Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3835
Revised: March 1, 2006
Accepted: March 10, 2006
Published online: June 28, 2006
AIM: To investigate the effects of experimental partial hepatectomy and normothermic ischemia-reperfusion damage on the time course of the expression of four different growth factor receptors in liver regeneration. This is relevant due to the potential therapeutic use of growth factors in stimulating liver regeneration.
METHODS: For partial hepatectomy (PH) 80% of the liver mass was resected in Sprague Dawley rats. Ischemia and reperfusion (I/R) were induced by occlusion of the portal vein and the hepatic artery for 15 min. The epidermal growth factor receptor, hepatic growth factor receptor, fibroblast growth factor receptor and tumour necrosis factor receptor-1 were analysed by immunohistochemistry up to 72 h after injury. Quantitative RT-PCR was performed at the time point of minimal receptor expression (24 h).
RESULTS: In immunohistochemistry, EGFR, HGFR, FGFR and TNFR1 showed biphasic kinetics after partial hepatectomy with a peak up to 12 h, a nadir after 24 h and another weak increase up to 72 h. During liver regeneration, after ischemia and reperfusion, the receptor expression was lower; the nadir at 24 h after reperfusion was the same. To evaluate whether this nadir was caused by a lack of mRNA transcription, or due to a posttranslational regulation, RT-PCR was performed at 24 h and compared to resting liver. In every probe there was specific mRNA for the receptors. EGFR, FGFR and TNFR1 mRNA expression was equal or lower than in resting liver, HGFR expression after I/R was stronger than in the control.
CONCLUSION: At least partially due to a post-transcrip-tional process, there is a nadir in the expression of the analysed receptors 24 h after liver injury. Therefore, a therapeutic use of growth factors to stimulate liver regeneration 24 h after the damage might be not successful.
- Citation: Baier P, Wolf-Vorbeck G, Hempel S, Hopt U, Dobschuetz EV. Effect of liver regeneration after partial hepatectomy and ischemia-reperfusion on expression of growth factor receptors. World J Gastroenterol 2006; 12(24): 3835-3840
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3835.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3835
Cytokines, hormones and growth factors are known to be involved in the liver’s regeneration. Until now, therapeutic use of growth factors, or anything else capable of stimulating regeneration in vivo, has not been successful[1]. Since not only ligands like cytokines, hormones and growth factors but also receptors could limit the influence of these factors, the aim of our research was the kinetics of growth factor receptors in liver regeneration. Epidermal growth factor receptors (EGFR) and hepatocyte growth factor receptors /c-Met (HGFR) are activated immediately after partial hepatectomy (PH)[2,3]. Research with c-met knockout mice demonstrates impaired liver regeneration after PH or toxic injury[4,5]. However, it seems that other signal transduction pathways could compensate for the lack of HGFR and induce liver growth. Furthermore, impaired liver regeneration in complicated situations seems to be more the consequence of alterations of the receptor site of the signalling pathway rather than a result of a decreased amount of ligands. In a model of fulminant hepatic failure due to a combination of PH and right liver lobe necrosis, Mizuguchi et al[6] found lower c-met expression compared to PH alone, but elevated levels of cytokines. The same is true after hepatectomy in rats with CCL(4)-induced cirrhosis[7,8]. HGF over-expression when c-Met is not altered leads to tumour formation in transgenic mice but could not promote liver regeneration[8]. From these references we hypothesised, that growth factor receptors, more than their ligands, are limiting in liver regeneration and therefore, it is more reasonable to look for the expression of the receptors.
There are several growth factor receptors, other than HGFR/c-Met, like EGFR, fibroblast growth factor receptor (FGFR) and tumour necrosis factor alpha receptor 1 (TNFR1) involved in liver regeneration about which, even less is known.As we know from recent experiments with transgenic animals, these receptors are required in liver regeneration[9,10]. Their kinetics during liver regeneration were unknown until now. Therefore, we analysed the kinetics of EGFR, HGFR, FGFR and TNFR1 in two models of liver regeneration: PH and reperfusion after warm 15 min ischemia (I/R).
The experiments were performed in male Sprague-Dawley rats weighing 350-500 g (Charles River, Sulzfeld, Germany) in accordance with the local regulation for animal experiments. All procedures were performed under isoflurane (Abbott, Wiesbaden, Germany) inhalation anaesthesia 2.5%-4%.
Partial hepatectomy (PH) was conducted after midline laparotomy. About 80% of the liver mass was resected, following ligation with a 3-0 polyglycolic acid ligature (Dexon,Braun, Melsungen, Germany). Then, the incision was closed by using a continuous two-layer suture technique.
Ischemia and reperfusion (I/R) were induced after midline laparotomy, by occlusion of the portal vein and the hepatic artery near the liver, by a vessel loop (Ethicon, Norderstedt, Germany). Care was taken not to occlude the bile duct. After 15 min, the loop was removed and the incision closed.
In each of the two models, regeneration after the injury was analysed at the following time points 0 h, 5 h, 2 h, 6 h, 12 h, 24 h, and 72 h. Each group consisted of five animals.
After regeneration, euthanasia was performed under general anaesthesia by blood collection and hepatectomy.
Receptors were identified by immunohistochemistry using 4 μm paraffin sections cleared with Rotihistol (Roth, Karlsruhe, Germany), passed through a graduated series of alcohol, and incubated for 5 min in room temperature water to rehydrate. Antigen retrieval for EGFR was accomplished using a microwave at 750 watts in target retrieval solution (pH 9.9) (vectastain, vectorlab, Burlingame, USA) and for TNFR1, HGFR, FGFR by incubation in 0.05% proteinase K for 15 min. Endogenous peroxidase activity was halted using 3% peroxide in methanol for 30 min, followed by endogenous biotin bound to avidine for 10 min and finally, in biotin for 10 min; non-specific binding was blocked by 1% BSA (Sigma, Munich, Germany). The antibodies for staining receptors were anti-TNFR1 (H-5, Santa Cruz Biotechnology, Santa Cruz, USA ), anti-EGFR (1161-1186,Calbiochem, San Diego, USA), anti-Met (B-2; Santa Cruz Biotechnology, Santa Cruz, USA), and anti-FGFR (Ab-1, Oncogene, Boston, USA). Antibodies were used at a dilution of 1:50-1:100 in PBS. Detection was performed using an avidine -biotin peroxidase system (vectastain, vectorlab, Burlingame, USA) with anti-mouse or anti-rabbit antibodies, and stained with 3-amino-9-rthyl-carbazole (Carbazol, Sigma, Munich, Germany).
Zone-selective cell isolation was performed after periportal or perivenous cell damage by ante- or retrograde perfusion with digitonin as described by Lindros[11].The portal vein and vena cava superioris were canulated under anaesthesia and washed out with approx. 300 mL oxygenated buffer I (5.36 mmol/L KCl, 1.17 mmol/L MgSO4, 0.79 mmol/L Na2HPO4, 0.15 mmol/L KH2HPO4, 10 mmol/L Hepes, 145 mmol/L NaCl, 0.1% glucose, and 1 mmol/L CaCl2, at pH 7.4). Afterwards, the liver was perfused via the portal vein or vena cava with 4 mM digitonin in oxygenated buffer I for 1-1.5 min. In a third step, the liver was washed out with 900 mL buffer II (5.36 mmol/L KCl, 0.77 mmol/L MgSO4, 0.93 mmol/L MgCl2, 0.34 mmol/L Na2HPO, 0.44 mmol/L KH2HPO, 0.2 mmol/L EDTA, 10 mmol/L Hepes, and 145 mmol/L NaCl, at pH 7.4). Afterwards, the liver was perfused with collagenase (0.025%) in buffer III (5.36 mmol/L KCl, 0.77 mmol/L MgSO4, 0.93 mmol/L MgCl2 , 0.34 mmol/L Na2HPO4, 0.44 mmol/L KH2HPO4, 10 mmol/L Hepes, 145 mmol/L NaCl, 0.2% glucose, 0.003% penicillin, 0.005% streptomycin, and 0.2% BSA, at pH 7.4), and one minute later, 4.3 mL of 200 mmol/L CaCl2 was added.
After mincing the liver into small particles in oxygenated buffer I, the liver cell-buffer mixture was pressed through a gauze and then through a 100 μm nylon filter. The cells were washed twice with 50 mL oxygenated buffer and centrifuged at 500 rpm for 5 min at 4°C. The pellet was brought to a Percoll gradient (Percoll, Amersham Biosciences d = 1.065) and centrifuged at 500 rpm for 15 min at 20°C . Hepatocytes were collected in the pellet and washed twice with 50 mL oxygenated buffer.
The correct discrimination between PP and PC hepatocytes was checked by performing the GS assay, as GS is located strictly pericentrally[12,13] . 250 000 hepatocytes were homogenized in oxygenated buffer I. A 100 μL sample was added to 400 μL test mix (2 × 250 mmol/L imidazole buffer, 2 × 250 mmol/L l-glutamic acid, 1 × 250 mmol/L hydroxylamine hydrochloride, 1 × 1.6 mmol/L adenosine-5-diphosphate, 1 × 250 mmol/L disodiumhydrogen arsenate, 1 × 10 mmol/L manganese chloride) and incubated 16-60 min at 37°C. The reaction was stopped by 100 μL stop-mix (2.42% iron III chloride, 1.45% trichloric acid in 5.4% HCl). The mixture was centrifuged (15 min, 3300 g) and analyzed in a photometer at 540 nm. GS activity was calculated as follows:
GS activity = ΔE × V × 1000 × (ε × d ×Δt × v × c ) -1 U/g with E = extinction, V = 1.5 mL
ε = 0.04395 l* mmol-1* mm-1, d = 10 mm , t = time, v = 0.1 mL, c = protein concentration.
PC hepatocytes were used with a GS activity above 300 U/g, PP hepatocytes were used with a GS activity below 30 U/g.
RNA was isolated using the RNeasy kit (QIAGEN, Hilden, D) and quantitative PCR was performed using the SYBER-Green technique (QuantiTect, QIAGEN, Hilden, D), according to the instructions given by the manufacturer. PCR was carried out by a one-step RT-PCR with the following cycle conditions: cycle 1: Temp.: 50°C 30:00 min, cycle 2: Temp.: 95°C for 15:00 min, cycle 3: Temp.: 94°C for 0:15 min, cycle 4: Temp.: 52°C for 0:30 min, cycle 5: Temp.: 72°C for 0:30 min, cycles 3-5 were repeated 45 times. Primer design was performed by Primer 3 (primer3_http://www.results.cgi) with sequences from NCBI:
HGFR (NM-031517): gtcttcaagtagccaagggc and aacatgcagtttcttgcagc
FGFR (NM-024146): gaagagcgacttccatagcc and acactgttacctgtctgcgg
TNFR1 (BC 086413): ctttgagcctttctaacccg and agtaagacgatcggaccagg
EGFR (M37394): tggacaaccctcatgtatgc and acatagtccaggaggcaacc
As an internal standard, we used 18 s rRNA: cgt agt tcc cga cat aaa cg and cag ctt tgc aac cat act cc. The difference between the threshold cycle CT of 18 s rRNA and the mRNA of interest is shown as Δ CT. A high Δ CT represents a low amount of specific RNA and vice versa.
Data management and t-test for unpaired data were carried out using PRISM® software. In case of semi-quantitative analysis in immunohistochemistry, a statistical analysis was omitted.
Unless otherwise stated, the chemicals used were from Sigma, Munich, Germany.
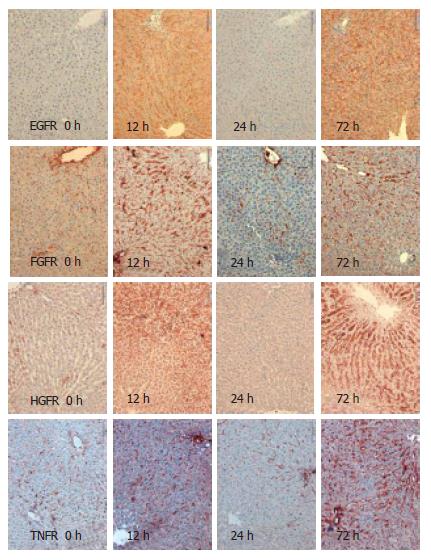
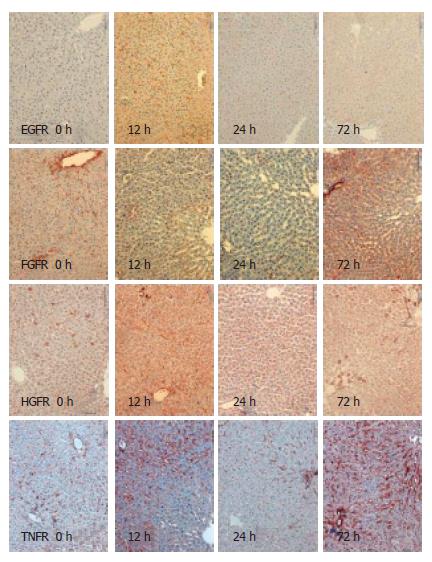
Using immunohistochemistry, we analysed the expression of EGFR, FGFR, HGFR and TNFR1 in the resting liver (tissue not influenced by proliferative stimuli) as control, during liver regeneration after partial hepatectomy, and during reperfusion after 15-min normothermic ischemia. Receptor expression was analysed semi-quantitatively with results shown in Table 1. For exemplary immunohistochemistry see Figure 1 and Figure 2. There is weak expression of FGFR but a strong one by TNFR1 in resting liver. Following PH, there is a strong expression of all receptors after 6 h and 12 h, and again after 72 h. Yet, there is no, or in case of FGFR and TNFR1, only weak expression of the receptors after 24 h. During regeneration after I/R, there is only a short and weak expression of EGFR, HGFR and TNFR1 in the first 12 h. TNFR is expressed strongly after 72 h again. After PH, as after I/R, there was no expression of any receptor, stronger than in the control after 24 h.
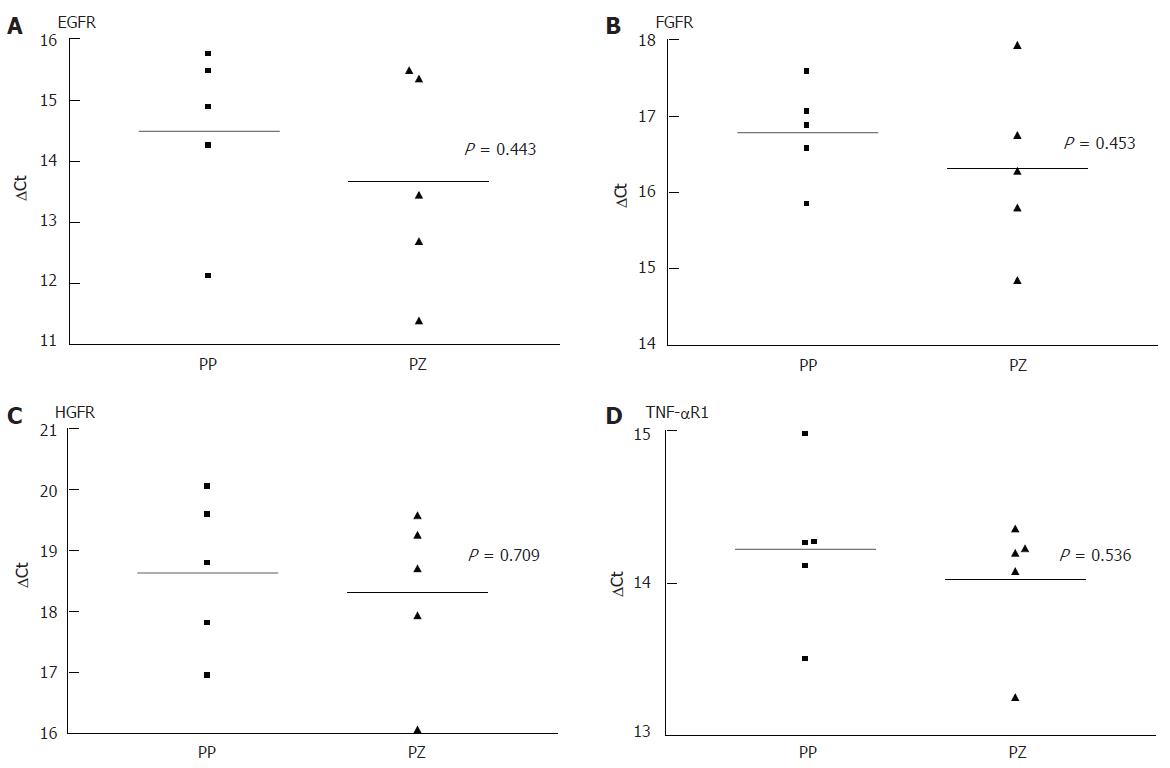
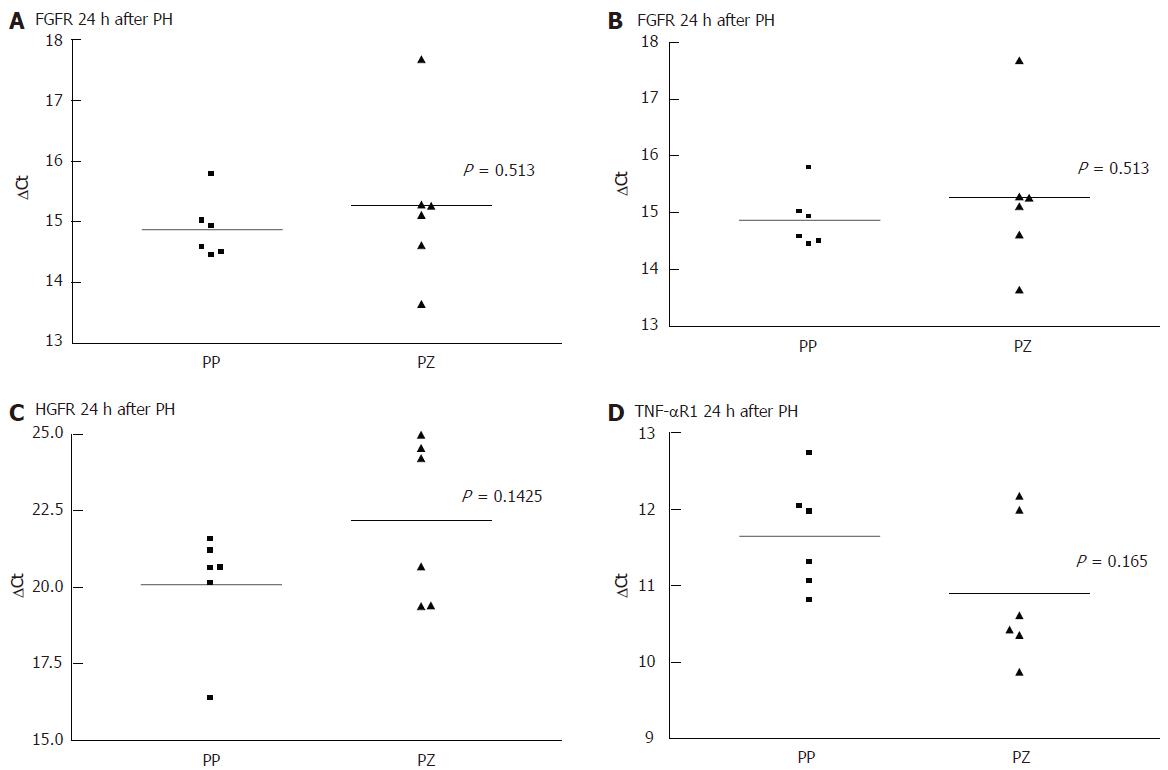
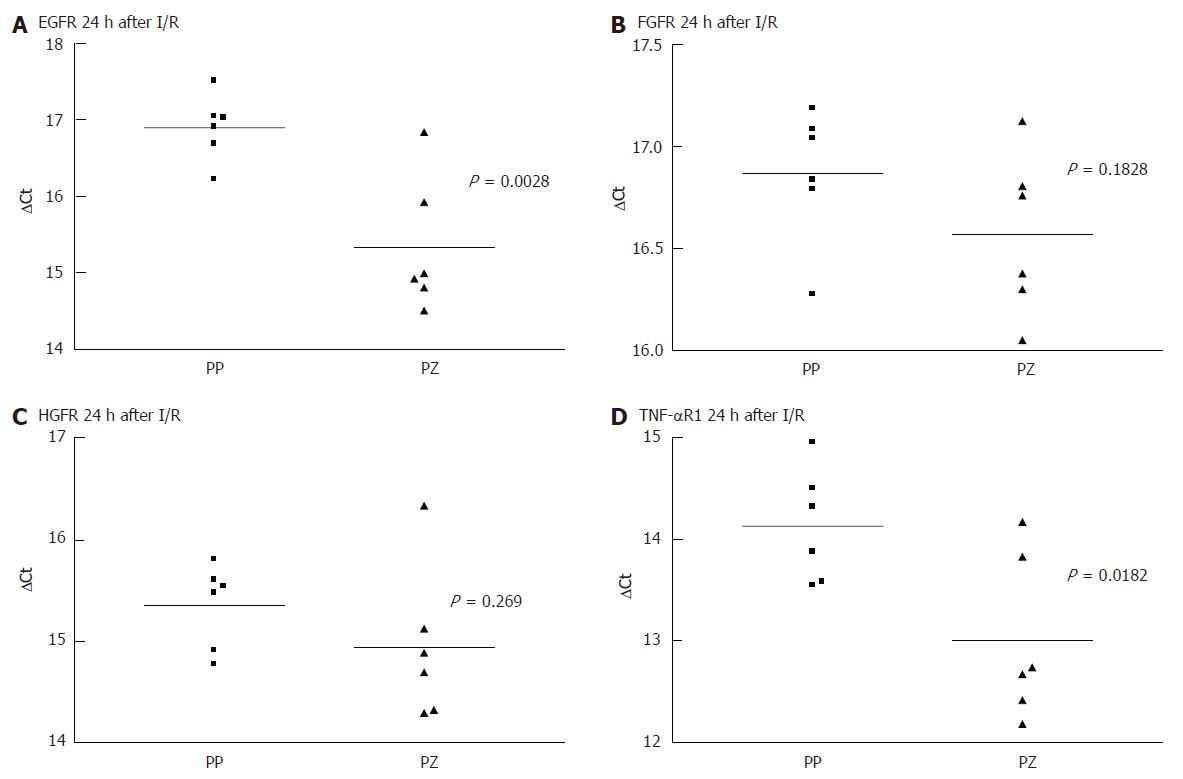
As there was a gap with no expression of any receptor, at 24 h after the liver injury, even when there was positive immunohistology at 72 h, we performed a quantitative PCR comparing the receptor mRNA in the resting liver and at 24 h. Each PCR was separately conducted for periportal and pericentral hepatocytes. As shown in Figure 3 and Figure 4, there was no difference in the expression of the analysed mRNA between periportal and pericentral hepatocytes in the resting liver or in regenerating liver after PH. In I/R there was a stronger pericentral expression of receptors. In case of EGFR and TNFR1 mRNA, this difference was significant (Figure 5).
Comparing receptor mRNA between resting liver and in regenerating liver, there was a significantly reduced expression of EGFR after PH and I/R, FGFR after I/R and TNFR1 after PH, represented by a greater ΔCT. Only HGFR was expressed more strongly 24 h after I/R than in resting liver. At every time point, there was a positive expression of receptor mRNA, even if immunohistochemically no receptor could be detected .
We analysed the expression of several growth factor receptors in two different models of liver injury and found a marked expression after 6 and 12 h with a nadir at 24 h and an increased expression after 72 h.
With no receptors to interact with, external growth factors might therefore not be able to promote the course of liver regeneration in vivo[1] when administered at the time of the nadir, at 24 h. It furthermore should be discussed, whether external growth factors influence receptor expression by negative feedback mechanisms. At 72 h, when the hepatocyte proliferation reaches its maximum, there is, once again, a (weak) expression of receptors. During this period, external growth factors do not promote regeneration, probably because proliferation is already at its maximum. Further research, investigating the meaningful application of growth factors in liver regeneration, is clearly warranted and should consider these two hypotheses.
The results demonstrated here correlate to previously published data of EGF binding studies. There, the observation was made that the EGF binding capacity of hepatocytes was reduced after PH, compared to control[14]. It is not yet clear why receptor kinetics during liver regeneration, after PH, are a biphasic process. Based on immohistochemical findings at 6 h after injury, we assume that there is an immediate expression of preformed receptors. It appears unlikely that de novo receptor synthesis can take place within 6 h. Later, after the injury, a de novo rebuilding of receptors might occur which correlates to the increase after 72 h.
After I/R, the expression of receptors is only very weak, only slightly more intensive than in the resting liver. Interestingly, the biphasic kinetics of TNFR1 after I/R are comparable to PH. In fact, the expression of FGFR and TNFR1 could be suppressed beyond the baseline using I/R.
The reason for the lack of receptor expression at 24 h after injury could be reduced mRNA expression, a posttranscriptional mechanism or shedding of the receptors. All of these mechanisms have been described in the context of receptor regulation processes[15,16]. In all samples, there is mRNA expression, yet at a reduced level. Therefore, a negative posttranscriptional effect may take place as well as a reduced translation of mRNA. In case of HGFR after I/R, this posttranscriptional effect is demonstrated, as more specific mRNA is detectable in regenerating liver tissue than in control.
In I/R model, we did not find the same increase in receptor expression as after PH. This is of particular interest because in myocardial and neuronal tissue, an up-regulation of growth factor receptors seems to be involved in ischemic preconditioning[17,18]. We could not find a similarly strong receptor expression after ischemic stimuli in liver regeneration. Although ischemic preconditioning is effective in the clinical setting of partial hepatectomy, it might not be due to a strong expression of growth factor receptors. As opposed to the resting liver and to the regenerating liver after PH, there is a zone-specific difference in the receptor mRNA expression after I/R. There is stronger expression in pericentral than in periportal hepatocytes. In immunohistochemistry, this difference is not found since there are no receptors at all. The observed variations in mRNA expression reflect the different impact of ischemia on the various parts of the liver acinus. The pericentral part is more sensitive than the periportal. In regeneration after I/R, there is stronger receptor mRNA expression in pericentral hepatocytes. Contrary to mRNA expression in I/R, there is no obvious predilection to any part of the liver acinus in the PH model, neither concerning the trauma nor the receptor expression (immunohistochemically and mRNA) during regeneration. This is of interest, as we know[19] that the proliferation after PH is stronger in periportal than in pericentral areas.
At 24 h after partial hepatectomy, many regulatory processes take place in the regenerating liver. As Xu et al[20] demonstrated by microarray, there are more genes expressed at 24 h than at any other time during liver regeneration. The receptors analysed here are not up-regulated at this time point, maybe they are part of the 135 genes Xu et al found to be down-regulated. As there should be a posttranscriptional regulation in case of the receptors, the approach of Liu et al[21], for analysing proteomics of regenerating liver, should be even more interesting than microarrays.
Concerning the expression of growth factor receptors, liver regeneration is a biphasic process with a nadir at 24 h. As a result, we hypothesise that the expression of receptors is an essential regulative factor in this process. This will have to be validated in subsequent studies.
Further research must focus on the mechanisms responsible for the decrease in receptor expression after 24 h. It should also be promising to analyse the expression of growth factor receptors in other models like sepsis or combined injuries such as, resection plus reperfusion or resection plus sepsis, since these are combinations correlated to complicated courses of liver regeneration in the clinical setting. In order to use therapeutic growth factors in liver regeneration, detailed knowledge about the kinetics of their corresponding receptors is of elementary importance.
| 1. | Court FG, Wemyss-Holden SA, Dennison AR, Maddern GJ. The mystery of liver regeneration. Br J Surg. 2002;89:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954-3960. [PubMed] |
| 3. | Skarpen E, Oksvold MP, Grøsvik H, Widnes C, Huitfeldt HS. Altered regulation of EGF receptor signaling following a partial hepatectomy. J Cell Physiol. 2005;202:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608-10613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 402] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477-4482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 624] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Mizuguchi T, Kamohara Y, Hui T, Neuman T, Mitaka T, Demetriou AA, Rozga J. Regulation of c-met expression in rats with acute hepatic failure. J Surg Res. 2001;99:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Masson S, Scotté M, François A, Coeffier M, Provot F, Hiron M, Ténière P, Fallu J, Salier JP, Daveau M. Changes in growth factor and cytokine mRNA levels after hepatectomy in rat with CCl(4)-induced cirrhosis. Am J Physiol. 1999;277:G838-G846. [PubMed] |
| 8. | Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 1997;94:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 335] [Article Influence: 11.6] [Reference Citation Analysis (10)] |
| 9. | Steiling H, Wüstefeld T, Bugnon P, Brauchle M, Fässler R, Teupser D, Thiery J, Gordon JI, Trautwein C, Werner S. Fibroblast growth factor receptor signalling is crucial for liver homeostasis and regeneration. Oncogene. 2003;22:4380-4388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 203] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Lindros KO, Penttilä KE. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985;228:757-760. [PubMed] |
| 12. | Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 396] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 307] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Earp HS, O'Keefe EJ. Epidermal growth factor receptor number decreases during rat liver regeneration. J Clin Invest. 1981;67:1580-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Xia M, Xue SB, Xu CS. Shedding of TNFR1 in regenerative liver can be induced with TNF alpha and PMA. World J Gastroenterol. 2002;8:1129-1133. [PubMed] |
| 16. | Johansson S, Andersson N, Andersson G. Pretranslational and posttranslational regulation of the EGF receptor during the prereplicative phase of liver regeneration. Hepatology. 1990;12:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Krieg T, Cui L, Qin Q, Cohen MV, Downey JM. Mitochondrial ROS generation following acetylcholine-induced EGF receptor transactivation requires metalloproteinase cleavage of proHB-EGF. J Mol Cell Cardiol. 2004;36:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Carmel JB, Kakinohana O, Mestril R, Young W, Marsala M, Hart RP. Mediators of ischemic preconditioning identified by microarray analysis of rat spinal cord. Exp Neurol. 2004;185:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Fausto N. Liver regeneration. J Hepatol. 2000;32:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 912] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 20. | Xu CS, Chang CF, Yuan JY, Li WQ, Han HP, Yang KJ, Zhao LF, Li YC, Zhang HY, Rahman S. Expressed genes in regenerating rat liver after partial hepatectomy. World J Gastroenterol. 2005;11:2932-2940. [PubMed] |
| 21. | Liu XW, Lu FG, Zhang GS, Wu XP, You Y, Ouyang CH, Yang DY. Proteomics to display tissue repair opposing injury response to LPS-induced liver injury. World J Gastroenterol. 2004;10:2701-2705. [PubMed] |
S- Editor Wang J L- Editor Lakatos PL E- Editor Bai SH