©The Author(s) 2025.
World J Gastroenterol. Dec 28, 2025; 31(48): 114355
Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.114355
Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.114355
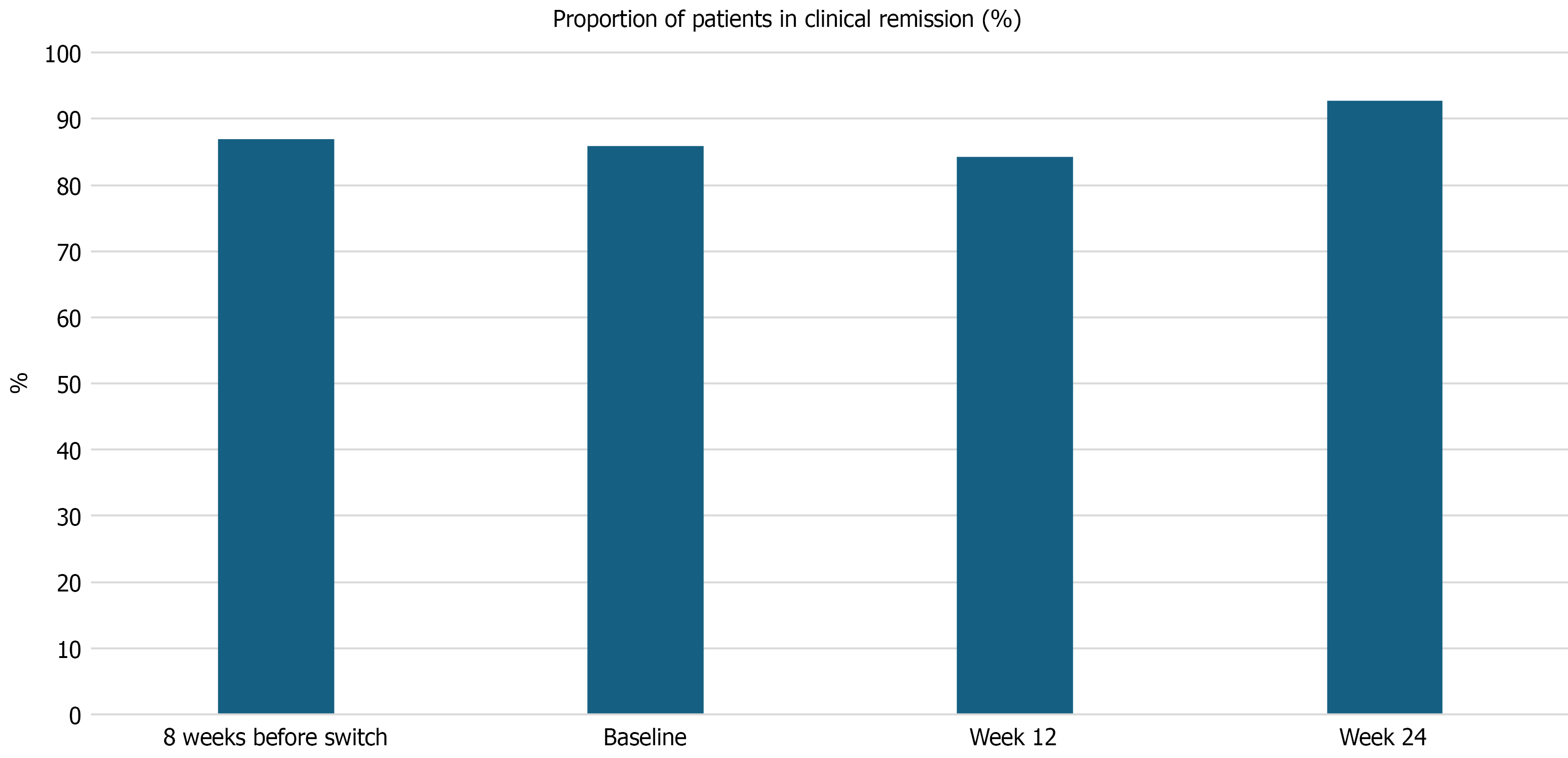
Figure 1 Clinical activity in inflammatory bowel disease patients at 8-weeks prior, time of switch, and weeks 12 and 24 following biosimilar switching.
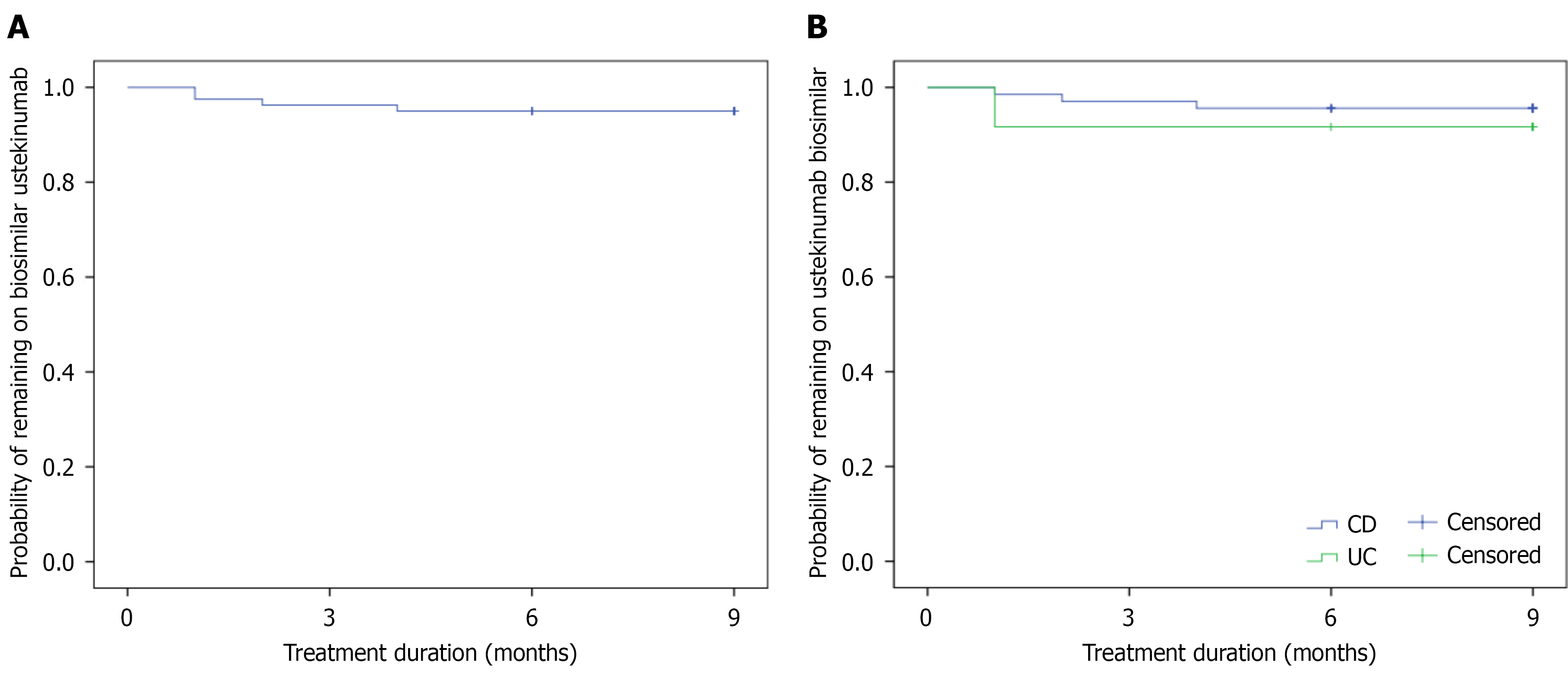
Figure 2 Probability of remaining on biosimilar ustekinumab after switching from the originator among all patients with inflammatory bowel disease and patients with ulcerative colitis or Crohn’s disease.
A: Patients with inflammatory bowel disease; B: Patients with ulcerative colitis or Crohn’s disease. UC: Ulcerative colitis; CD: Crohn’s disease.
- Citation: Kritzinger J, Candel I, Kotrri G, Nadeem H, Afif W, Bitton A, Wild G, Bessissow T, Lakatos PL. Clinical outcomes and drug sustainability after non-medical switch from ustekinumab originator to biosimilars in inflammatory bowel disease. World J Gastroenterol 2025; 31(48): 114355
- URL: https://www.wjgnet.com/1007-9327/full/v31/i48/114355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i48.114355