©The Author(s) 2025.
World J Gastroenterol. Dec 28, 2025; 31(48): 113856
Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.113856
Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.113856
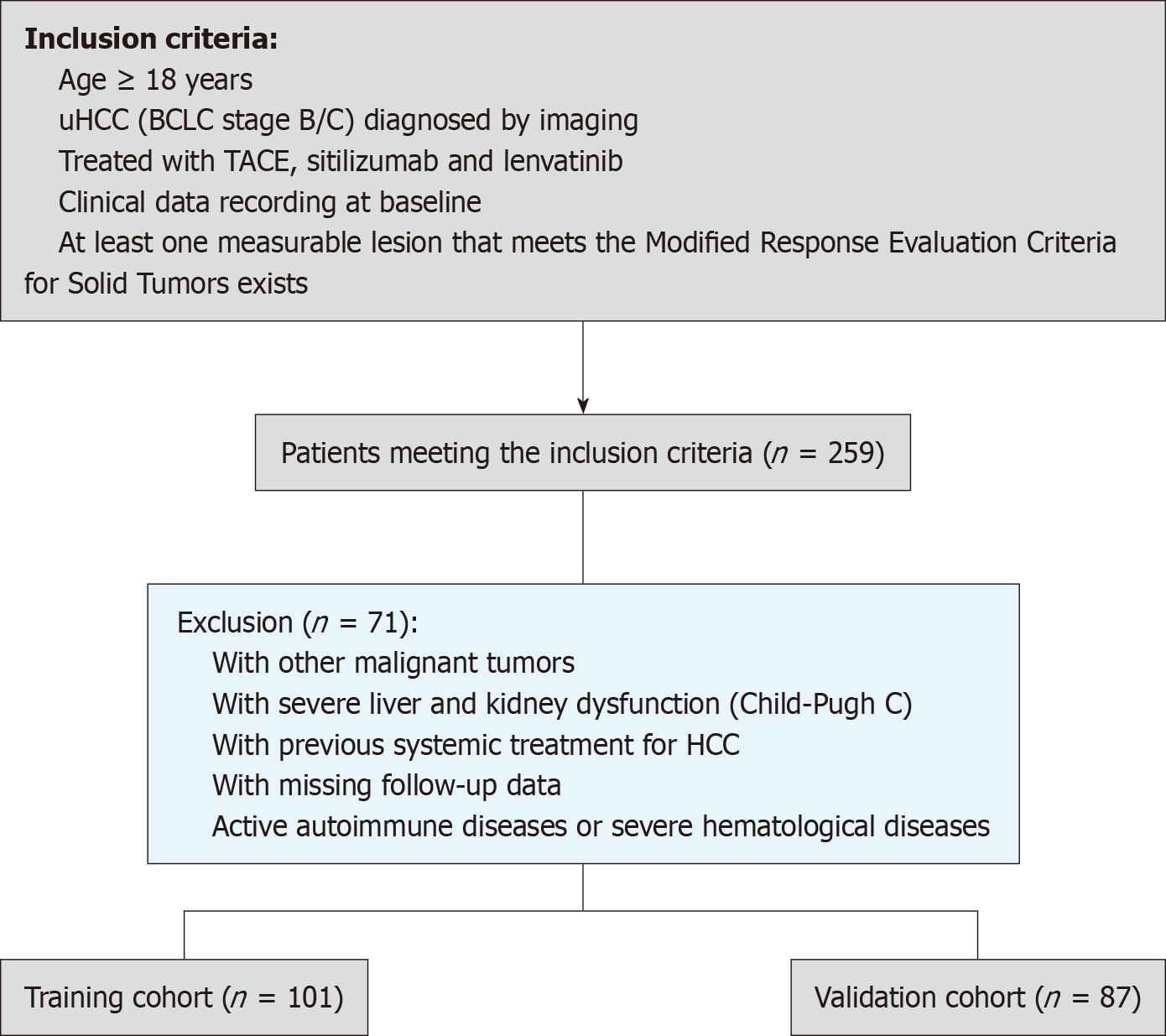
Figure 1 Flowchart of the research cohort.
uHCC: Unresectable hepatocellular carcinoma; BCLC: Barcelona Clinic Liver Cancer; TACE: Transarterial chemoembolization; mRECIST: Modified Response Evaluation Criteria for Solid Tumors; HCC: Hepatocellular carcinoma.
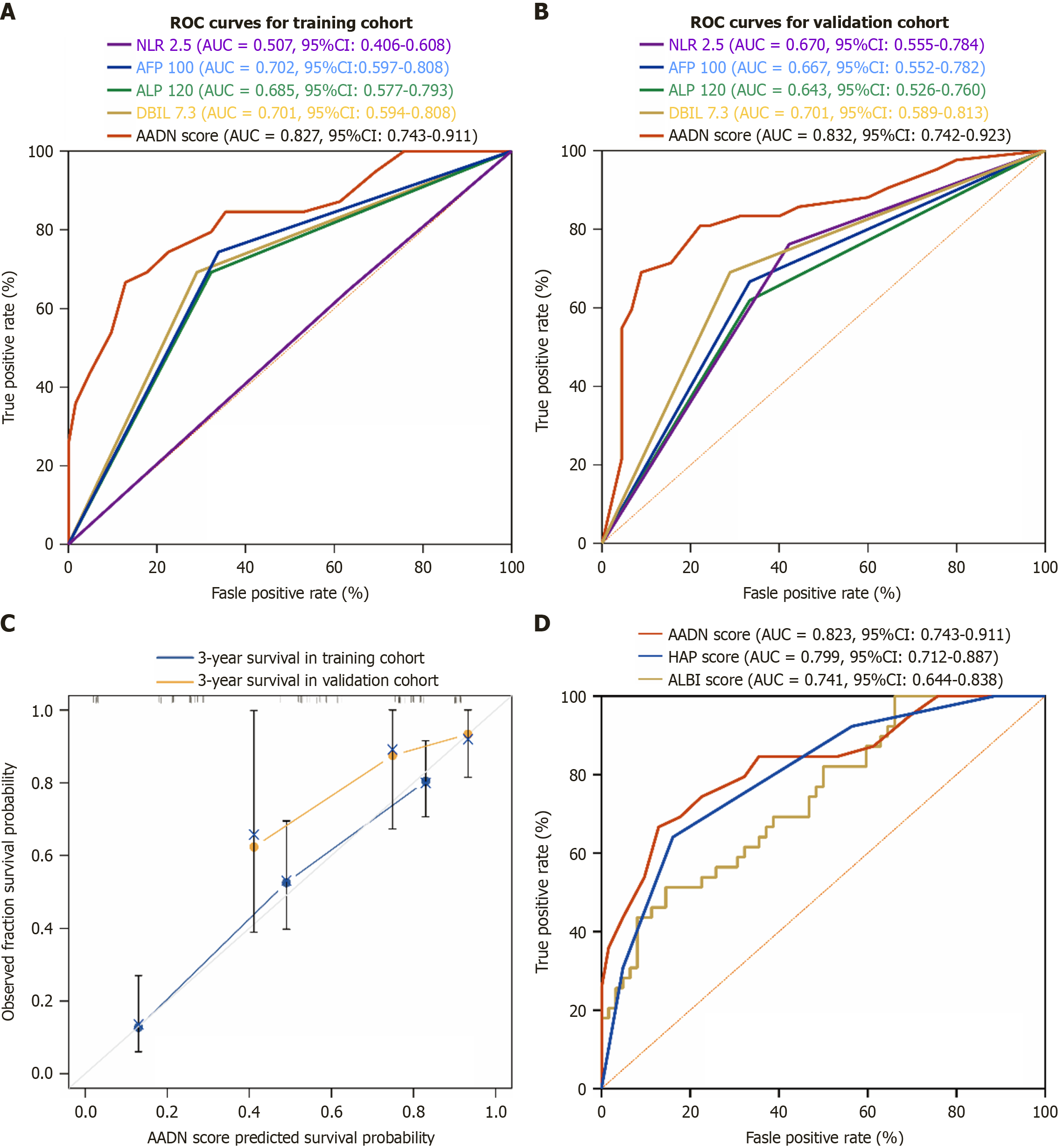
Figure 2 Receiver operating characteristic curve analysis of the predictive performance of the AADN score in the training cohort and validation cohort.
A: Receiver operating characteristic (ROC) curve analysis of the predictive performance of the AADN score in the training cohort; B: ROC curve analysis of the predictive performance of the AADN score in the validation cohort; C: Calibration curve of the AADN score for predicting 3-year survival; D: ROC curve was used to compare the diagnostic efficacy of AADN score, hepatic arterial infusion chemotherapy score and albumin-bilirubin score. ROC: Receiver operating characteristic; NLR: Neutrophil to lymphocyte ratio; AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; DBIL: Direct bilirubin; AUC: Area under the curve; CI: Confidence interval; HAP: Hepatic arterial infusion chemotherapy; ALBI: Albumin-bilirubin.
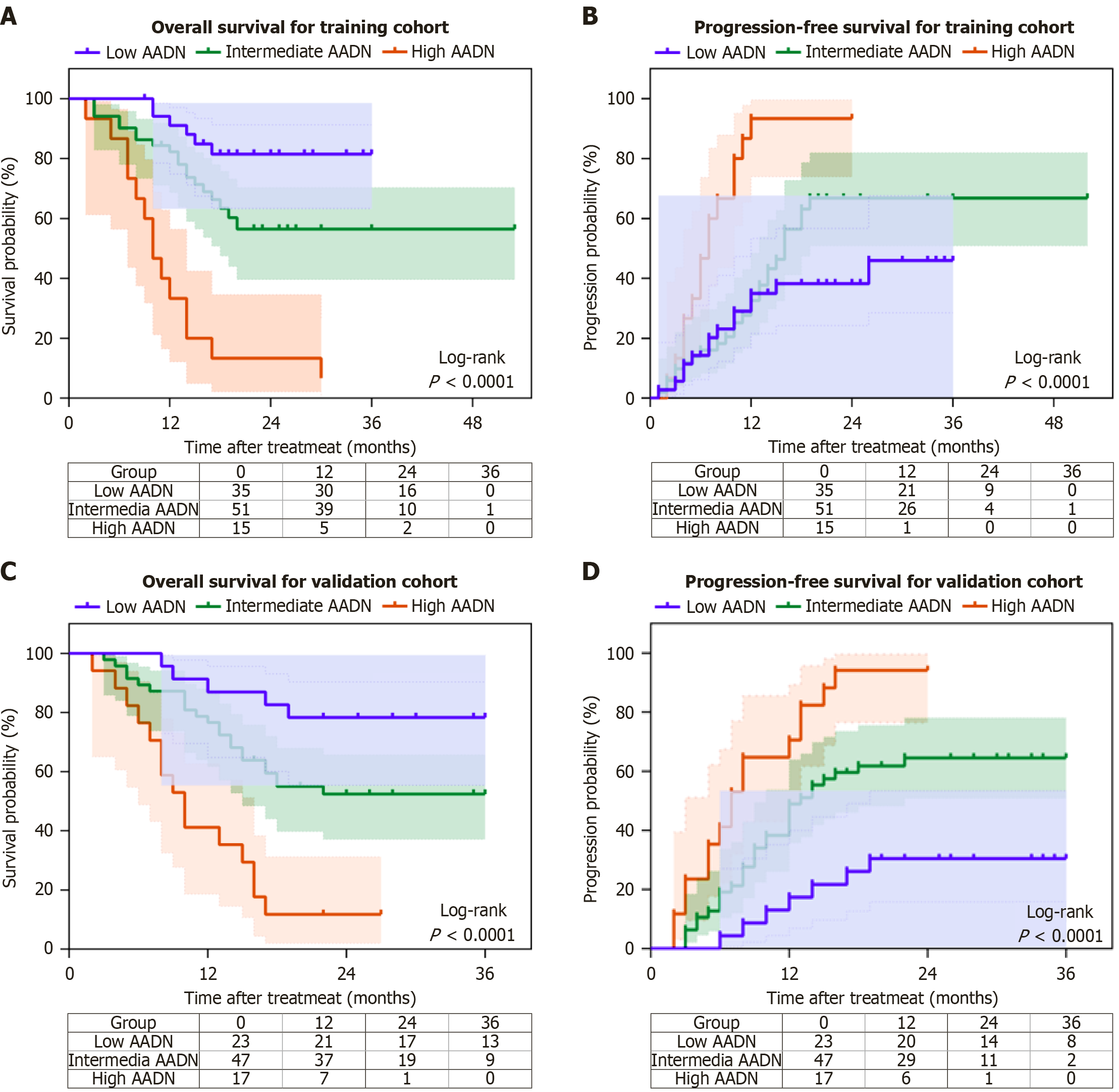
Figure 3 The prognosis of both the training cohort and the validation cohort based on the AADN score.
A: The Kaplan-Meier overall survival curves for the training cohort classify patients into low-risk, intermediate-risk, and high-risk groups according to their AADN score (log-rank P < 0.0001); B: The Kaplan-Meier progression-free survival curves for the training cohort classify patients into low-risk, intermediate-risk, and high-risk groups according to their AADN score (log-rank P < 0.0001); C: The Kaplan-Meier overall survival curves for the validation cohort classify patients into low-risk, intermediate-risk, and high-risk groups according to their AADN score (log-rank P < 0.0001); D: The Kaplan-Meier progression-free survival curves for the validation cohort classify patients into low-risk, intermediate-risk, and high-risk groups according to their AADN score (log-rank P < 0.0001).
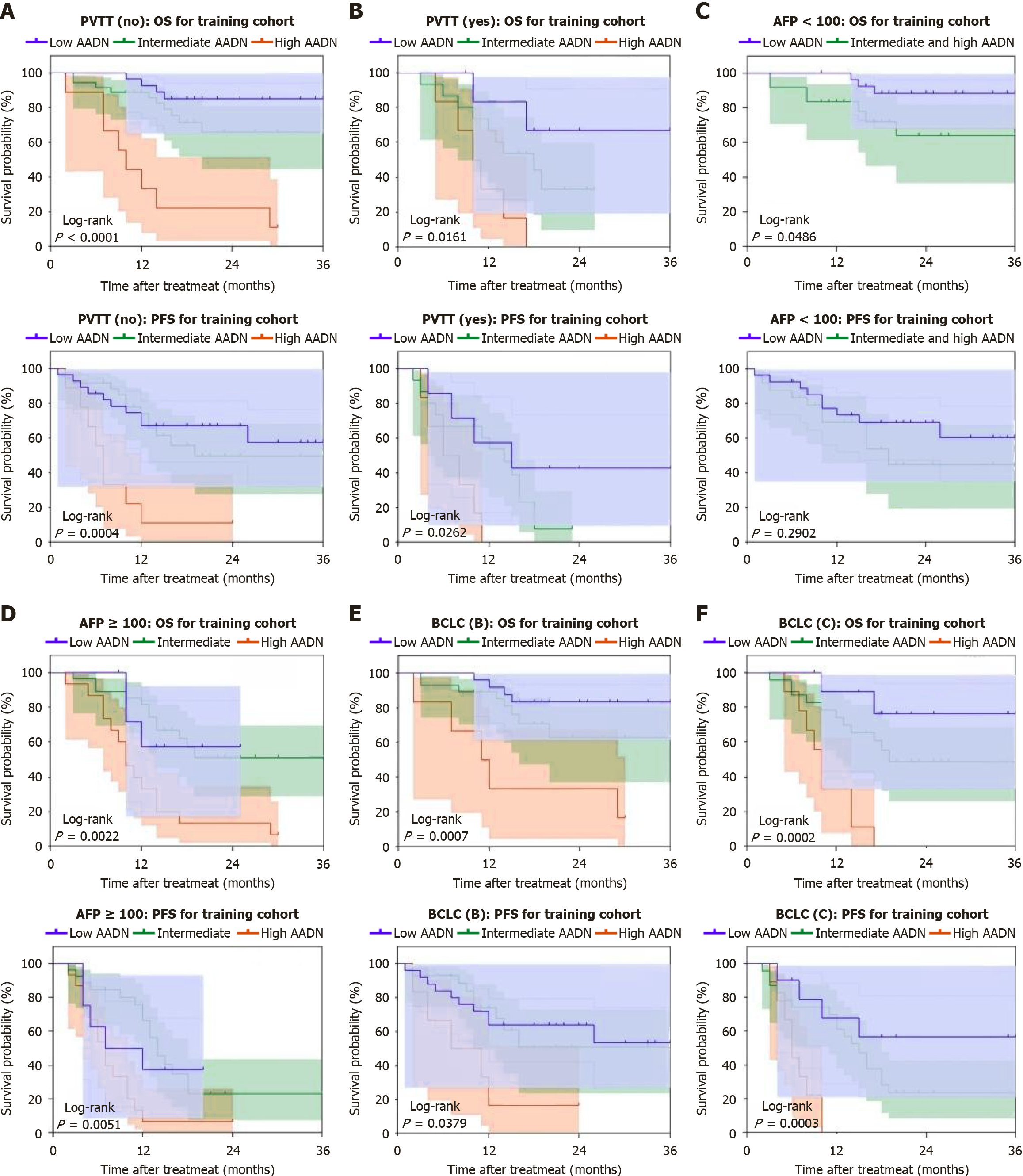
Figure 4 Subgroup analysis of survival outcomes stratified by clinical and pathological characteristics.
A: Overall survival (OS) and progression-free survival (PFS) of patients without portal vein tumor thrombus in the training cohort based on the AADN score (log-rank, OS: P < 0.0001, PFS: P = 0.0004); B: OS and PFS of patients with portal vein tumor thrombus in the training cohort based on the AADN score (log-rank, OS: P = 0.0161, PFS: P = 0.0262); C: OS and PFS of patients with AFP < 100 ng/mL in the training cohort based on the AADN score (log-rank, OS: P = 0.0486, PFS: P = 0.2902); D: OS and PFS of patients with AFP ≥ 100 ng/mL in the training cohort based on the AADN score (log-rank, OS: P = 0.0022, PFS: P = 0.0051); E: OS and PFS of Barcelona Clinic Liver Cancer stage B patients in the training cohort based on the AADN score (log-rank, OS: P = 0.0007, PFS: P = 0.0379); F: OS and PFS of Barcelona Clinic Liver Cancer stage C patients in the training cohort based on the AADN score (log-rank, OS: P = 0.0002, PFS: P = 0.0003). PVTT: Portal vein tumor thrombus; OS: Overall survival; AFP: Alpha-fetoprotein; PFS: Progression-free survival; BCLC: Barcelona Clinic Liver Cancer.
- Citation: Zhang X, Liao MJ, Ren LY, Qin WY, Mu SW, She SP, Fei R, Cong X, Zhou YP, Chen DB, Chen HS. AADN score: Predicting response to transarterial chemoembolization, sintilimab and lenvatinib in patients with hepatocellular carcinoma. World J Gastroenterol 2025; 31(48): 113856
- URL: https://www.wjgnet.com/1007-9327/full/v31/i48/113856.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i48.113856