Copyright
©The Author(s) 2025.
World J Gastroenterol. Oct 14, 2025; 31(38): 109802
Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.109802
Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.109802
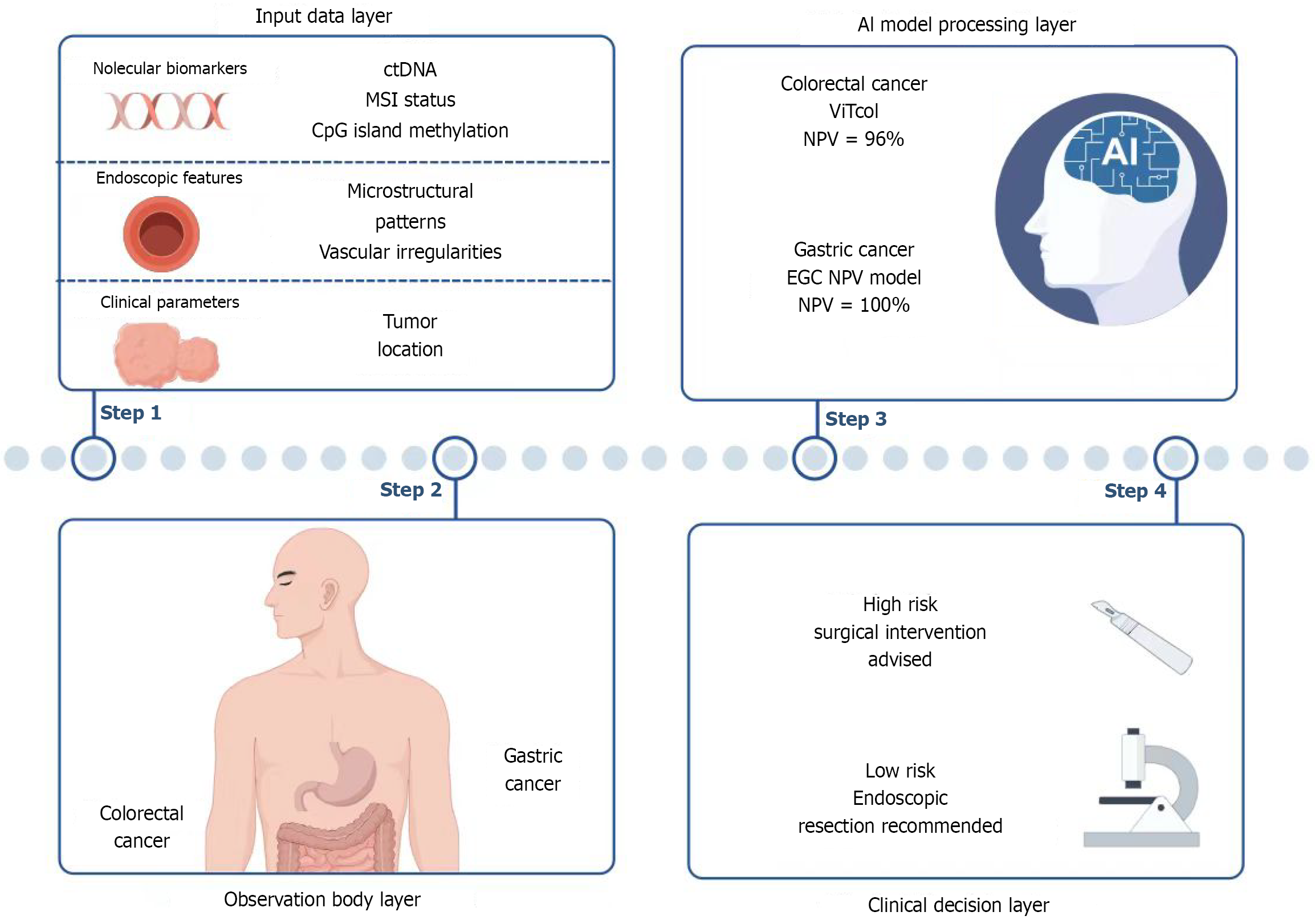
Figure 1 Artificial intelligence-assisted diagnostic and clinical decision integration workflow for gastrointestinal cancers.
Step 1: Input data layer: Integration of multi-dimensional data including molecular biomarkers (circulating tumor DNA, microsatellite instability status, CpG island methylation), endoscopic features (microstructural patterns, vascular abnormalities), and clinical parameters (tumor location). Step 2: Macroscopic observation layer: Visual identification of gastrointestinal lesion regions to facilitate subsequent artificial intelligence (AI) processing. Step 3: AI model processing layer: Gastric cancer analysis: Multimodal model incorporating ulcer-free status, high differentiation, and proximal tumor location [negative predictive value (NPV) = 100%] supports function-preserving surgical decisions. Colorectal cancer analysis: Vision Transformer-based model (ViTCol, NPV = 96%) combines 7 indicators including age and tumor location for precise lymph node metastasis risk stratification. Step 4: Clinical decision layer: AI-generated risk stratification guides therapeutic recommendations: Surgical resection for high-risk cases and endoscopic resection for low-risk cases. ctDNA: Circulating tumor DNA; MSI: Microsatellite instability; AI: Artificial intelligence; NPV: Negative predictive value; EGC: Early gastric cancer; NPV: Negative predictive value; ViT: Vision Transformer; LNM: Lymph node metastasis.
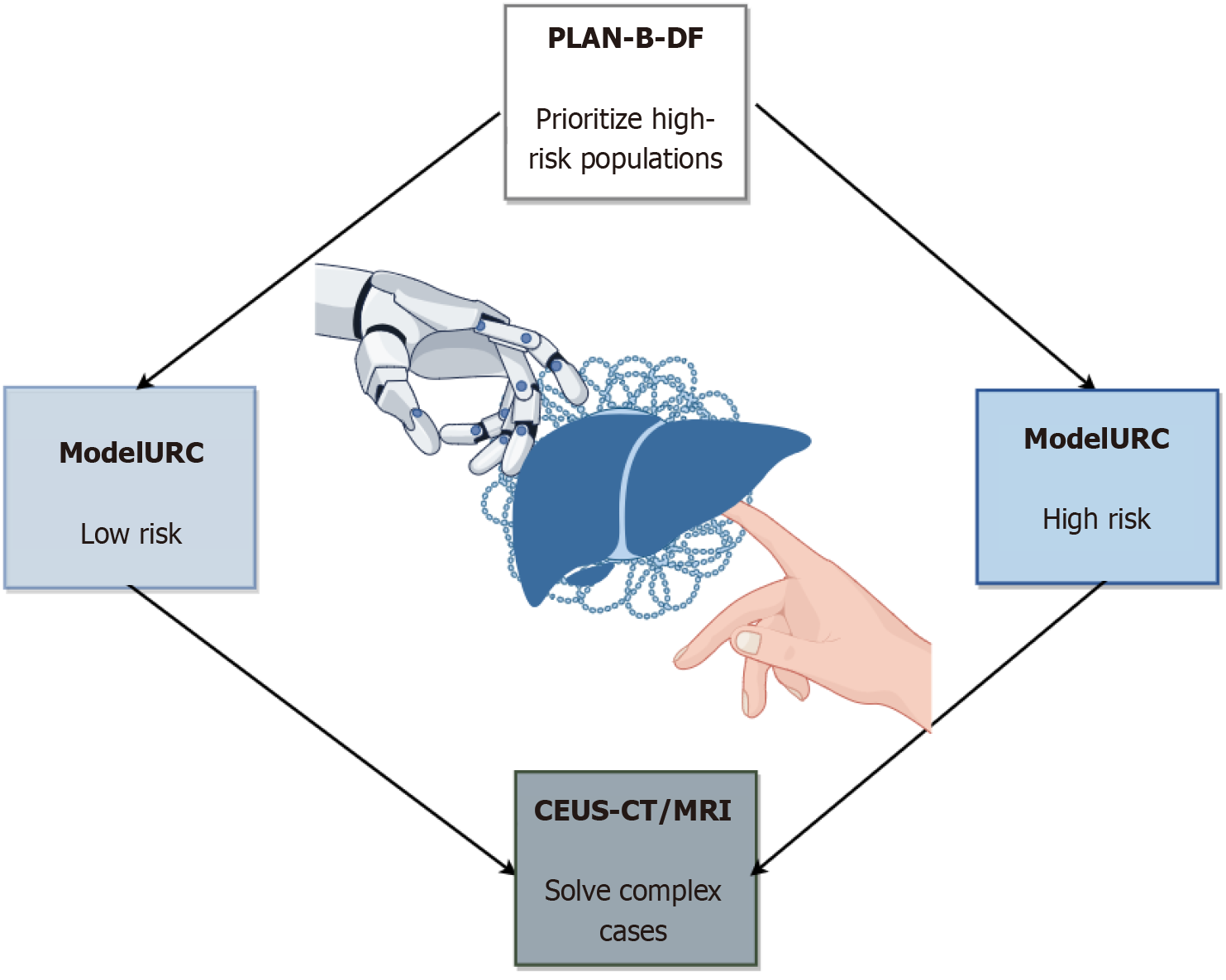
Figure 2 Integration of artificial intelligence-assisted diagnostic models and imaging techniques for liver disease evaluation.
This flowchart illustrates the risk stratification and multimodal imaging workflow for liver disease diagnosis. The PLAN-B-DF model (gradient boosting algorithm) identifies high-risk populations of hepatocellular carcinoma (HCC) in chronic hepatitis B patients by analyzing computed tomography (CT)-derived biomarkers (e.g., visceral fat distribution, spleen volume) and clinical data, achieving a C-index of 0.91. ModelURC stratifies patients into low-risk and high-risk groups for differential surveillance protocols. For complex cases (e.g., sub-3 cm lesions or atypical imaging features), contrast-enhanced ultrasound combined with CT/magnetic resonance imaging LI-RADS classification enhances diagnostic sensitivity to 88.8%, minimizing unnecessary biopsies. The framework integrates artificial intelligence-driven quantitative analysis (e.g., deep learning-based fibrosis staging, radiomics) with multimodal imaging to optimize precision in liver fibrosis grading, HCC screening, and longitudinal monitoring. CEUS: Contrast-enhanced ultrasound; CT: Computed tomography; MRI: Magnetic resonance imaging.
- Citation: Wu YM, Tang FY, Qi ZX. Multimodal artificial intelligence technology in the precision diagnosis and treatment of gastroenterology and hepatology: Innovative applications and challenges. World J Gastroenterol 2025; 31(38): 109802
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/109802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.109802