©The Author(s) 2025.
World J Gastroenterol. Sep 14, 2025; 31(34): 108617
Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.108617
Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.108617
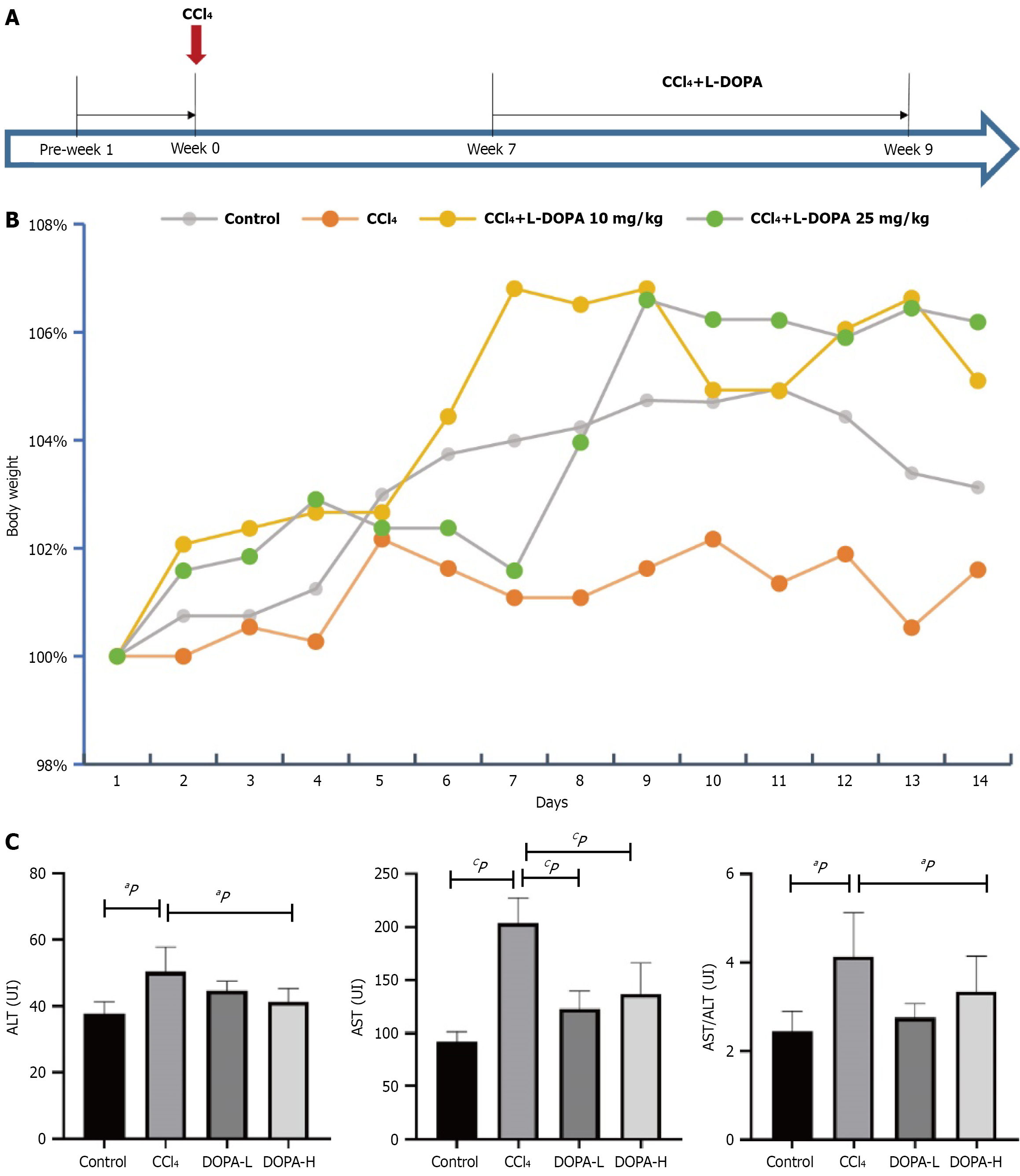
Figure 1 Evaluation of the anti-fibrosis effect of L-DOPA in carbon tetrachloride-induced liver fibrosis rat model.
A: The rats were divided into four groups. On day 0, 40% carbon tetrachloride (CCl4) was injected intraperitoneally once every three days. L-DOPA (10 mg/kg) was intraperitoneally injected daily from the 7th to the 9th week in the low-dose and the injection group, while L-DOPA (25 mg/kg) was intraperitoneally injected daily from the 7th to the 9th week in the high dose group. The intervention effect of L-DOPA on liver fibrosis throughout the entire process of fibrosis and inflammation was observed with normal saline as a control; B: Changes were visualized during the experiment, the body weight of the three groups of rats was measured and standardized to the initial weight at week 7; C: Tukey's multiple comparison tests were performed to detect the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and the ratio of AST to ALT in serum of rats in different groups. aP < 0.05; cP < 0.001; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; CCl4: Carbon tetrachloride.
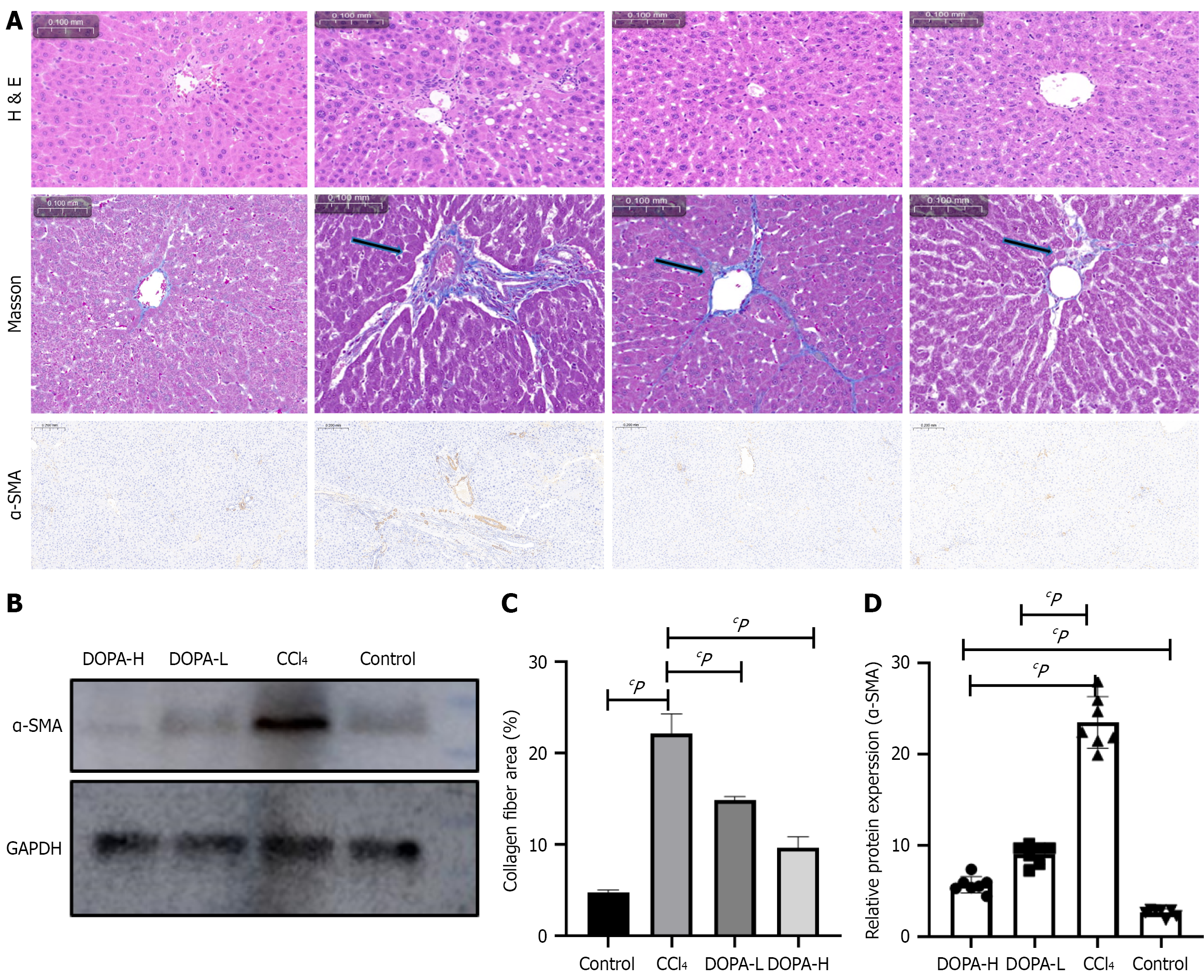
Figure 2 Morphological, histopathological and immunohistochemical analysis of the effect of L-DOPA on carbon tetrachloride-induced liver fibrosis.
A: Representative images of rat liver treated with hematoxylin-eosin and Masson's staining, and α-smooth muscle actin (α-SMA) immunohistochemical staining; B: Western blotting analysis of α-SMA in lysed liver tissue; C and D: The results were standardized relative to of GAPDH expression, and the representative protein bands are displayed on the corresponding histogram. ImageJ software (NIH) was used for quantitative analysis of α-SMA positive area, n = 8/group. cP < 0.001; NS: Not significant; H&E: Hematoxylin and eosin; α-SMA: Α-smooth muscle actin; CCl4: Carbon tetrachloride.
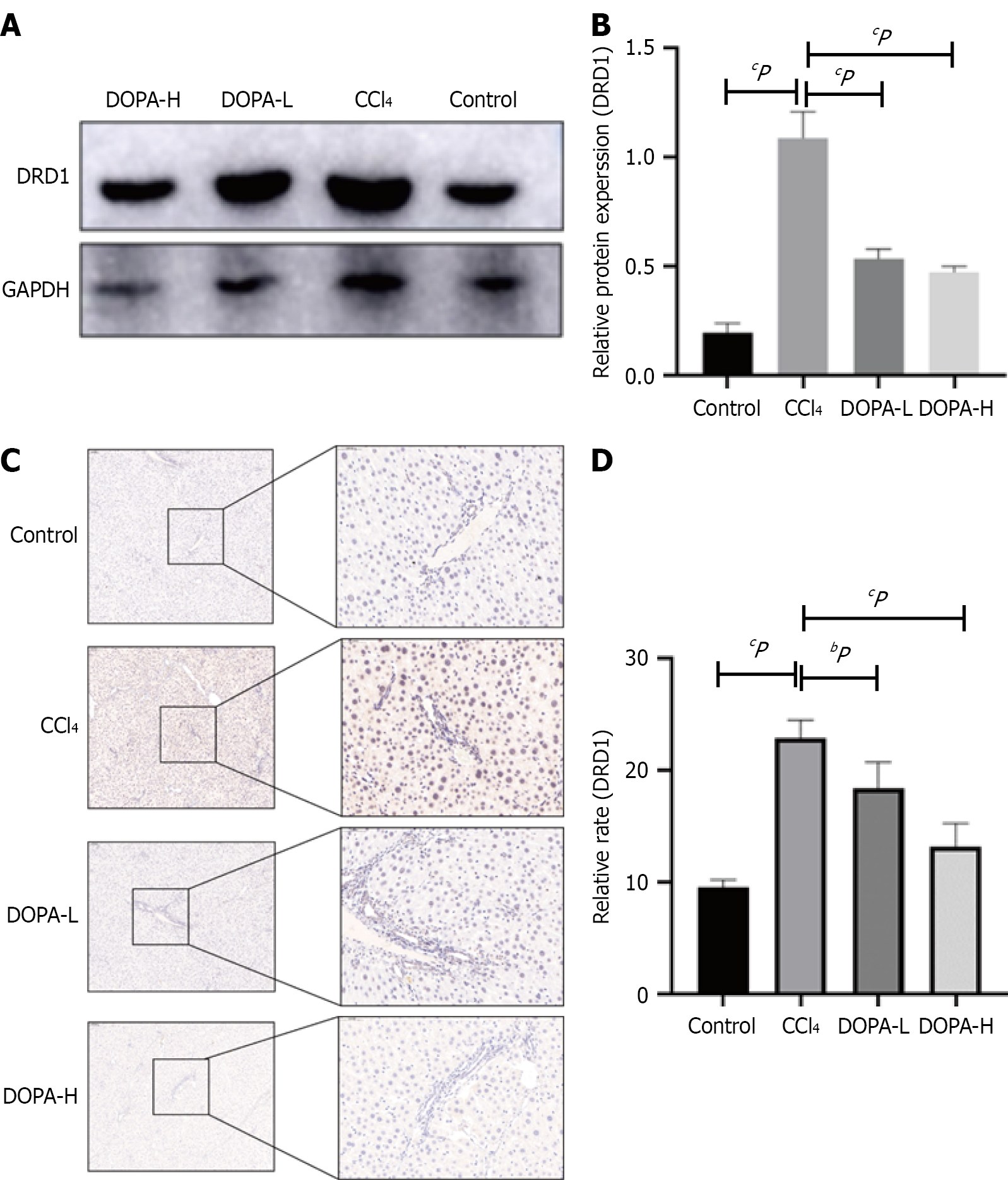
Figure 3 L-DOPA treatment of liver dopamine D1 pathway.
A and B: Western blotting analysis of dopamine D1 (DRD1) in lysed liver tissue, the results were standardized relative to GAPDH expression, and the representative protein bands (B) are displayed on the corresponding histogram; C: Representative fiber images of DRD1 immunohistochemistry in the liver (original magnification, × 100). Scale bar = 100 μm; D: ImageJ software (NIH) was used for quantitative analysis of DRD1 positive area, n = 8/group. bP < 0.01; cP < 0.001; NS: Not significant; CCl4: Carbon tetrachloride; DRD1: Dopamine D1.
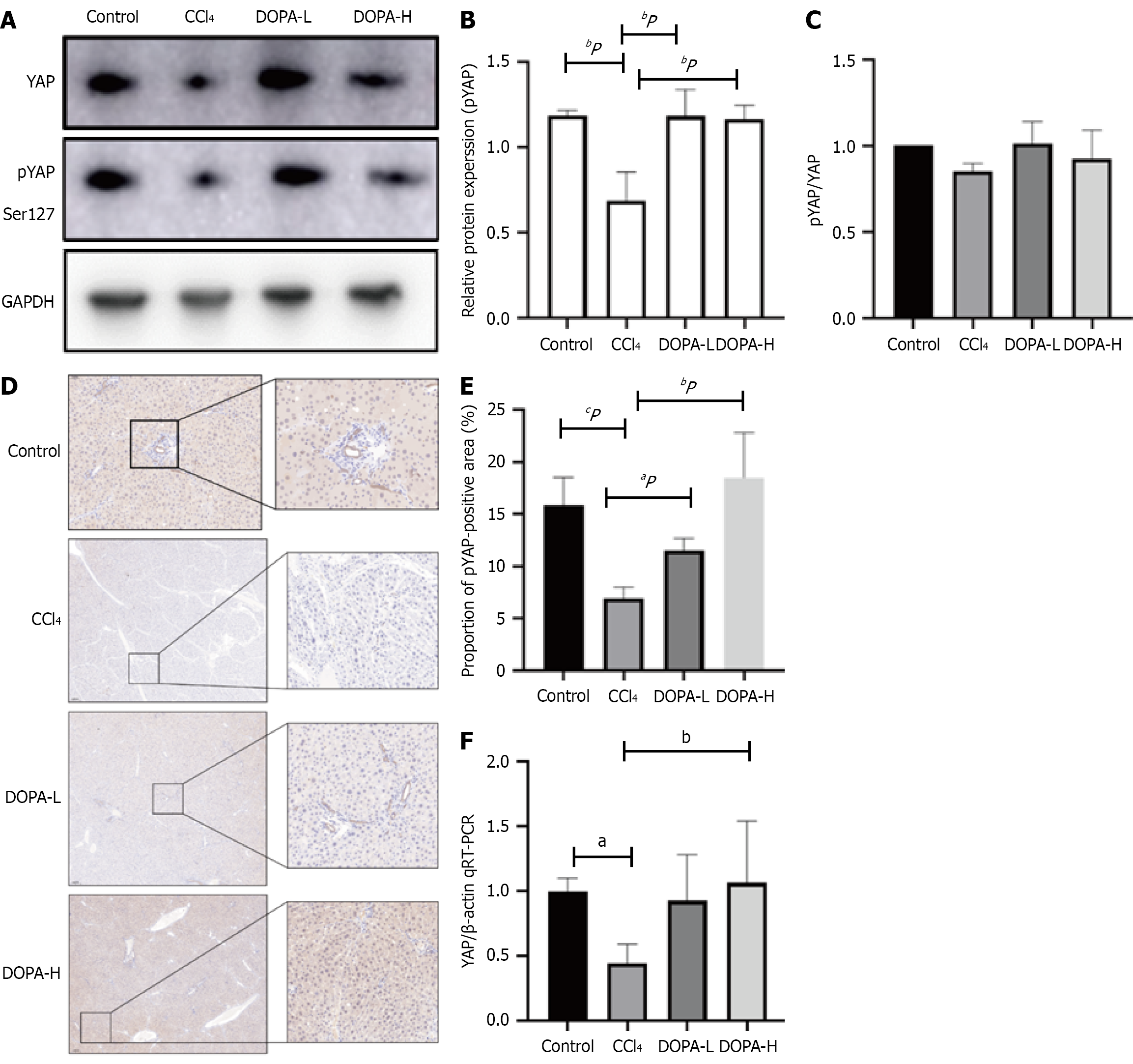
Figure 4 Phosphorylation of Yes-associated protein 1 in Hippo signaling pathway.
A: Western blotting was used to analyze the expression of p-Yes-associated protein 1 (YAP) in lysed liver tissue, and the results were standardized relative to GAPDH expression (n = 8); B: The representative protein bands were shown on the corresponding histogram; C: The ratio of pYAP to YAP is shown; D: Representative fiber images of liver pYAP immunohistochemistry (original magnification, × 100). Scale bar = 100 μm; E: ImageJ software (NIH) was used for quantitative analysis of DRD1 positive area, n = 8/group; F: Quantitative real-time polymerase chain reaction was used to detect the mRNA expression level of YAP. aP < 0.05; bP < 0.01; cP < 0.001; NS: Not significant; CCl4: Carbon tetrachloride; YAP: Yes-associated protein 1; qRT-PCR: Reverse transcription-quantitative real-time polymerase chain reaction.
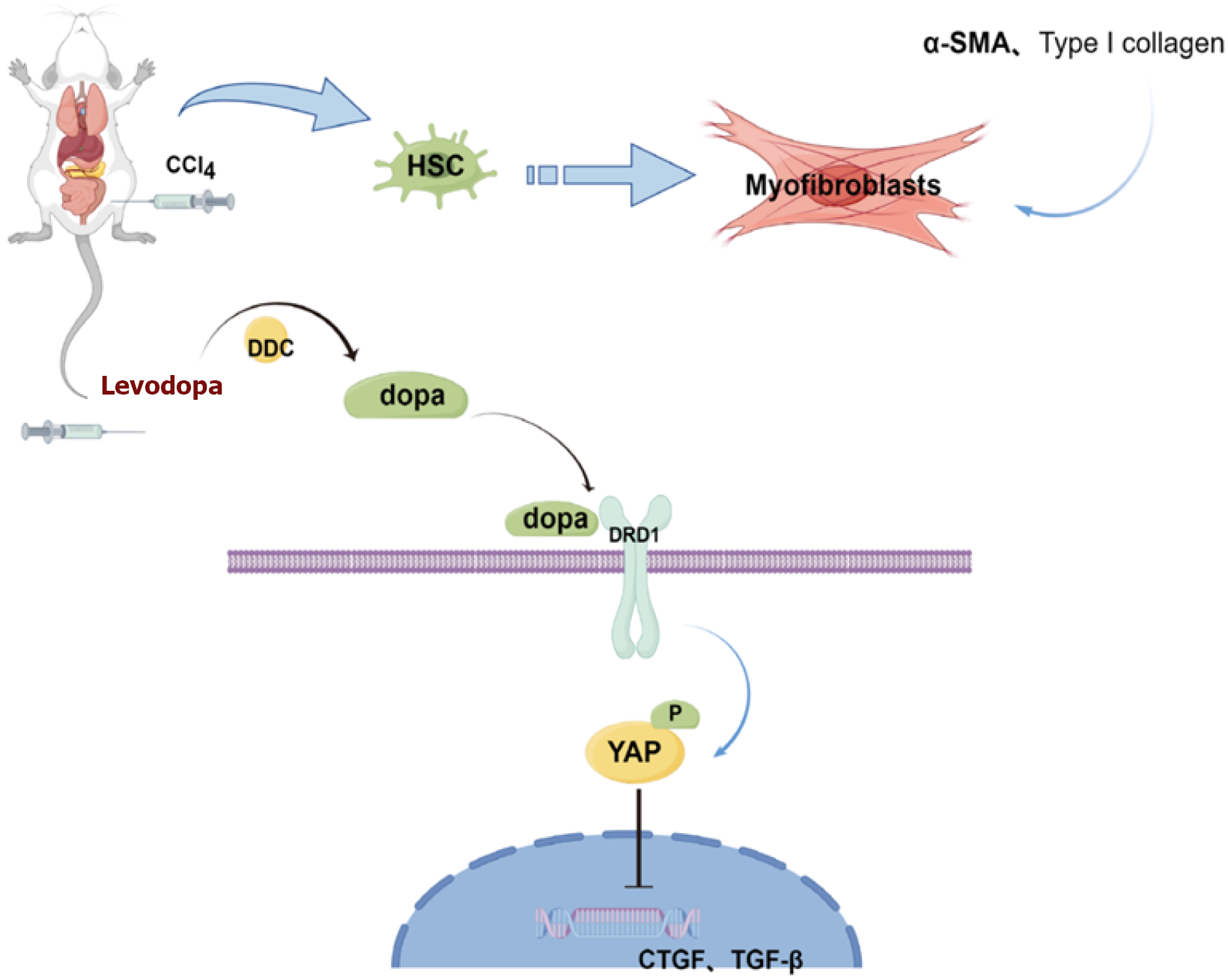
Figure 5 Schematic diagram illustrates the protective mechanism of levodopa on liver fibrosis by inhibiting the Hippo/Yes-associated protein 1 signaling pathway.
HSC: Hepatic stellate cell; CCl4: Carbon tetrachloride; α-SMA: Α-smooth muscle actin; DDC: Dopa decarboxylase, dopamine dopa; DRD1: Dopamine receptor 1; YAP: Yes-associated protein 1.
- Citation: Wang HY, Qi MM, Zhang K, Zhu YZ, Zhang J. Dopamine receptor D1-mediated suppression of liver fibrosis via Hippo/Yes-associated protein 1 signaling in levodopa treatment. World J Gastroenterol 2025; 31(34): 108617
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/108617.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.108617