular pattern of positivity in cytoplasm of hepatocytes in all lobular regions (-400 ×).
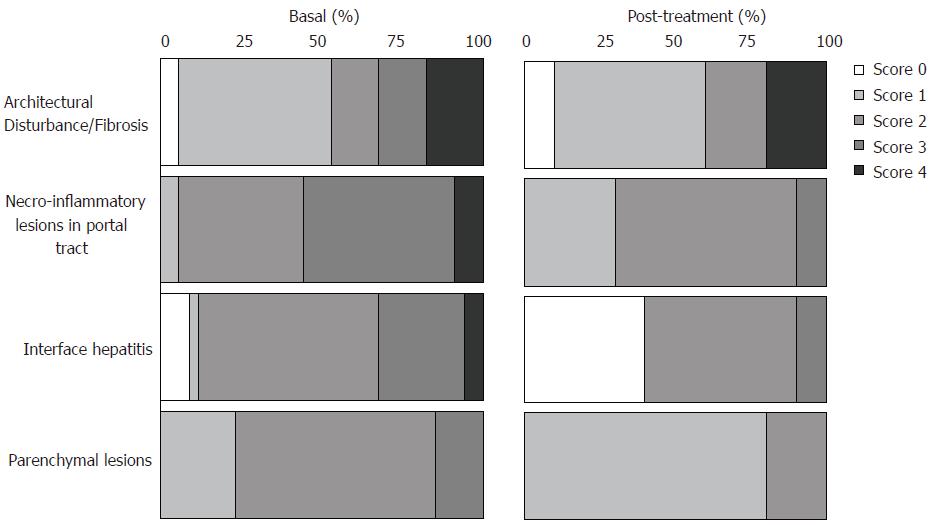
Histological and immunohistochemical findings before treatment (samples from 34 patients) and post-treatment (samples from 10 patients) are shown in Figures 3 and 4. Immunohistochemical analyses demonstrated of IGF-IR positivity in hepatocytes in 85% (29/34) samples of CHC before treatment; twenty-three of these 29 samples showed positive immunostaining in hepatocytes located in all regions of the lobe (zones I, II and III of the Rappaport’s acinus), while in 6 of these 29 samples revealed positive immunostaining with grade 1 in periportal region, zone 1 (Figure 2E). On contrary, no IGF-IR positivity in hepatocytes was observed in 15% (5/34) samples of CHC before treatment.
Figure 3 Percentage of score distribution obtained by histological analyses in liver biopsies from pre- (34 cases) and post-treatment (10 cases, 5 sustained virological responders and 5 non-responders) according to Brazilian Consensus on Chronic Hepatitis[21].
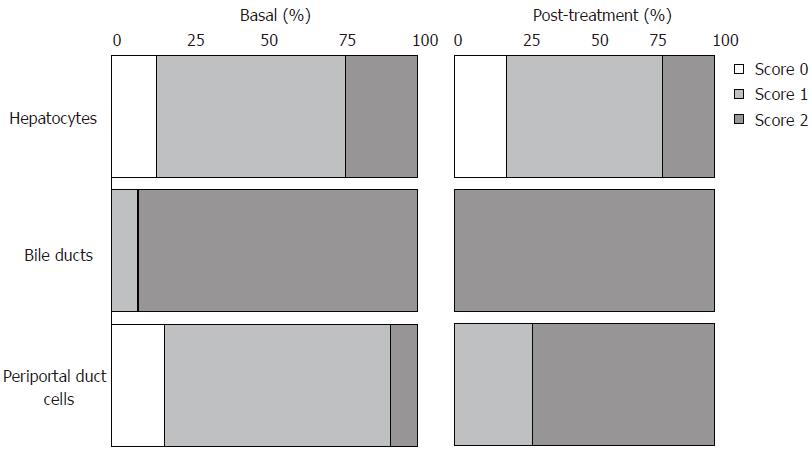
Figure 4 Percentage of score distribution obtained by IGF-IR immunohistochemical analyses in liver biopsies from pre- (34 cases) and post-treatment (10 cases, 5 sustained virological responders and 5 non-responders).
Among the patients with fibrosis of degree 0, 1 and 2, 13% (3/23) did not show positivity in hepatocytes, 70% (16/23) showed weak staining and 17% (4/23) showed intense staining. Within the group with fibrosis of degree 3 and 4, 9% (1/11) did not show any staining, 55% (6/11) showed weak staining and 36% (4/11) showed intense staining. In some cases, especially in cirrhotic livers, a granular pattern of positivity in hepatocytes cytoplasm from all lobular regions was visualized (Figure 2F).
All of the pre-treatment samples (34/34) showed IGF-IR positivity in bile ducts, with strong positivity (degree 2) in 91% (31/34) of the positive cases. Twenty-eight of 34 (82%) samples showed IGF-IR positivity in periportal duct cells (canals of Hering), with weak positivity (degree 1) in 25 of 28 samples and strong positivity (degree 2) in 3 of 28 samples.
Only 50% (17/34) and 15% (5/34) of the samples showed positivity in portal lymphocytes and Kupffer cells, respectively, with a weak (degree 1) positivity pattern. No staining in lobular lymphocytes and in endothelial cells was observed.
Histopathological and immunohistochemical variables did not show any statistically significant correlation. There were no statistically significant differences on IGF-IR immunohistochemical pattern in hepatic tissues from patients with and without sustained virological response between pre- and post-treatment periods (Figure 4).
mRNA expression of IGF-IR and IGF-I
An increased IGF-IR mRNA content was detected in hepatic tissue of all 34 patients with CHC before treatment as compared with normal liver (Clontech Laboratories) which did not express IGF-IR mRNA (Figure 5). There were no relevant modifications in IGF-I mRNA content in hepatic tissue from patients with CHC as compared with normal liver, however, since there was only one sample of normal hepatic tissue, it was not possible to perform a statistical evaluation (data not shown).
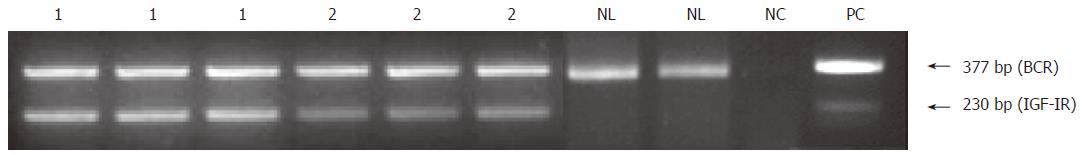
Figure 5 Semi-quantitative RT-PCR analysis of IGF-IR mRNA in hepatic fragments obtained from 2 patients with chronic hepatitis C (lanes 1 and 2) and normal human liver (NL).
The experiments for the patients with CHC were carried out in triplicates and for normal human liver (NL) in duplicates. Human placenta was used as a positive control (PC) and no DNA template as negative control (NC).
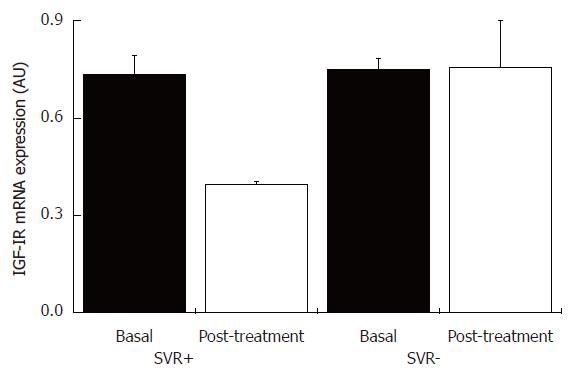
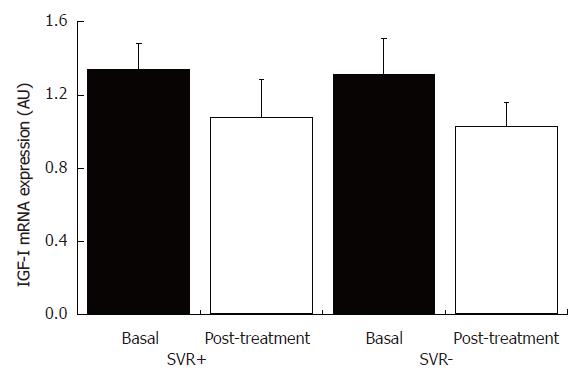
IGF-IR and IGF-I mRNA contents were evaluated in hepatic tissue obtained within one month of the end of treatment in ten patients. There was a statistically significant decrease (P = 0.05) in mean IGF-IR mRNA content in hepatic tissues from patients who achieved sustained virological response in comparison to non-responders (Figure 6), while there was no statistically significant difference in IGF-I mRNA content in hepatic tissues from patients with and without sustained virological response between pre- and post-treatment periods (Figure 7). Furthermore, no statistically significant correlations were observed between IGF-IR and IGF-I mRNA contents and histopathological or immunohistochemical variables.
Figure 6 IGF-IR mRNA content in liver tissue from patients with chronic hepatitis C with and without sustained virological response, and pre- and post-treatment with INF-α and ribavirin.
SVR+: With sustained virological response (n = 5); SVR-: without sustained virological response (n = 5).
Figure 7 IGF-I mRNA content in liver tissue from patients with chronic hepatitis C with and without sustained virological response, and pre- and post-treatment with INF-α and ribavirin.
SVR+: With sustained virological response (n = 5); SVR-: without sustained virological response (n = 5).
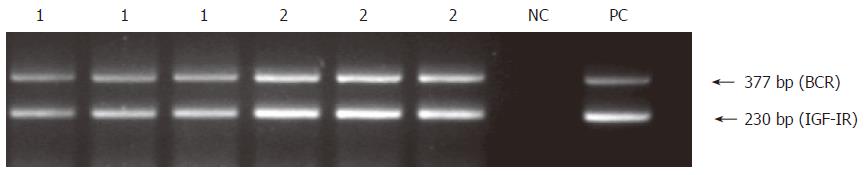
In an attempt to better characterize the expression pattern of these two genes in normal hepatic tissues, IGF-IR and IGF-I mRNA contents were evaluated in hepatic fragments from six cadaveric liver donors (8-12 h of cold ischemia) following orthopic transplantation. Interestingly, despite being considered as normal livers by histopathological examination, all samples presented an increased IGF-IR mRNA content (Figure 8), regardless the degree of ischemia-reperfusion injury, which ranged from 1 to 3, accordingly to Gayotto & Leitão classification[23]. In addition, there were no relevant modifications in IGF-I mRNA content in hepatic tissue from liver donors following orthopic transplantation in comparison to normal liver (data not shown).
Figure 8 Preservation-reperfusion injury: Representative results of semi- quantitative RT-PCR analysis of IGF-IR mRNA in 2 hepatic fragments from cadaveric liver donor following orthopic transplantation (lanes 1 and 2).
The experiment was carried out in triplicates. Human placenta was used as a positive control (PC) and no DNA template as negative control (NC).
DISCUSSION
Although almost all tissues and cells express IGF-IR during embryogenesis, in the normal adult liver, IGF-IR mRNA is practically undetectable[5] and very low IGF-I binding has been shown in cultured hepatocytes[8], possibly due to IGF-IR down-regulation by the locally produced IGF-I. However, hepatic non-parenchymal cells, such as Kupffer cells, endothelial cells and HSC, express IGF-IR and are susceptible to their mitogenic effects[11,24,25].
In the current investigation, we demonstrated an increase of IGF-IR mRNA content in hepatic tissue from patients with different CHC staging. The immunohistochemical findings suggested this augmented mRNA content might be secondary to ductular proliferation, since these epithelial cells expressed IGF-IR, and to increased IGF-IR expression in hepatocytes. Although IGF-IR immunoreactivity was low in Kupffer cells and in portal lymphocytes, we could not rule out that the expression of this receptor in these cell types has also contributed to increase the level of IGF-IR mRNA observed in all cases.
The proliferative response to several types of liver injury in human liver is characterized by an increase in bile duct–like structures, termed “ductular reaction”[26,27]. Ductular reaction located at the limiting plate takes place in hepatitis C[28], where a significant correlation between the extent of ductular proliferation and the severity of inflammation was observed[29]. Eleazar et al[30] have also demonstrated a positive correlation between ductular reaction and disease activity in chronic hepatitis, including CHC. Although in the current study, immunohistochemical staining for specific epithelial cell markers such as cytokeratin 19 has not been performed, IGF-IR immunostaining and H&E analysis have clearly shown ductular reaction. Previous findings showing an increase in the number of cells that originate newly proliferated ductal cells, the hepatic progenitor cells (“oval cells”), as hepatitis C severity progresses[31] as well as the recent report of a strong correlation between portal fibrosis and periportal ductular reaction with progenitor cells expansion in CHC[32] illustrate the need for further studies addressing the mechanisms underlying progenitor cells activation and liver remodelling in CHC.
The up-regulation of IGF-IR expression in hepatocytes of patients with CHC could constitute an attempt to stimulate hepatocyte regeneration in response to liver damage. The role of IGF-IR in liver regeneration following subtotal hepatectomy has been demonstrated in animal models. Caro et al[8] showed a significant increase in IGF-I binding in freshly isolated hepatocytes from regenerating adult rat liver. In addition, Santos et al[33] demonstrated both a transient increase in IGF-I binding to liver membranes and an increase on IGF-IR mRNA concentrations. Up-regulation of IGF-IR as well as IGF-I was also observed in hepatocytes of rats chronically treated with carbon tetrachloride (CCl4) and immunoreactivity increased proportionally to fibrosis development, which might be a compensatory reaction to the continuous loss of hepatocytes[34]. However, the role of IGF-I system in the injured liver has not been completely understood. While experiments in cultured HSC support the participation of IGF-I in HSC transformation toward a fibrogenic phenotype, promoting HSC proliferation[25,35] and collagen synthesis[11], targeted over-expression of IGF-I in activated HSCs attenuates fibrogenesis and accelerates liver regeneration after acute CCl4-induced injury. These effects appear to be mediated in part by up-regulation of HGF and down-regulation of TGF-β1, suggesting that IGF-I can modulate the cytokine response to liver injury, facilitating regeneration and reducing fibrosis[36]. Based on our finding of increased IGF-IR expression even after an acute challenge such as ischemia-reperfusion injury, we question if a direct effect of HSC-derived IGF-I on hepatocytes expressing IGF-IR might contribute to the accelerated hepatic regeneration observed in this model. Additionally, Desbois-Mouthon et al[37] have recently reported that hepatocyte proliferation during liver regeneration is impaired in liver-specific IGF-IR knockout mice as evidence that IGF-IR contributes to liver regeneration.
In the liver, it has been shown that ischemia-reperfusion injury occurs as biphasic pattern, with an initial acute phase characterized by hepatocellular damage at 3-6 h and a subacute phase characterized by massive neutrophil infiltration at 18-24 h[38]. The ischemia-reperfusion injury also induces the activation of intracellular pathways clearly involved in the hepatocellular regenerative response[39], such as expression of tumor necrosis factor α (TNF-α)[40,41] and IL-6-associated signalling pathways[42], in an attempt to replace cells lost to ischemic and immunologic damage[43]. To our knowledge, augmented IGF-IR expression has never been reported in animal or human livers submitted to ischemia-reperfusion injury, while it was described in a murine model of renal ischemia-reperfusion injury[44]. Considering that liver is the organ with the highest levels of IGF-I expression, our finding of increased IGF-IR expression in an acute model of hepatic damage such as ischemia-reperfusion injury as well as in the condition of chronic hepatitis C highlights the need for additional studies to elucidate the role of IGF-I in the complex events of liver regeneration.
Regarding IGF-IR expression in the post-treatment period, although few patients were available for this analysis, the decrease in IGF-IR mRNA content observed in the patients who achieved sustained virological response suggests an improvement in hepatic injury without returning to the expression pattern observed in normal liver, which exhibits undetectable levels of IGF-IR mRNA. We hypothesized that the change on IGF-IR mRNA content in the absence of modifications on the immunostaining pattern, as well as the positivity of IGF-IR mRNA in all studied samples even in the absence of IGF-IR immunostaining may be explained by the higher sensibility of RT-PCR in comparison to immunohistochemical analysis. It remains to be clarified if, as observed for histopathological changes, longer periods following sustained virological response are require for detecting additional reduction in IGF-IR mRNA content.
Because of the important role of the IGF system in mediating not only normal growth and tissue repair but also carcinogenesis, the expression and function of all components of this system must be strictly controlled. Although we speculate that up-regulation of IGF-IR might participate in hepatocyte regeneration, the suggestion that HCV replication might mediate expression of the IGF-IR ligand IGF-II in cirrhotic livers[45] rise concerns that these two simultaneous changes could act as possible factors for HCV-associated hepatocarcinogenesis in cases of advanced hepatic lesion, since an autocrine and/or paracrine activation of the IGF-II/IGF-IR axis has been implicated in the development of HCC[46]. This hypothesis is corroborated by the finding that hepatitis B virus (HBV) X protein (HBx), which participates in the process of HBV-associated liver carcinogenesis, activates the IGF-IR gene expression[47,48]. It remains to be elucidated if a direct activation of IGF-IR gene by HCV could contribute to the increased IGF-IR expression in hepatocytes in CHC.