©The Author(s) 2025.
World J Gastroenterol. Oct 14, 2025; 31(38): 109486
Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.109486
Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.109486
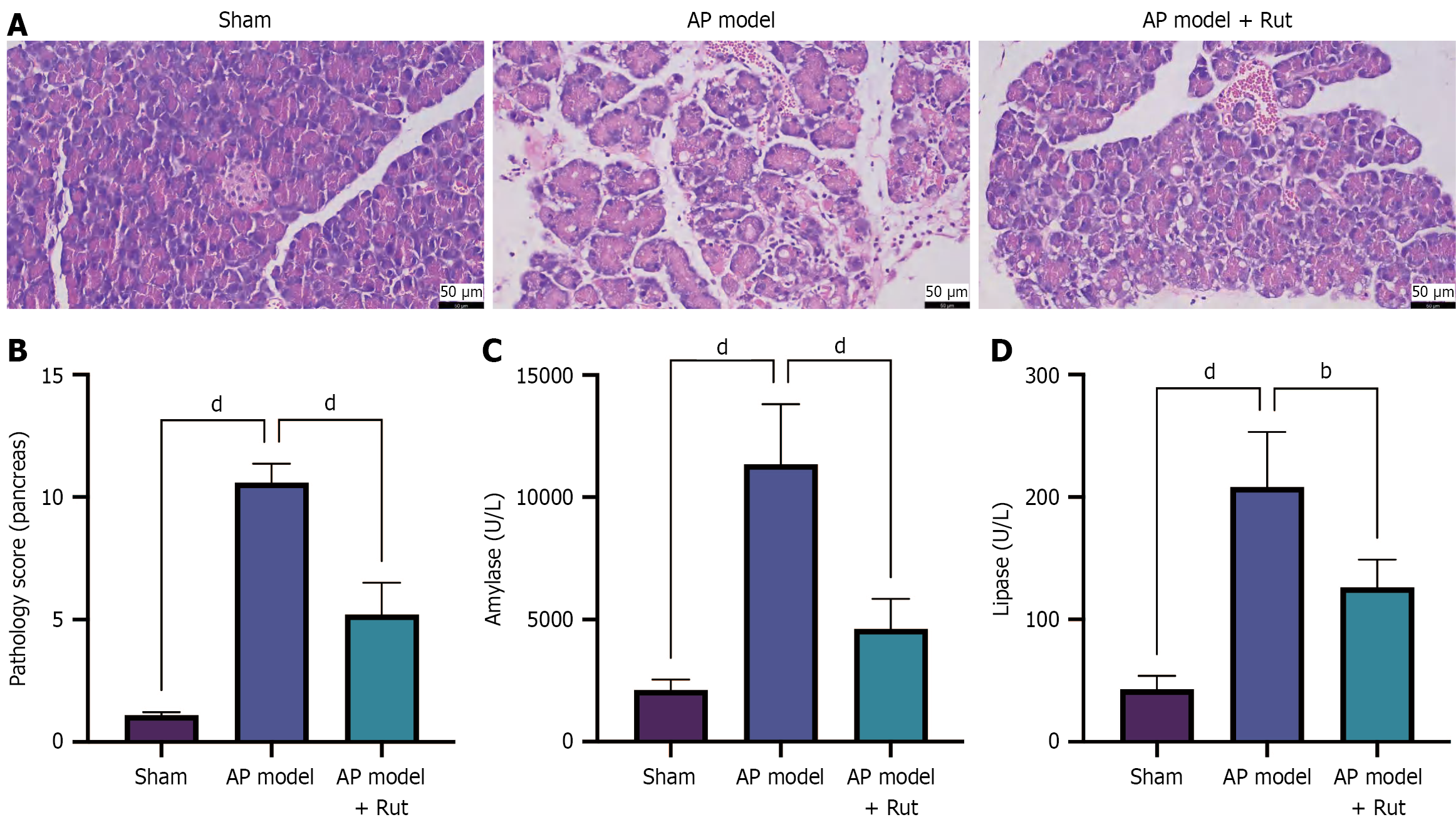
Figure 1 Rutaecarpine reduces tissue injury in rats with acute pancreatitis.
A rat model of acute pancreatitis was established and treated with rutaecarpine. A: Hematoxylin and eosin staining to observe pancreatic tissue injury; B: Pathology score of pancreas; C and D: Biochemical analysis to measure serum amylase and lipase levels. n = 5; bP < 0.01, dP < 0.0001. AP: Acute pancreatitis; Rut: Rutaecarpine.
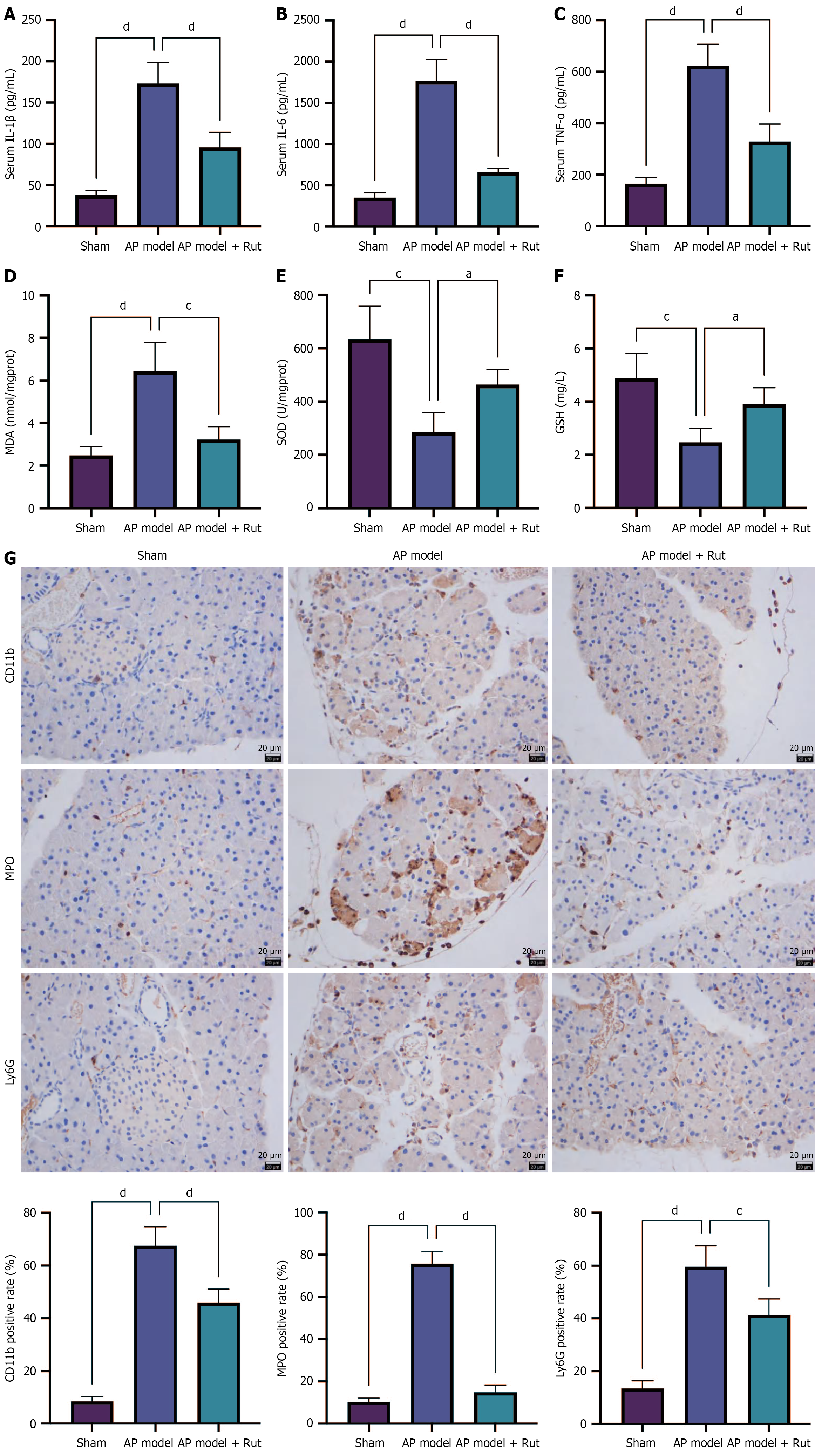
Figure 2 Rutaecarpine reduces inflammation and oxidative stress in rats with acute pancreatitis.
An acute pancreatitis rat model was established and treated with rutaecarpine. A-C: Assay kits were used to measure the levels of serum inflammatory factors interleukin-1 beta, interleukin-6, and tumor necrosis factor alpha; D-F: The levels of oxidative stress-related malondialdehyde, superoxide dismutase, and glutathione in pancreatic tissue; G: Immunohistochemistry staining was performed to detect CD11b, myeloperoxidase, and Ly6G in pancreatic tissue. n = 5; aP < 0.05, cP < 0.001, dP < 0.0001. AP: Acute pancreatitis; Rut: Rutaecarpine; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor alpha; MDA: Malondialdehyde; SOD: Superoxide dismutase; GSH: Glutathione; MPO: Myeloperoxidase.
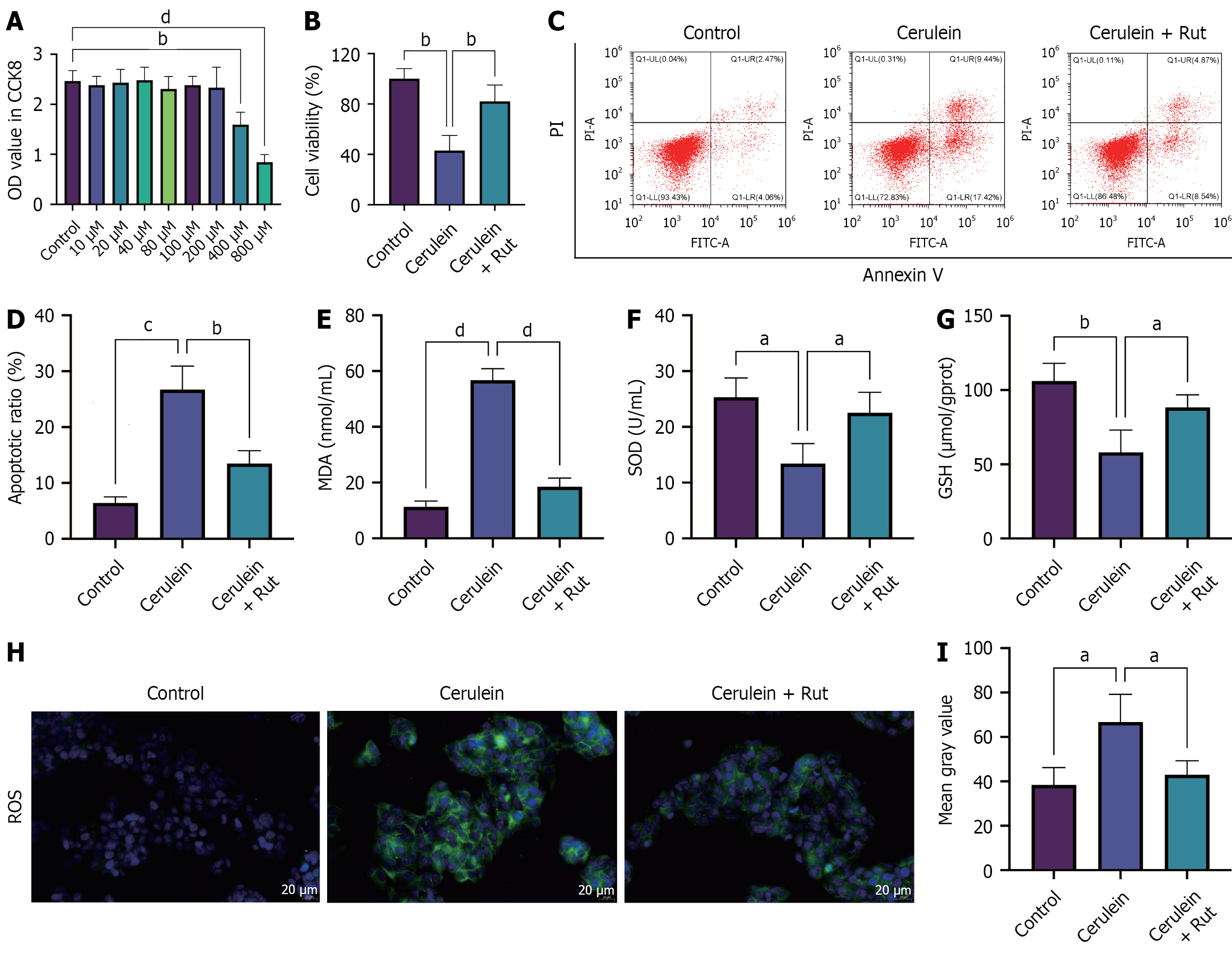
Figure 3 Rutaecarpine alleviates cerulein-induced AR42J cell damage.
A cell model of acute pancreatitis was established in AR42J cells that were pre-incubated with rutaecarpine. A and B: Cell Counting Kit-8 was used to determine the safe concentration of rutaecarpine (A) and assess the viability of AR42J cells with optimal concentration (B); C and D: Flow cytometry was used to detect cell apoptosis; E-G: Assay kits were utilized to detect the levels of malondialdehyde, superoxide dismutase, and glutathione; H and I: The positive rate of reactive oxygen species was measured through immunofluorescence staining. n = 3; aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. OD: Optical density; Rut: Rutaecarpine; PI: Propidium iodide; MDA: Malondialdehyde; SOD: Superoxide dismutase; GSH: Glutathione; ROS: Reactive oxygen species.
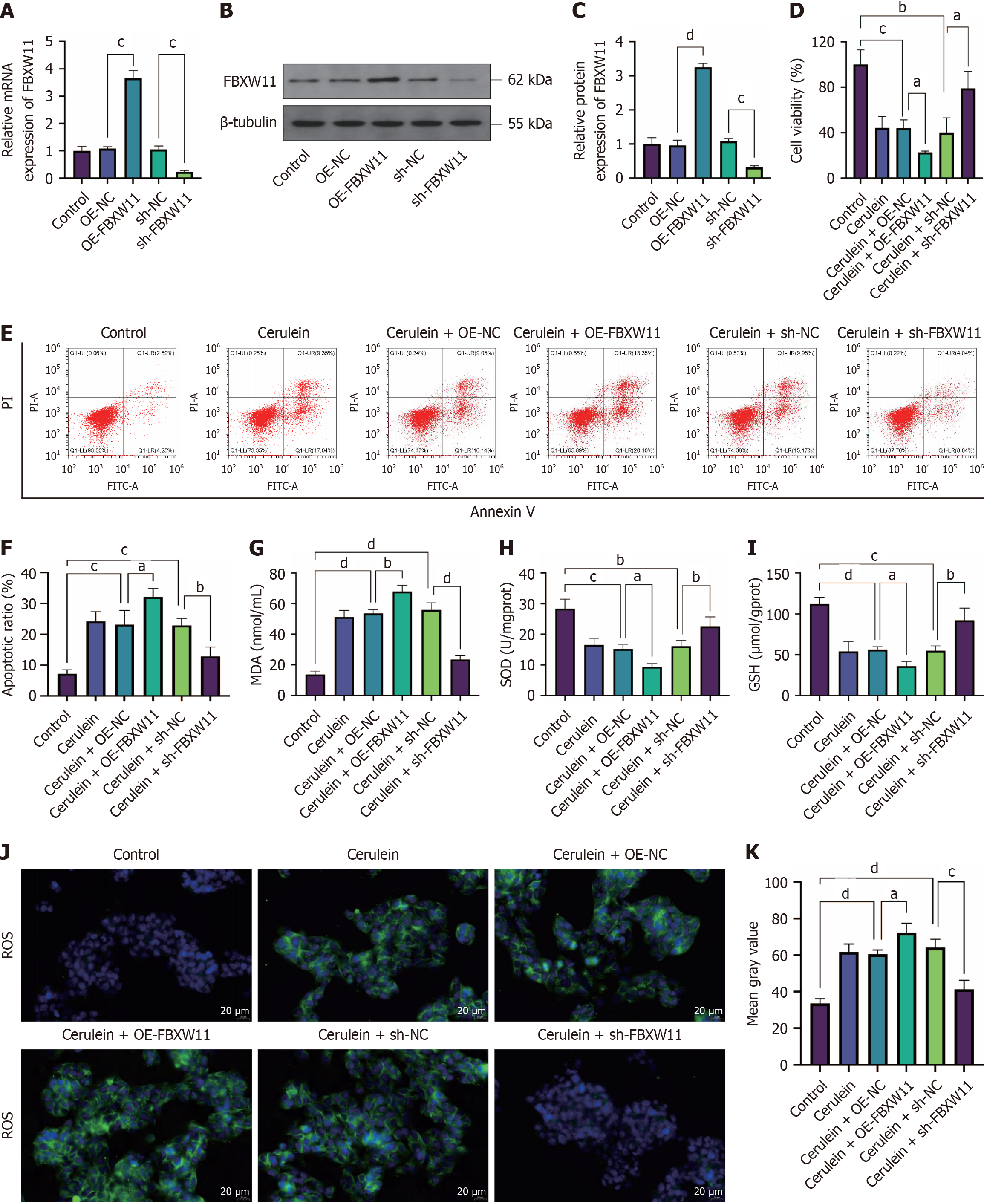
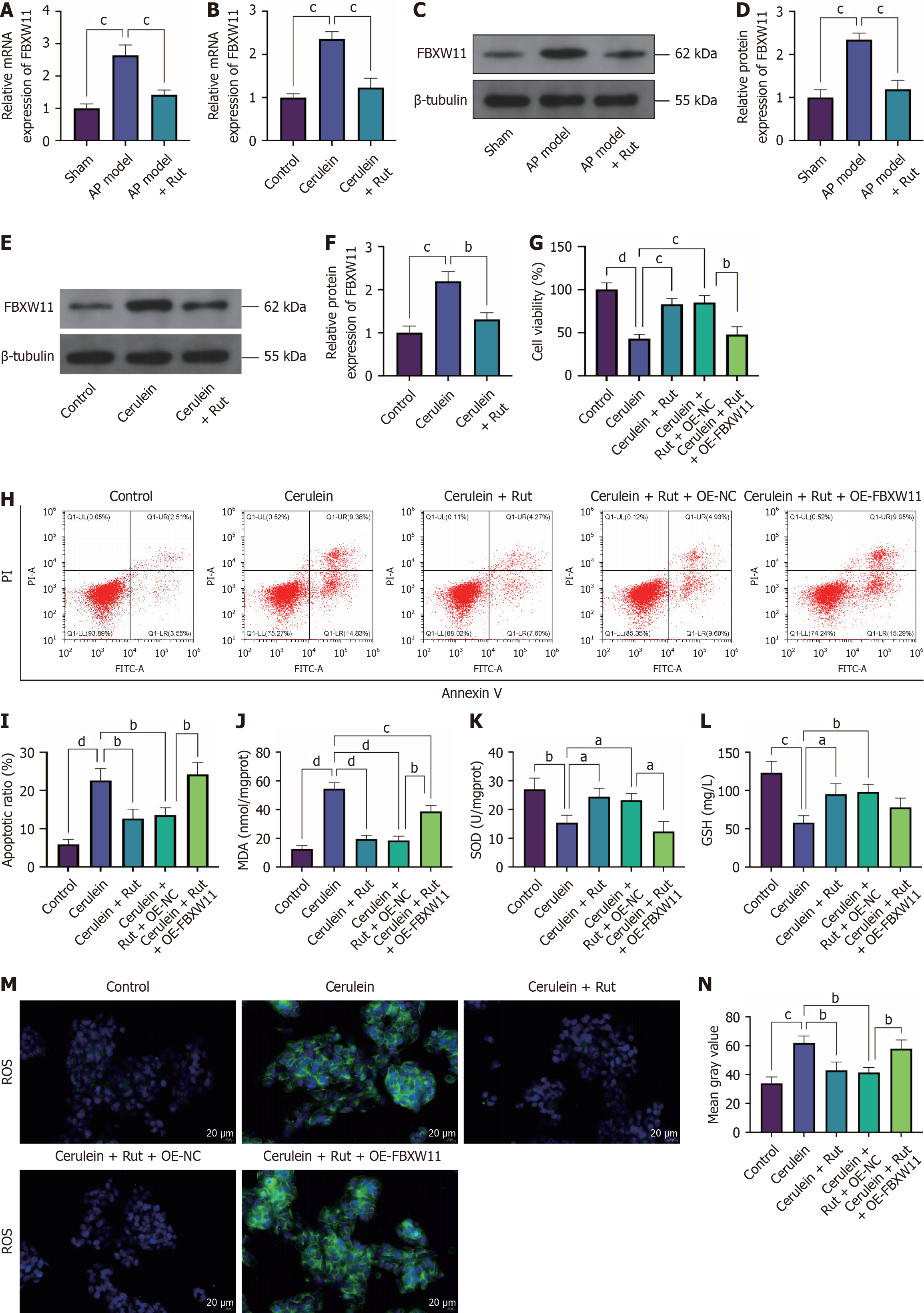
Figure 4 Impact of F-box and WD repeat domain containing 11 on acute pancreatitis cells.
AR42J cells were transfected with F-box and WD repeat domain containing 11-related vectors before cerulein treatment. A-C: Reverse transcription-quantitative polymerase chain reaction (A) and western blotting (B and C) detection of F-box and WD repeat domain containing 11 mRNA and protein; D-F: Cell Counting Kit-8 (D) and flow cytometry (E and F) assays to detect cell viability and apoptosis, respectively; G-I: Malondialdehyde, superoxide dismutase, and glutathione levels; J and K: Immunofluorescence detection of reactive oxygen species. n = 3; aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. FBXW11: F-box and WD repeat domain containing 11; OE-NC: Overexpression negative control; OE-FBXW11: F-box and WD repeat domain containing 11 overexpression; shNC: Short hairpin RNA negative control; shFBXW11: F-box and WD repeat domain containing 11 knockdown; PI: Propidium iodide; MDA: Malondialdehyde; SOD: Superoxide dismutase; GSH: Glutathione; ROS: Reactive oxygen species.
Figure 5 F-box and WD repeat domain containing 11 eliminates the protective effect of rutaecarpine on acute pancreatitis.
AR42J cells were transfected with F-box and WD repeat domain containing 11 overexpression and then pre-incubated with rutaecarpine before cerulein treatment. A-F: Reverse transcription-quantitative polymerase chain reaction (A and B) and western blotting (C-F) detection of F-box and WD repeat domain containing 11 mRNA and protein in both animal and cell models of acute pancreatitis; G-I: Cell Counting Kit-8 (G) and flow cytometry (H and I) assays to detect cell viability and apoptosis, respectively; J-L: Malondialdehyde, superoxide dismutase, and glutathione levels; M and N: Immunofluorescence detection of reactive oxygen species. n = 3; aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. FBXW11: F-box and WD repeat domain containing 11; AP: Acute pancreatitis; Rut: Rutaecarpine; OE-NC: Overexpression negative control; OE-FBXW11: F-box and WD repeat domain containing 11 overexpression; PI: Propidium iodide; MDA: Malondialdehyde; SOD: Superoxide dismutase; GSH: Glutathione; ROS: Reactive oxygen species.
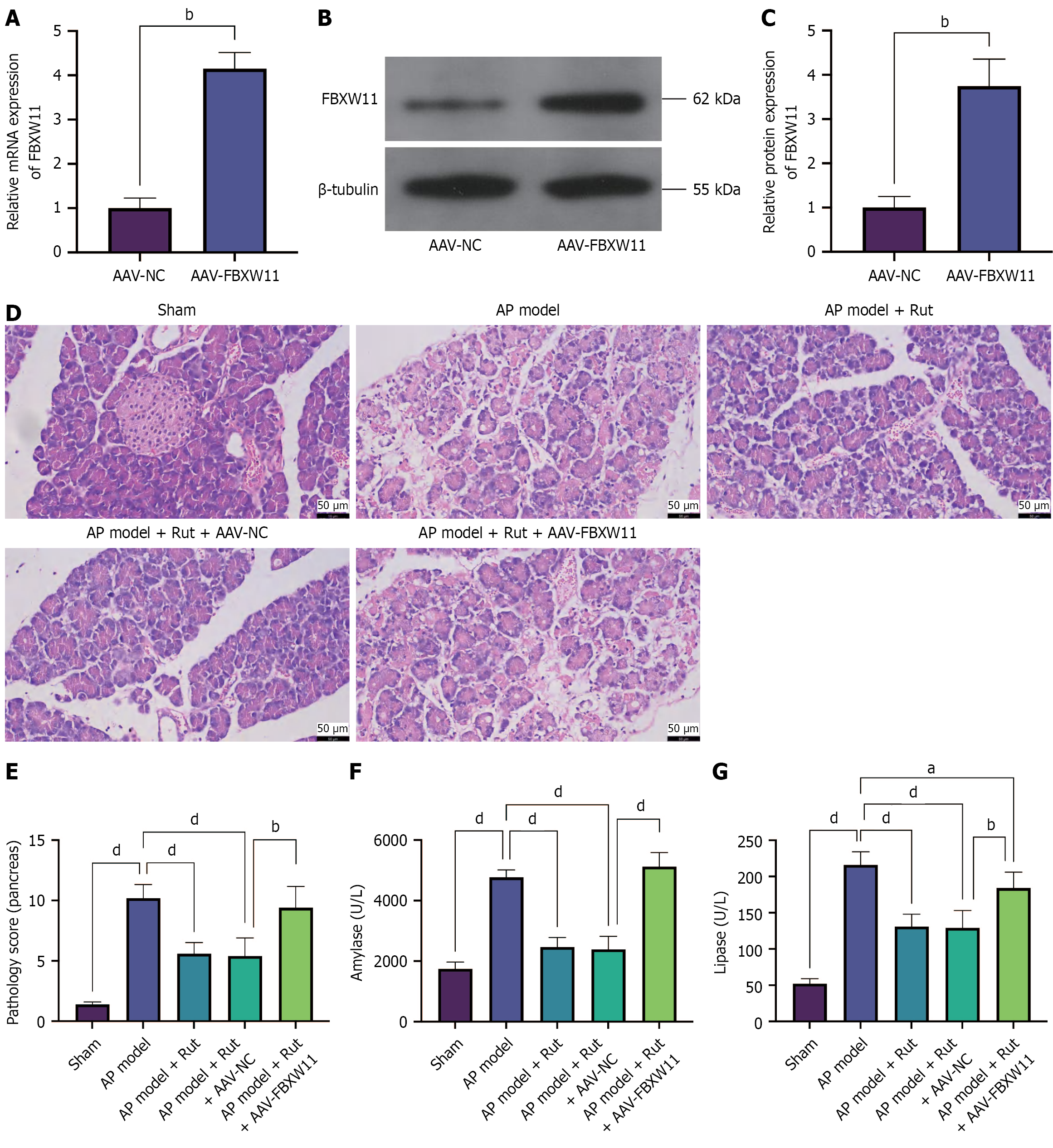
Figure 6 Rutaecarpine inhibits F-box and WD repeat domain containing 11 to reduce tissue injury in acute pancreatitis rats.
An acute pancreatitis model was established in rats infected with adeno-associated viral-F-box and WD repeat domain containing 11 and treated with rutaecarpine. A-C: Reverse transcription-quantitative polymerase chain reaction (A) and western blotting (B and C) to detect F-box and WD repeat domain containing 11 mRNA and protein; D: Hematoxylin and eosin staining to detect pancreatic tissue injury; E: Pathology score of pancreas; F and G: Biochemical analysis to determine serum amylase and lipase levels; n = 5; aP < 0.05, bP < 0.01, dP < 0.0001. AAV-NC: Adeno-associated viral-negative control; FBXW11: F-box and WD repeat domain containing 11; AAV-FBXW11: Adeno-associated viral-F-box and WD repeat domain containing 11; AP: Acute pancreatitis; Rut: Rutaecarpine.
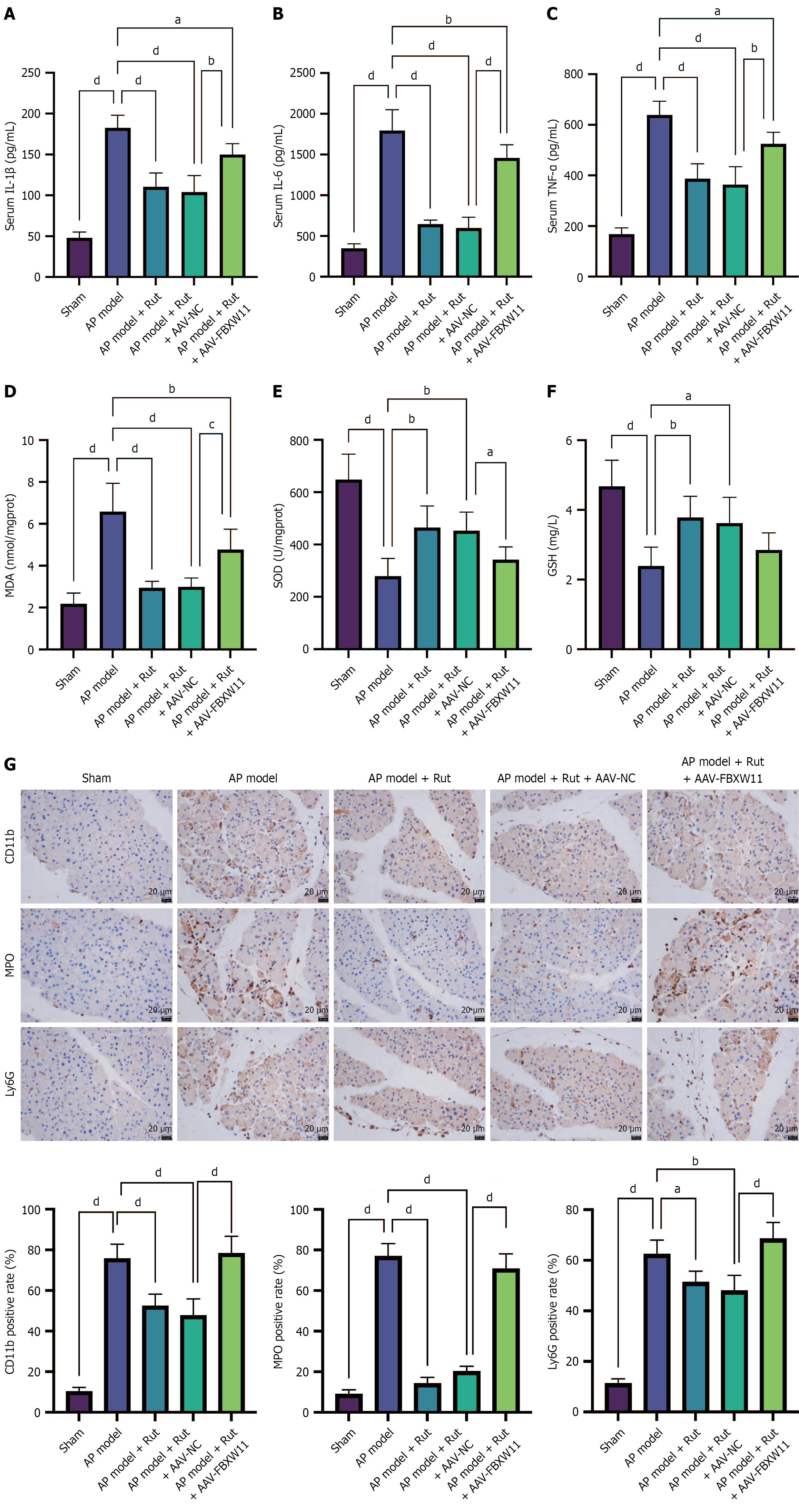
Figure 7 Rutaecarpine inhibits F-box and WD repeat domain containing 11 to reduce inflammation and oxidative stress in acute pancreatitis rats.
An acute pancreatitis model was established in rats infected with adeno-associated viral -F-box and WD repeat domain containing 11 and treated with rutaecarpine. A-C: Assay kits were used to measure the levels of serum inflammatory factors interleukin-1 beta, interleukin-6, and tumor necrosis factor alpha; D-F: The levels of pancreatic malondialdehyde, superoxide dismutase, and glutathione; G: Immunohistochemistry staining was performed to detect pancreatic CD11b, myeloperoxidase, and Ly6G. n = 5; aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. AP: Acute pancreatitis; Rut: Rutaecarpine; AAV-NC: Adeno-associated viral-negative control; FBXW11: F-box and WD repeat domain containing 11; AAV-FBXW11: Adeno-associated viral-F-box and WD repeat domain containing 11; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor alpha; MDA: Malondialdehyde; SOD: Superoxide dismutase; GSH: Glutathione; MPO: Myeloperoxidase.
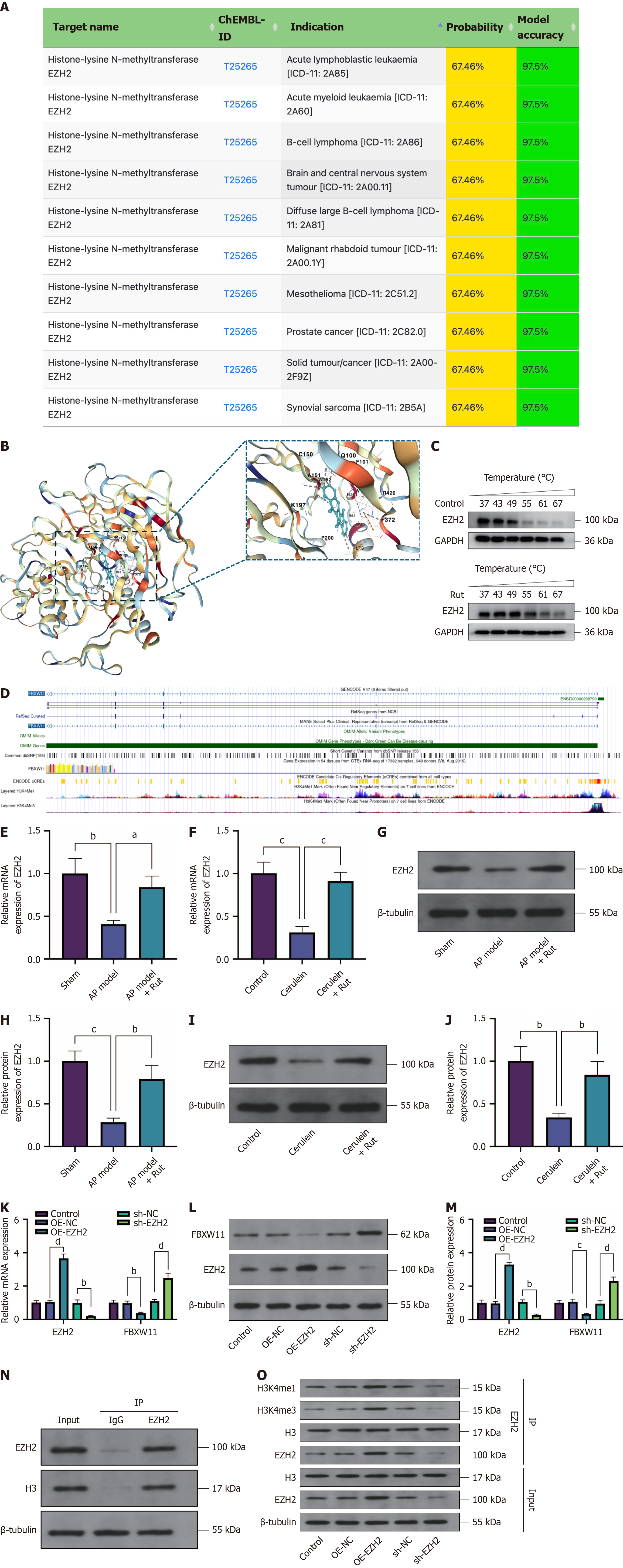
Figure 8 Rutaecarpine modulates F-box and WD repeat domain containing 11 expression through enhancer of zeste homolog 2.
The mechanism underlying rutaecarpine’s regulation of F-box and WD repeat domain containing 11 was detected. A: SuperPred prediction of proteins targeted by rutaecarpine (Rut); B: Binding interactions of Rut with enhancer of zeste homolog 2 (EZH2) (molecular docking simulation of Rut binding to EZH2 protein); C: Cellular thermal shift assay verification of Rut binding to EZH2; D: The University of California Santa Cruz database analysis of the FBXW11 promoter; E-J: Reverse transcription-quantitative polymerase chain reaction (E and F) and western blotting (G-J) detection of EZH2 mRNA and protein expression in both animal and cell models of acute pancreatitis treated with Rut; K-M: Effects of EZH2 overexpression and EZH2 knockdown transfection on FBXW11 expression; N and O: Co-immunoprecipitation detection of EZH2-histone H3 binding (N) and H3 methylation (O). n = 3; aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. EZH2: Enhancer of zeste homolog 2; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; Rut: Rutaecarpine; AP: Acute pancreatitis; FBXW11: F-box and WD repeat domain containing 11; OE-NC: Overexpression negative control; OE-EZH2: Enhancer of zeste homolog 2 overexpression; shNC: Short hairpin RNA negative control; sh-EZH2: Enhancer of zeste homolog 2 knockdown; IgG: Immunoglobulin G; H3: Histone H3; IP: Immunoprecipitation.
- Citation: Jia Y, Shi YX, Gu H, Liu Y, Peng J, Yan L. Rutaecarpine targets F-box and WD repeat domain containing 11 to inhibit inflammatory infiltration and alleviate acute pancreatitis. World J Gastroenterol 2025; 31(38): 109486
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/109486.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.109486