©The Author(s) 2026.
World J Gastroenterol. Jan 14, 2026; 32(2): 114097
Published online Jan 14, 2026. doi: 10.3748/wjg.v32.i2.114097
Published online Jan 14, 2026. doi: 10.3748/wjg.v32.i2.114097
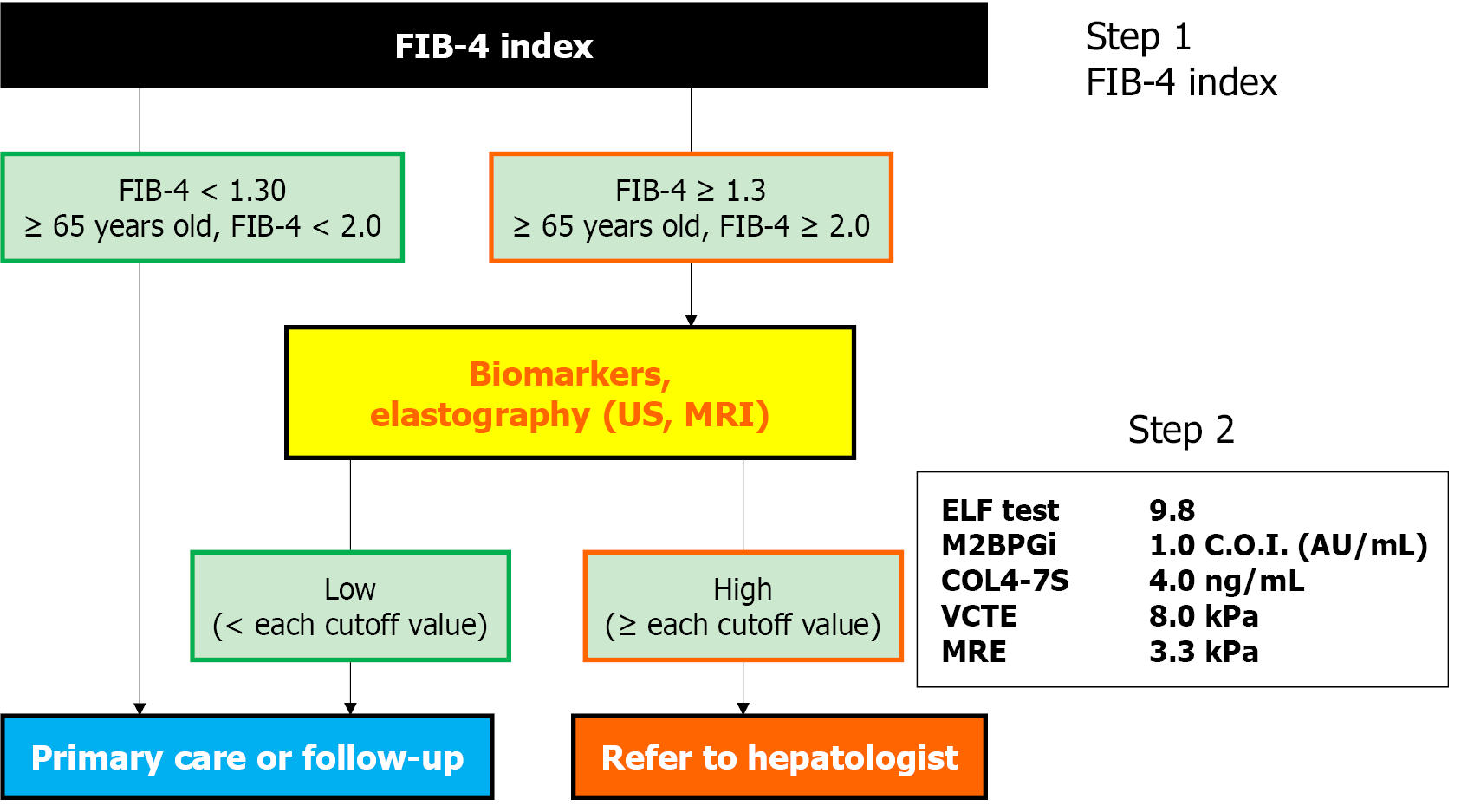
Figure 1 Algorithm for referral of patients with metabolic dysfunction-associated steatotic liver disease using noninvasive tests for liver fibrosis.
The algorithm for referral from a primary care physician to a hepatologist to narrow down cases of metabolic dysfunction-associated steatotic liver disease with advanced liver fibrosis is shown. First, the fibrosis-4 index is evaluated (step 1), and if the score is 1.30 or equal to or greater than 2.00 age 65 or older, the patient is evaluated using one of the various noninvasive tests (step 2). Patients with high values are referred to a hepatologist (refer to hepatologist). In cases followed by a family physician or a regular physician (primary care or follow-up), it is recommended to repeat the above procedure every 2-3 years. FIB-4: Fibrosis-4; US: Ultrasound; MRI: Magnetic resonance imaging; ELF: Enhanced liver fibrosis; M2BPGi: Mac-2-binding protein glycosylation isomer; C.O.I.: Cutoff index; COL4-7S: Type IV collagen 7S; VCTE: Vibration-controlled transient elastography; MRE: Magnetic resonance elastography.
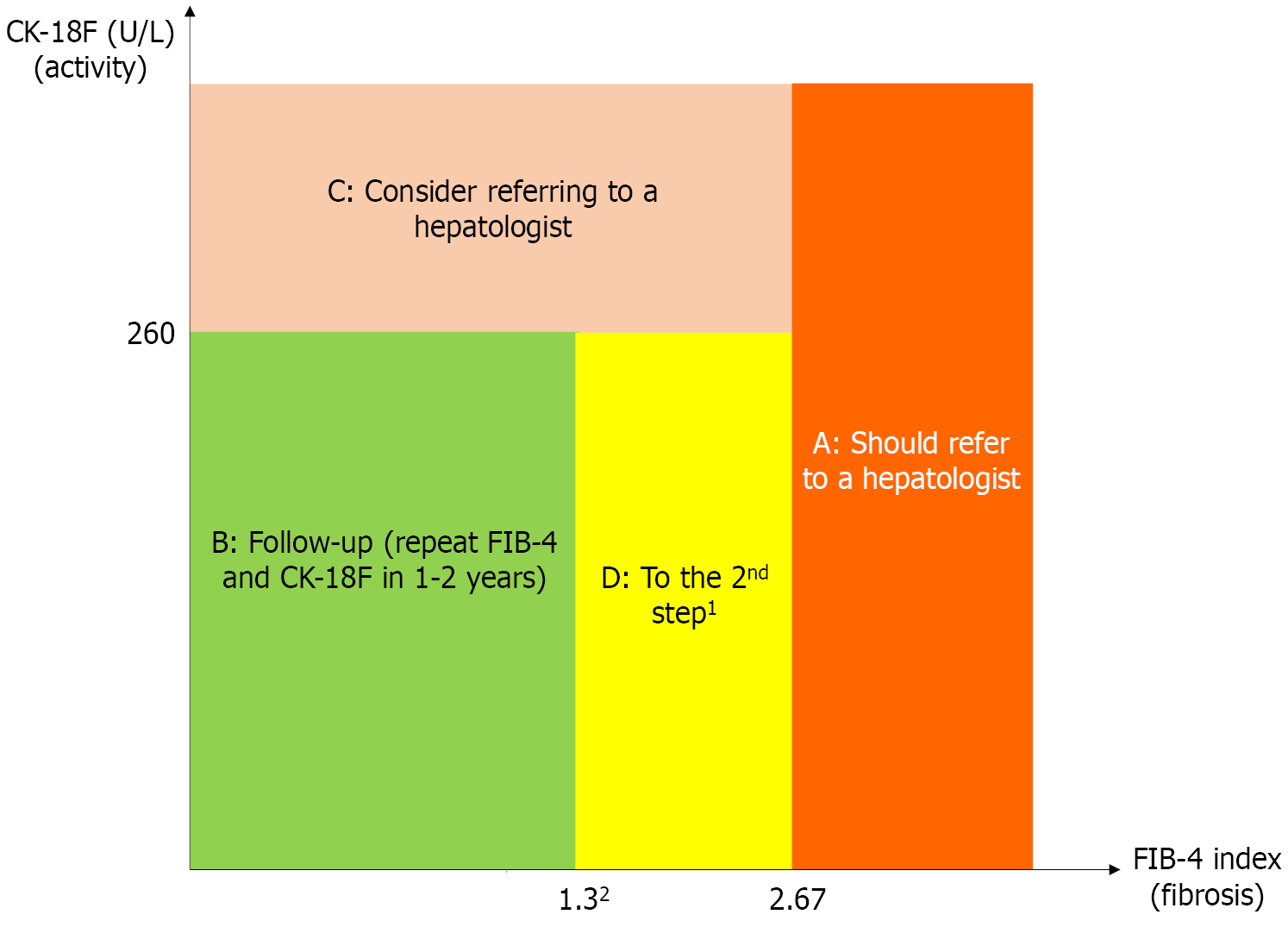
Figure 2 Stepwise approach for evaluating metabolic dysfunction-associated steatotic liver disease using the fibrosis-4 index and the cytokeratin 18 fragment biomarker.
A: High-risk group (orange). Patients with fibrosis-4 (FIB-4) > 2.67 should be referred to a hepatologist regardless of cytokeratin 18 fragment (CK-18F) levels due to the high risk of advanced fibrosis; B: Low-risk group (green). Patients with FIB-4 < 1.3 and CK-18F < 260 U/L are considered to have minimal fibrosis and inflammation. Follow-up with repeat FIB-4 and CK-18F testing every 1-2 years is recommended; C: Referral consideration group (pink). Patients with CK-18F ≥ 260 U/L may have progressive inflammation. Consider referring to a hepatologist for pathologic evaluation; D: Intermediate-risk group (yellow). Patients with FIB-4 of 1.30-2.67 and CK-18F < 260 U/L may include cases of full-blown metabolic dysfunction-associated steatohepatitis with advanced fibrosis. 1Indicates further evaluation with the enhanced liver fibrosis test, Mac-2-binding protein glycosylation isomer, type IV collagen 7S, vibration-controlled transient elastography, or magnetic resonance elastography will be necessary to confirm fibrosis status. 2Indicates a fibrosis-4 index of 2.0 is considered intermediate risk for patients age 65 or older. CK-18F: Cytokeratin 18 fragment; FIB-4: Fibrosis-4.
- Citation: Kamada Y, Sumida Y, Takahashi H, Ishiba H, Kawanaka M, Tada T, Yoneda M, Imajo K, Seko Y, Fujii H, Nakajima A. Noninvasive strategies for metabolic dysfunction-associated steatotic liver disease assessment and referral in Japan. World J Gastroenterol 2026; 32(2): 114097
- URL: https://www.wjgnet.com/1007-9327/full/v32/i2/114097.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i2.114097