Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.5032
Revised: December 4, 2004
Accepted: December 9, 2004
Published online: August 28, 2005
AIM: To explore the expression and correlation of CD44v6, vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-2 and matrix metalloproteinase (MMP)-9 in Krukenberg and primary epithelial ovarian carcinoma.
METHODS: The expressions of CD44v6, VEGF, MMP-2 and MMP-9 were detected by immunohistochemical method in 20 cases of normal ovarian tissues, 38 cases of Krukenberg tumor and 45 cases of primary epithelial ovarian carcinoma.
RESULTS: The expression of CD44v6 (primary epithelial ovarian carcinoma tissue vs normal ovarian tissue: χ2 = 4.516, P = 0.034; Krukenberg tumor tissue vs normal ovarian tissue: χ2 = 19.537, P = 0.001) and VEGF (primary epithelial ovarian carcinoma tissue vs normal ovarian tissue: P = 0.026; Krukenberg tumor tissue vs normal ovarian tissue: χ2 = 22.895, P = 0.001) was significantly higher in primary epithelial ovarian carcinoma tissue and Krukenberg tumor tissue than in normal ovarian tissue. The positive expression rate of MMP-2 and MMP-9 was 0% in the normal ovarian tissue. The positive expression rate of CD44v6 (χ2 = 10.398, P = 0.001), VEGF (χ2 = 13.149, P = 0.001), MMP-2 (χ2 = 33.668, P = 0.001) and MMP-9 (χ2 = 38.839, P = 0.001) was remarkably higher in Krukenberg tumor than in primary epithelial ovarian carcinoma. The correlation of CD44v6, VEGF, MMP-2, and MMP-9 was observed in primary epithelial ovarian carcinoma and Krukenberg tumor.
CONCLUSION: CD44v6, VEGF, MMP-2, and MMP-9 are involved in ovarian carcinoma, gastric cancer and Krukenberg tumor. Detection of CD44v6, VEGF, MMP-2 and MMP-9 may contribute to the diagnosis of ovarian carcinoma, gastric cancer, and Krukenberg tumor.
- Citation: Lou G, Gao Y, Ning XM, Zhang QF. Expression and correlation of CD44v6, vascular endothelial growth factor, matrix metalloproteinase-2, and matrix metalloproteinase-9 in Krukenberg tumor. World J Gastroenterol 2005; 11(32): 5032-5036
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/5032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.5032
Gastric cancer is one of the common malignancies in gastrointestinal tract[1-3]. Its metastasis rate is 64.2% in China[4]. Krukenberg tumor is an ovary metastatic cancer from gastrointestinal cancer. Krukenberg tumor is highly malignant with a poor prognosis and its mechanism is not clear.
Invasion and metastasis are the leading biological characteristics of malignant tumor, and have a close relation with factors such as movement of tumor cells, apoptosis and metastasis-associated genes. VEGF is an important angiogenic factor, which may induce angiogenesis in tumor, and has a higher expression in tumor tissues, which is closely related with invasion and metastasis of tumor[5-7]. CD44v6 is one of the numerous adhesive molecules and a transmembrane glycoprotein located on cell surface. It induces homing of lymphocytes and participates in adhesion between cells, influencing invasion and metastasis of tumor[8-10]. MMP is one of the proteolytic enzymes and plays an important role in occurrence and development of tumor[11-13].
Patients were selected from Tumor Hospital of Harbin Medical University from 1992 to 2001. All patients were informed of the purpose of the study and gave their informed consent. Forty-five cases of primary epithelia ovarian carcinoma (15 cases of serious adenocarcinoma, 16 cases of mucous adenocarcinoma, and 14 cases of others pathologic types) and 35 cases of Krukenberg tumor were included in the study. All ovarian cancers had metastasis to other organs and all Krukenberg tumors came from gastric cancer. The age of the patients was 20-75 years, averaged 41 years. All cases were diagnosed by histology or cytology, and received no chemotherapy and radiotherapy before operation. Specimens were embedded in paraffin.
Monoclonal antibody was purchased from Bossed Company of Wuhan. Immunohistochemical method was used to detect the expression of CD44v6, VEGF, and MMPs. Staining was performed following the manufacturer’s instructions. The first antigen of negative control was replaced by PBS.
The cells with unambiguous brown and yellow particles present in cytoplasm of tumor cells under optical microscope were defined as positive cells. Positive intensity was divided into three grades: weak positive (counting score was 1), strong positive (counting score was 3) and moderately positive (counting score was 2). At the same time, the number of positive cells was calculated. Zero to four grades represented the number of positive cells less than 5%, 5-25%, 26-50%, 51-75% and more than 75%, respectively. The last counting scores were intensity scores. If the product had one or more scores, it was positive. Otherwise, it was negative.
Analysis of variance was used to analyze the difference between groups. Data were analyzed by χ2 test or Fisher’s exact test. Correlation among variables was tested by Pearson of bivariate.
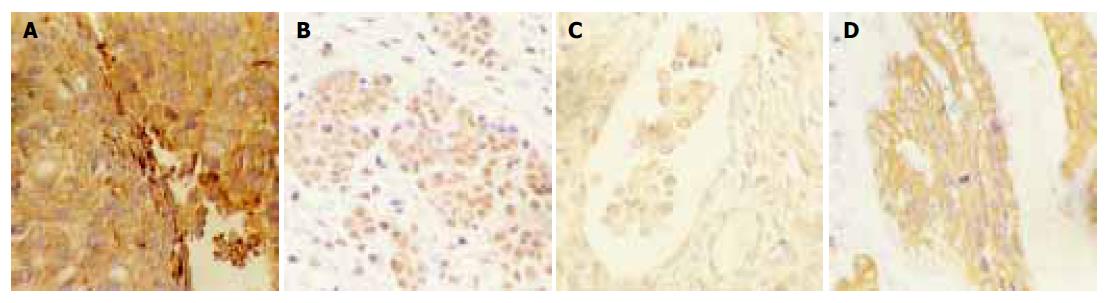
Positively staining particles of Cd44v6 were mainly distributed in plasmalemma of tumor, some of which were expressed in cytoplasm (Figure 1A). Significant difference in positive expression was observed between normal ovarian tissue and primary epithelial ovarian carcinoma, Krukenberg tumor, and gastric carcinoma (P<0.05). The positive expression of CD44v6 had no significant difference in ovarian carcinoma, ovarian mucous carcinoma and other carcinomas. No significant difference was found in moderately- and poorly-differentiated Krukenberg tumor.
Positive-staining particles of VEGF were mainly distributed in cytoplasm (Figure 1B). The positive expression rate was higher in primary epithelial ovarian carcinoma, Krukenberg tumor, and gastric carcinoma than in normal ovarian tissue (P<0.05). The positive expression rates of VEGF were 31.1% and 71.1% respectively for primary epithelial ovarian carcinoma and Krukenberg tumor (P<0.05). No significant difference was found in ovarian carcinoma. No significant difference in positive expression rate was observed in moderately-and poorly-differentiated Krukenberg tumor.
Positive-staining particles of MMP-2 and MMP-9 were distributed in cytoplasm (Figures 1C and D). Positive expression rate of MMP-2 and MMP-9 was 0% in normal ovarian carcinoma (0/20). The positive expression rate of MMP-2 and MMP-9 was significantly higher in Krukenberg tumor, than in primary epithelial ovarian carcinoma (P<0.05 for all of them). There was no relation between positive expression rate of MMPs and pathological types of primary epithelial ovarian carcinoma and between positive expression rate of MMPs and differentiation degree of Krukenberg tumor.
There was no significant difference in positive expression rate of VEGF, CD44v6, and MMP-2 between gastric carcinoma and Krukenberg tumor. The positive expression rate of MMP-9 was remarkably higher in Krukenberg tumor than in gastric carcinoma (P<0.05). Obvious difference of positive expression rate of MMP-2 and MMP-9 was found in different differentiation degrees of gastric carcinoma. The positive expression rate was significantly higher in poorly-differentiated gastric carcinoma than in well- and moderately-differentiated gastric carcinoma (P<0.05, Table 1).
| CD44v6 | VEGF | MMP-2 | MMP-9 | ||||||
| n | n | % | n | % | n | % | n | % | |
| Normal ovarian tissue | 20 | 2 | 10.0 | 1 | 5.0 | – | – | ||
| Epithelial ovarian cancer | 45 | 16 | 35.6 | 14 | 31.1 | 8 | 17.8 | 6 | 13.3 |
| Serous adenocarcinoma | 15 | 6 | 40.0 | 7 | 46.7 | 4 | 26.7 | 3 | 20.0 |
| Mucous adenocarcinoma | 16 | 4 | 25.0 | 3 | 18.8 | 2 | 12.5 | 2 | 12.5 |
| Other types | 14 | 6 | 42.9 | 4 | 28.6 | 2 | 14.3 | 1 | 7.1 |
| Krukenberg tumor | 38 | 27 | 71.1 | 27 | 71.1 | 31 | 81.6 | 31 | 81.6 |
| Moderate differentiation | 14 | 9 | 64.3 | 10 | 71.4 | 12 | 85.6 | 10 | 71.4 |
| Poor differentiation | 24 | 18 | 75.0 | 17 | 70.8 | 19 | 79.2 | 21 | 87.5 |
| Gastric carcinoma | 38 | 30 | 78.9 | 27 | 73.7 | 25 | 65.8 | 23 | 60.5 |
| High and moderate differentiation | 16 | 12 | 75.0 | 9 | 56.3 | 7 | 31.8 | 6 | 37.5 |
| Poor differentiation | 22 | 18 | 81.8 | 16 | 72.7 | 18 | 81.8 | 17 | 77.3 |
Positive expression was graded by rank correlation method. The results indicated that there was a remarkable relation between positive expressions of VEGF and CD44v6, CD44v6 and MMP-2 and MMP-9, MMP-2, and MMP-9 (Table 2).
| CD44v6 | MMP-2 | MMP-9 | ||||
| r | P | r | P | r | P | |
| VEGF | 0.342 | 0.023 | 0.498 | 0.000 | 0.498 | 0.000 |
| CD44v6 | 0.419 | 0.005 | 0.213 | 0.212 | ||
| MMP-2 | 0.488 | 0.001 | ||||
In primary epithelial ovarian carcinoma, there was a significant relation between expressions of CD44v6, VEGF, MMP-2, and MMP-9 (Table 3). VEGF vs MMP-9, and CD44v6 vs MMP-9.
| CD44v6 | MMP-2 | MMP-9 | ||||
| r | P | r | P | r | P | |
| VEGF | 0.605 | 0.004 | 0.608 | 0.003 | 0.711 | 0 |
| CD44v6 | 0.684 | 0.001 | 0.804 | 0 | ||
| MMP-2 | 0.457 | 0.037 | ||||
The relation between variables was significant in gastric carcinoma (Table 4).
| CD44v6 | MMP-2 | MMP-9 | ||||
| r | P | r | P | r | P | |
| VEGF | 0.366 | 0.046 | 0.25 | 0.378 | 0.385 | 0.035 |
| CD44v6 | 0.46 | 0.011 | 0.200 | 0.475 | ||
| MMP-2 | 0.439 | 0.015 | ||||
CD44v6 is highly expressed in serum and tissues of ovarian carcinoma and correlates with development of ovarian carcinoma[14,15]. In the present study, the expression of CD44v6 was significantly higher in primary epithelial ovarian carcinoma and Krukenberg tumor than in normal ovarian tissue (P<0.05), suggesting that expression of CD44v6 is related with malignant behaviors of ovarian carcinoma. The high expression of CD44v6 correlates with formation, development and transfer of ovarian carcinoma.
CD44 plays an important role in regulation of progress and metastasis of primary gastric carcinoma. Our study found that there was a significant difference in positive expression rate of CD44 between primary epithelial ovarian carcinoma and Krukenberg tumor (P<0.05). The positive expression rate of CD44v6 was higher in primary gastric carcinoma (78.9%), indicating that higher expression of CD44v6 has a close correlation with metastasis of gastric carcinoma. It was reported that the positive expression rate of CD44v6 is 64-77% in gastric carcinoma tissue[16-18]. Studies indicate that superfluous expression of CD44v6 correlates closely with occurrence, development, infiltration and metastasis of cancers.
VEGF is one of the agents accelerating the formation of blood vessels[19-21], and has multiple functions after it binds to specific receptors of endothelial cell surface, indicating that the development, infiltration and metastasis of cancer is related with higher expression of CD44v6 in cancer[5-7,22].
VEGF is highly expressed in serum and tissues of ovarian carcinoma[23-28]. In the present study, the expression of VEGF was significantly higher in primary epithelial ovarian carcinoma and Krukenberg tumor than in normal ovarian tissue (P<0.05), indicating that the occurrence, development and metastasis of ovarian carcinoma is closely related with high expression of VEGF.
It has been identified that tumor metastasis is accelerated by VEGF, which is highly expressed in gastric carcinoma. VEGF may be used as an index for poor prognosis of gastric carcinoma[29-34]. The positive expression is significantly different between primary epithelial ovarian carcinoma and Krukenberg tumor (P<0.05). There is no significant difference between mucous and mixed epithelial tumor and differentiation of Krukenberg tumor (P>0.05). The expression of VEGF is higher in primary gastric cancer, suggesting that cancer metastasis may be accelerated by VEGF. It was reported that the expression of VEGF is higher in carcinoma of colon with metastasis, than in carcinoma of colon without metastasis[35-38], suggesting that metastasis of colonic carcinoma is closely related with positive expression of VEGF.
MMPs play an important role among enzymes breaking the extracellular matrix. MMP-2 and MMP-9 are closely related with metastasis of tumor[39-41]. Collagenase has enzymolysis not only for matrix component of cells, but also for main component of membrana basalis. The expression of collagenase increases obviously in tumor tissues, metastasis and serum[42-44].
In this study, MMP-2 and MMP-9 were not expressed in ovarian normal tissue. The expression was low in primary epithelial ovarian carcinoma, the reasons might be that the samples were stored for a long time and the staining was not ideal. Expression of MMP-2 and MMP-9 was higher in malignant tumor tissues than in normal tissues. There was a significant difference in expression of MMP-2 and MMP-9 between Krukenberg tumor and normal tissue, and primary epithelial ovarian carcinoma and normal tissue (P<0.05). The expression rate was higher in primary gastric cancer. The results indicate that invasion and metastasis of tumor are accelerated by MMP-2 and MMP-9, and MMP-2 and MMP-9 play an important role in the metastasis of gastric carcinoma. It was reported that invasion and metastasis of tumors are related to the expression of MMP-2 and MMP-9[45,46]. High expression of MMP-2 and MMP-9 may be the molecular basis of invasion and metastasis of tumor cells. Invasion and metastasis are present, if there is overexpression of MMP-2 and MMP-9 in tumor tissue.
There was not a significant difference in MMP-2 expression between moderately- and poorly-differentiated Krukenberg tumor (P>0.05), indicating that expression of MMP-2 is not related with tumor differentiation.
Tumor metastasis nvolves a series of complex processes. Many gene products take part in the process and play an important role in forming metastasis. The significant correlations were obtained between variables in primary epithelial ovarian carcinoma and Krukenberg tumor, but not in CD44v6 and MMP-9 in our study, indicating that the above-mentioned factors participate in tumor invasion and metastasis.
| 1. | Zhao GH, Li TC, Shi LH, Xia YB, Lu LM, Huang WB, Sun HL, Zhang YS. Relationship between inactivation of p16 gene and gastric carcinoma. World J Gastroenterol. 2003;9:905-909. [PubMed] |
| 2. | Wang MW, Yang SB, Zhang ZQ, Zhu QF, Wang GS, Li H, Yao C, Wu BY, You WD. Gastroscopy follow-up study of premalignant gastric lesions in senile patients. Shijie Huaren Xiaohua Zazhi. 2003;11:1279-1281. |
| 3. | Shen B, Zhu JS. Study progress of providing with blood and intervene chemotherapy by arteries. Shijie Huaren Xiaohua Zazhi. 2003;11:1425-1428. |
| 4. | National gastric cancer pathology cooperation. Pathology study in 360 gastric cancer corpses examination. Chin J Pathol. 1983;12:124-128. |
| 5. | Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med (Berl). 2003;81:20-31. [PubMed] |
| 6. | Vacca A, Ria R, Ribatti D, Semeraro F, Djonov V, Di Raimondo F, Dammacco F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003;88:176-185. [PubMed] |
| 7. | Conti CJ. Vascular endothelial growth factor: regulation in the mouse skin carcinogenesis model and use in antiangiogenesis cancer therapy. Oncologist. 2002;7 Suppl 3:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Mi JQ, Zhang ZH, Shen MC. Significance of CD44v6 protein expression in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:156-158. |
| 9. | Jüngling B, Menges M, Goebel R, Wittig BM, Weg-Remers S, Pistorius G, Schilling M, Bauer M, König J, Zeitz M. Expression of CD44v6 has no prognostic value in patients with colorectal cancer. Z Gastroenterol. 2002;40:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Morrin M, Delaney PV. CD44v6 is not relevant in colorectal tumour progression. Int J Colorectal Dis. 2002;17:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Waas ET, Lomme RM, DeGroot J, Wobbes T, Hendriks T. Tissue levels of active matrix metalloproteinase-2 and -9 in colorectal cancer. Br J Cancer. 2002;86:1876-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Kuittinen O, Soini Y, Turpeenniemi-Hujanen T. Diverse role of MMP-2 and MMP-9 in the clinicopathological behavior of Hodgkin's lymphoma. Eur J Haematol. 2002;69:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Jiang YS, Gao Y. Biologic characteristic of matrix metallopro-teinase and its effects in soakage and metastasis of hepatic carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:1403-1404. |
| 14. | Li J, Liu J, Lu HO, Jiang Y, Guo L, Li H. Immunohistochemistry analysis of CD44 aberrance type in ovarian cancer. Chin J Clini Oncol. 1998;25:738-740. |
| 15. | Wang J, Sui LH, Gao QY. Expression of transmembrane glycopreotein V6 and its signification in epithelia ovarian cancer. Chin J Clini Obs Gynecol. 2001;2:16-18. |
| 16. | Cai Q, Lu HF, Sun MH, Du X, Fan YZ, Shi DR. Expression of CD44 v3 and v6 proteins in human colorectal carcinoma and its relevance with prognosis. Shijie Huaren Xiaohua Zazhi. 2000;8:1255-1258. |
| 17. | Gu HP, Ni CR, Zhan RZ. Relationship of expressions of CD15, CD44v6 and nm23H1 mRNA with metastasis and prognosis of colon carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:887-891. |
| 18. | Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Bellamy WT. Expression of vascular endothelial growth factor and its receptors in multiple myeloma and other hematopoietic malignancies. Semin Oncol. 2001;28:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Taraboletti G, Poli M, Dossi R, Manenti L, Borsotti P, Faircloth GT, Broggini M, D'Incalci M, Ribatti D, Giavazzi R. Antiangiogenic activity of aplidine, a new agent of marine origin. Br J Cancer. 2004;90:2418-2424. [PubMed] |
| 23. | Gadducci A, Viacava P, Cosio S, Cecchetti D, Fanelli G, Fanucchi A, Teti G, Genazzani AR. Vascular endothelial growth factor (VEGF) expression in primary tumors and peritoneal metastases from patients with advanced ovarian carcinoma. Anticancer Res. 2003;23:3001-3008. [PubMed] |
| 24. | Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol. 2003;33:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Kakeji Y, Koga T, Sumiyoshi Y, Shibahara K, Oda S, Maehara Y, Sugimachi K. Clinical significance of vascular endothelial growth factor expression in gastric cancer. J Exp Clin Cancer Res. 2002;21:125-129. [PubMed] |
| 26. | Duan LX, Zhong DW, Hu FZ, Zhao H, Yang ZL, Yi W, Shu GS, Hua SW. Relationship between expression of VEGF, Flt1, bFGF and P53and outcome in patients with gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 2004;12:546-549. |
| 27. | Zhang HT, Hu X. Relationship between VEGF and incursions and metastasis ingastric cancer. Shijie Huaren Xiaohua Zazhi. 2003;11:344-345. |
| 28. | Li QM, Yu Q, Min CY. Expression of mutant P53 and VEGF in experimental gastric cancer in rats and the effect of decoction Weikang-ning. Shijie Huaren Xiaohua Zazhi. 2003;11:997-1000. |
| 29. | Mao ZB, Xiao MB, Huang JF, Ni HB, Ni RZ, Wei Q, Zhang H. Expression of VEGF and its signification in serum of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:1220-1221. |
| 30. | Takahashi Y, Mai M. Significance of angiogenesis and clinical application of anti-angiogenesis. Nihon Geka Gakkai Zasshi. 2001;102:381-384. [PubMed] |
| 31. | Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K, Matsutani N, Yasui W, Chayama K. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001;93:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Linderholm BK, Lindh B, Beckman L, Erlanson M, Edin K, Travelin B, Bergh J, Grankvist K, Henriksson R. Prognostic correlation of basic fibroblast growth factor and vascular endothelial growth factor in 1307 primary breast cancers. Clin Breast Cancer. 2003;4:340-347. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Liu F, Zhang YJ. Roles of VEGF-C and its receptor Flt-4 in proliferation and metastasis of primary breast cancer. Ai Zheng. 2003;22:1053-1056. [PubMed] |
| 34. | Yu CY, Pam KF, Xing DY, Liang G, Tan W, Zhang L, Lin D. Correlation between a single nucleotide polymorphism in the matrix metalloproteinase-2 promoter and risk of lung cancer. Cancer Res. 2002;15:6430-6433. |
| 35. | Hao YD, Zhao YW, Kong LF, Zhang YP. Chang of MMP-2 enzymologic activity in tissues of human hepatocellular cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:952-953. |
| 36. | Ylisirniö S, Höyhtyä M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer Res. 2000;20:1311-1316. [PubMed] |
| 37. | Mönig SP, Baldus SE, Hennecken JK, Spiecker DB, Grass G, Schneider PM, Thiele J, Dienes HP, Hölscher AH. Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2001;39:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Kabashima A, Maehara Y, Kakeji Y, Baba H, Koga T, Sugimachi K. Clinicopathological features and overexpression of matrix metalloproteinases in intramucosal gastric carcinoma with lymph node metastasis. Clin Cancer Res. 2000;6:3581-3584. [PubMed] |
| 39. | Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Relationship between biologic behavior and phenotypic expression in intramucosal gastric carcinomas. Hum Pathol. 2002;33:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Cai H, Kong ZR, Chen HM. Matrix metalloproteinase-2 and angiogenesis in gastric cancer. Ai Zheng. 2002;21:25-28. [PubMed] |
| 41. | Hirvonen R, Talvensaari-Mattila A, Pääkkö P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) in T(1-2)N0 breast carcinoma. Breast Cancer Res Treat. 2003;77:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Takahashi Y, Kitadai Y, Ellis LM, Bucana CD, Fidler IJ, Mai M. Multiparametric in situ mRNA hybridization analysis of gastric biopsies predicts lymph node metastasis in patients with gastric carcinoma. Jpn J Cancer Res. 2002;93:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Takahashi M, Oka N, Naroda T, Nishitani MA, Kanda K, Kanayama HO, Kagawa S. Prognostic significance of matrix metalloproteinases-2 activation ratio in renal cell carcinoma. Int J Urol. 2002;9:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Matsuyama Y, Takao S, Aikou T. Comparison of matrix metalloproteinase expression between primary tumors with or without liver metastasis in pancreatic and colorectal carcinomas. J Surg Oncol. 2002;80:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Liang YY, Zhao T, He EST. Relationship between MMP-9, MMP-2 and metastasis of gastric cancer. Chin J General Surg. 2000;15:119. |
| 46. | Zhang CW, Zou SC, Xu WJ, Zhao CS. Expression of MMP-9 and its clinical signification. Zhongguo Weichang Waike Zazhi. 2000;3:25-27. |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK