©The Author(s) 2025.
World J Gastroenterol. Aug 28, 2025; 31(32): 108827
Published online Aug 28, 2025. doi: 10.3748/wjg.v31.i32.108827
Published online Aug 28, 2025. doi: 10.3748/wjg.v31.i32.108827
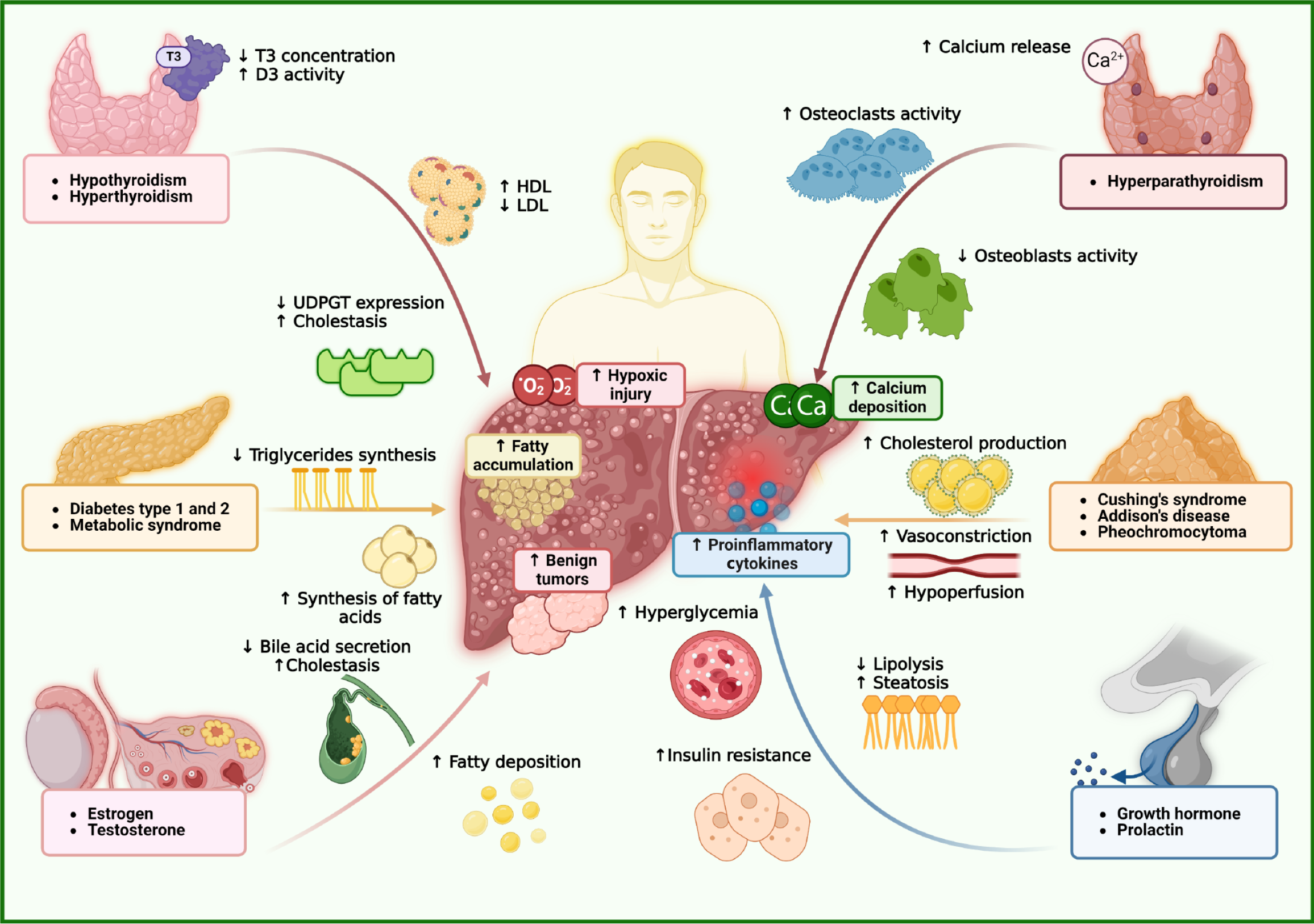
Figure 1 Interactions between endocrine organs and hepatic function.
In hypothyroidism, reduced triiodothyronine levels impair β-oxidation and downregulate uridine diphosphate-glucuronosyltransferase, leading to lipid accumulation and defective bilirubin conjugation, which contribute to cholestasis. Elevated tumor necrosis factor-α and decreased deiodinase activity further exacerbate liver dysfunction. In contrast, hyperthyroidism increases metabolic rate and hepatic oxygen demand, accelerating lipid turnover but inducing hepatocellular injury through oxidative stress and hypoxia. This results in reduced low-density lipoprotein levels and paradoxically elevated high-density lipoprotein, possibly due to increased apolipoprotein A1 synthesis. In adrenal dysfunction, hyperaldosteronism activates mineralocorticoid receptor and epithelial sodium channel, causing sodium retention, ascites, and edema compounded by oxidative stress. Hypercortisolism enhances gluconeogenesis and hepatic lipid accumulation, raising the risk of metabolic dysfunction-associated steatotic liver disease, while adrenal insufficiency impairs anti-inflammatory responses, promoting inflammation. Sex hormones also modulate hepatic physiology. Low testosterone decreases glucose transporter type 4 activity, contributing to insulin resistance and sarcopenia. Increased aromatase activity converts testosterone to estradiol, leading to hyperestrogenism, cholestasis, and benign hepatic tumors. Estrogen signaling through estrogen receptor α offers protective effects by reducing oxidative stress and inflammation, thereby mitigating steatosis and fibrosis. In contrast, growth hormone and prolactin have been associated with increased hepatic steatosis. D3: Type 3 deiodinase; Ca: Calcium; O2: Oxygen; T3: Triiodothyronine; UDPGT: Uridine diphosphate-glucuronosyltransferase; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
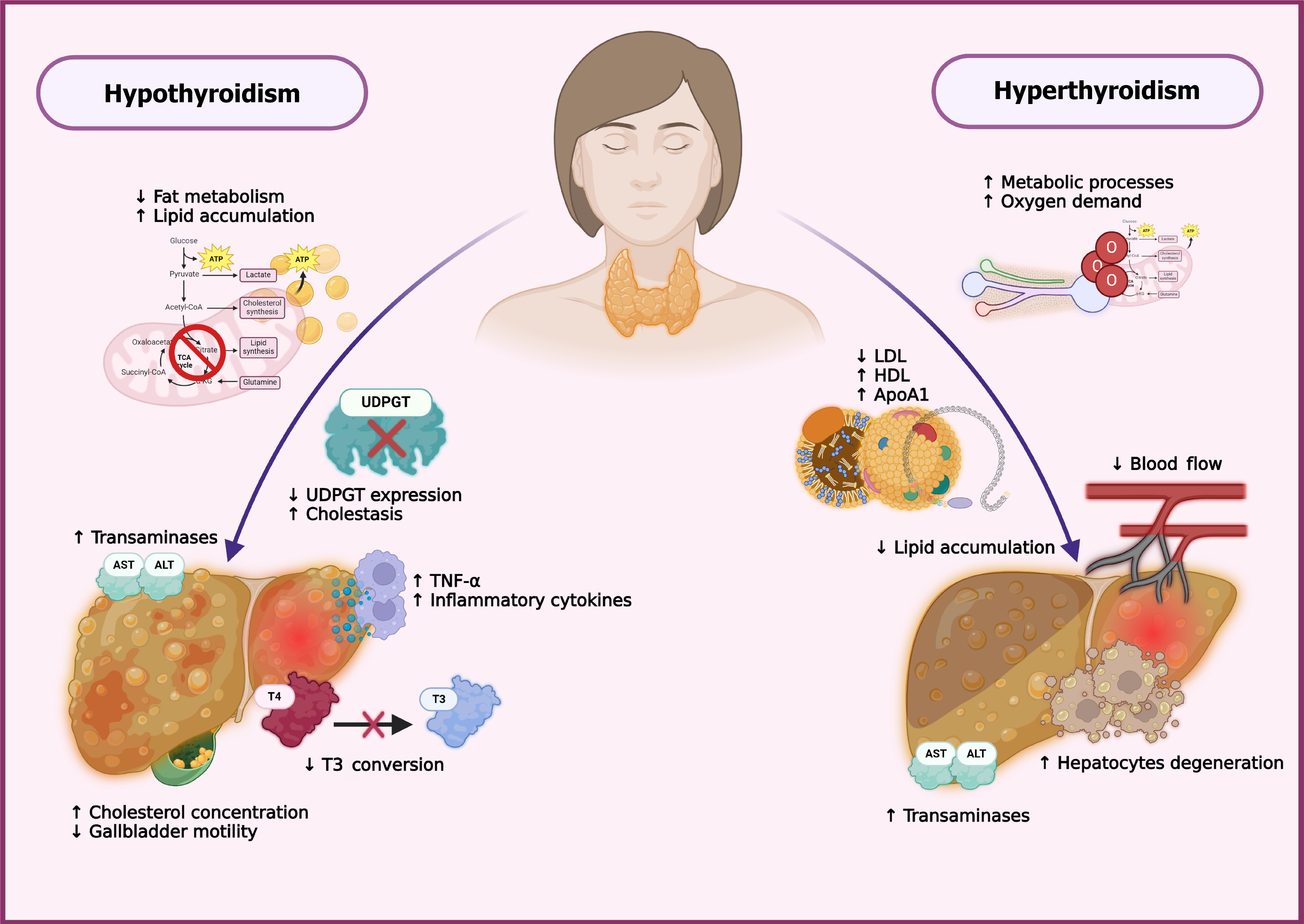
Figure 2 The role of the thyroid gland.
In hypothyroidism, reduced levels of thyroxine (T4) and triiodothyronine (T3) lower the basal metabolic rate and impair lipid metabolism, leading to hepatic lipid accumulation due to decreased β-oxidation and suppressed lipolytic gene expression. It also reduces uridine diphosphate-glucuronosyltransferase activity, impairing bilirubin conjugation and promoting cholestasis. Diminished D1 activity limits T4-to-T3 conversion, and elevated proinflammatory cytokines like tumor necrosis factor-α contribute to hepatocellular injury and increased transaminase levels. Hypothyroidism also impairs low-density lipoprotein (LDL) clearance and bile acid synthesis, raising serum cholesterol and reducing gallbladder motility. Conversely, hyperthyroidism accelerates lipid metabolism and stimulates lipoprotein lipase activity, which decreases LDL levels. Despite this, high-density lipoprotein concentrations tend to rise, possibly due to enhanced synthesis of apolipoprotein A1. However, increased oxidative stress and hepatic oxygen demand can lead to hepatocyte damage, particularly in centrilobular zones. These contrasting effects emphasize the bidirectional relationship between thyroid status and liver health. ATP: Adenosine triphosphate; TCA: Tricarboxylic acid cycle; T3: Triiodothyronine; UDPGT: Uridine diphosphate-glucuronosyltransferase; T4: Thyroxine; TNF: Tumor necrosis factor; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ApoA1: Apolipoprotein A1; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
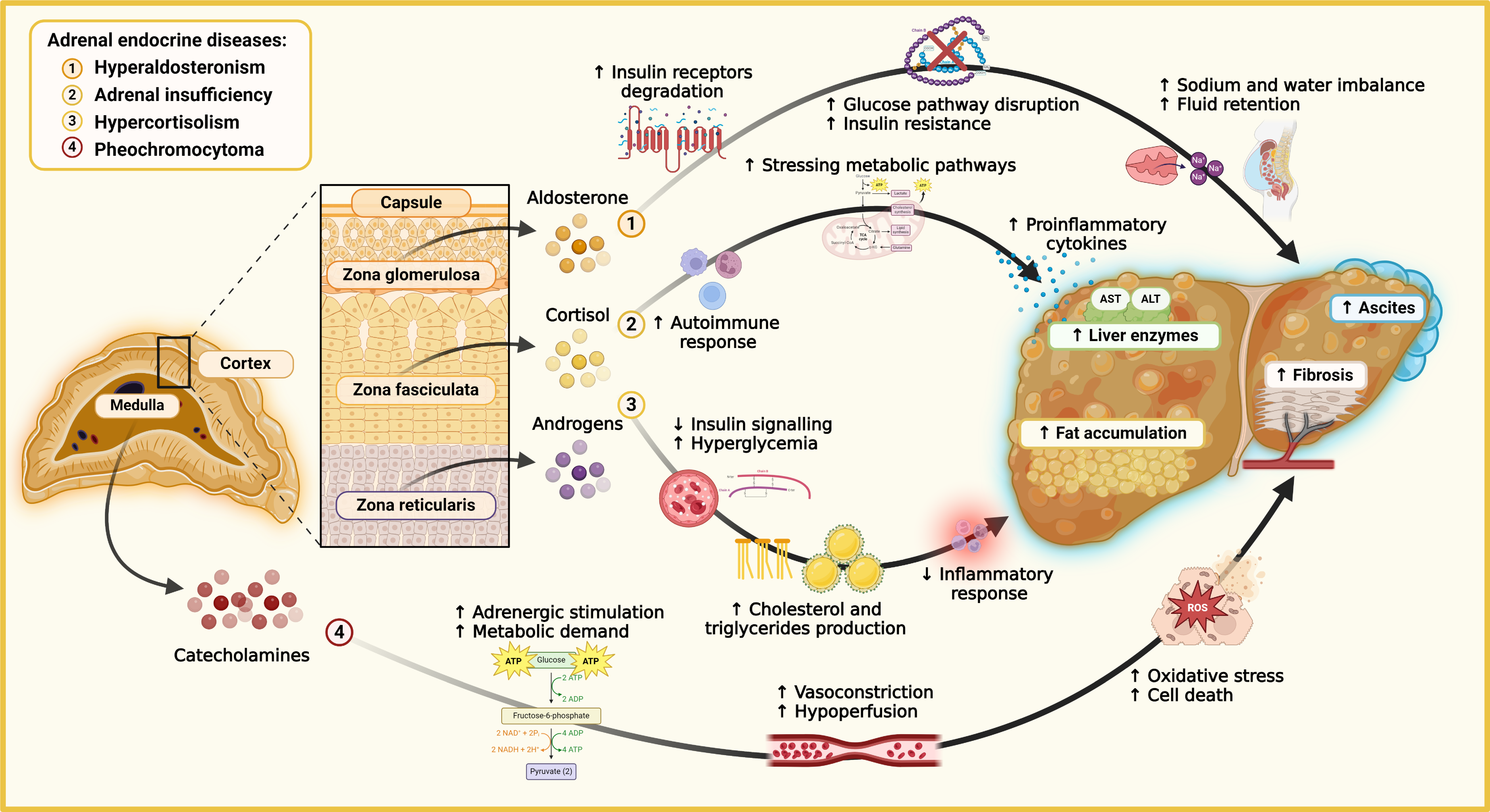
Figure 3 The link between adrenal diseases and liver dysfunction.
Molecular pathways connecting adrenal endocrine disorders with liver dysfunction and metabolic complications. In secondary hyperaldosteronism, excess aldosterone from the zona glomerulosa activates mineralocorticoid receptors, leading to sodium and water retention, ascites, and worsening portal hypertension. Aldosterone also increases insulin receptor degradation, contributing to insulin resistance and disrupting glucose homeostasis. In adrenal insufficiency, reduced cortisol from the zona fasciculata impairs anti-inflammatory responses, promoting autoimmune reactions and proinflammatory cytokines like interleukin-6 and tumor necrosis factor-α, which drive liver inflammation and fibrosis. Excess cortisol disrupts insulin signaling pathways in hypercortisolism, causing hyperglycemia, increased cholesterol and triglyceride synthesis, and ectopic hepatic fat accumulation, leading to steatotic liver disease. In pheochromocytoma, excessive catecholamines from the adrenal medulla increase adrenergic stimulation, resulting in lipolysis, oxidative stress, and reactive oxygen species production, causing vasoconstriction, hypoperfusion, and liver cell damage. These combined effects manifest as elevated liver enzymes, ascites formation, and fibrosis. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ATP: Adenosine triphosphate; ROS: Reactive oxygen species; ADP: Adenosine diphosphate; NAD: Nicotinamide adenine dinucleotide.
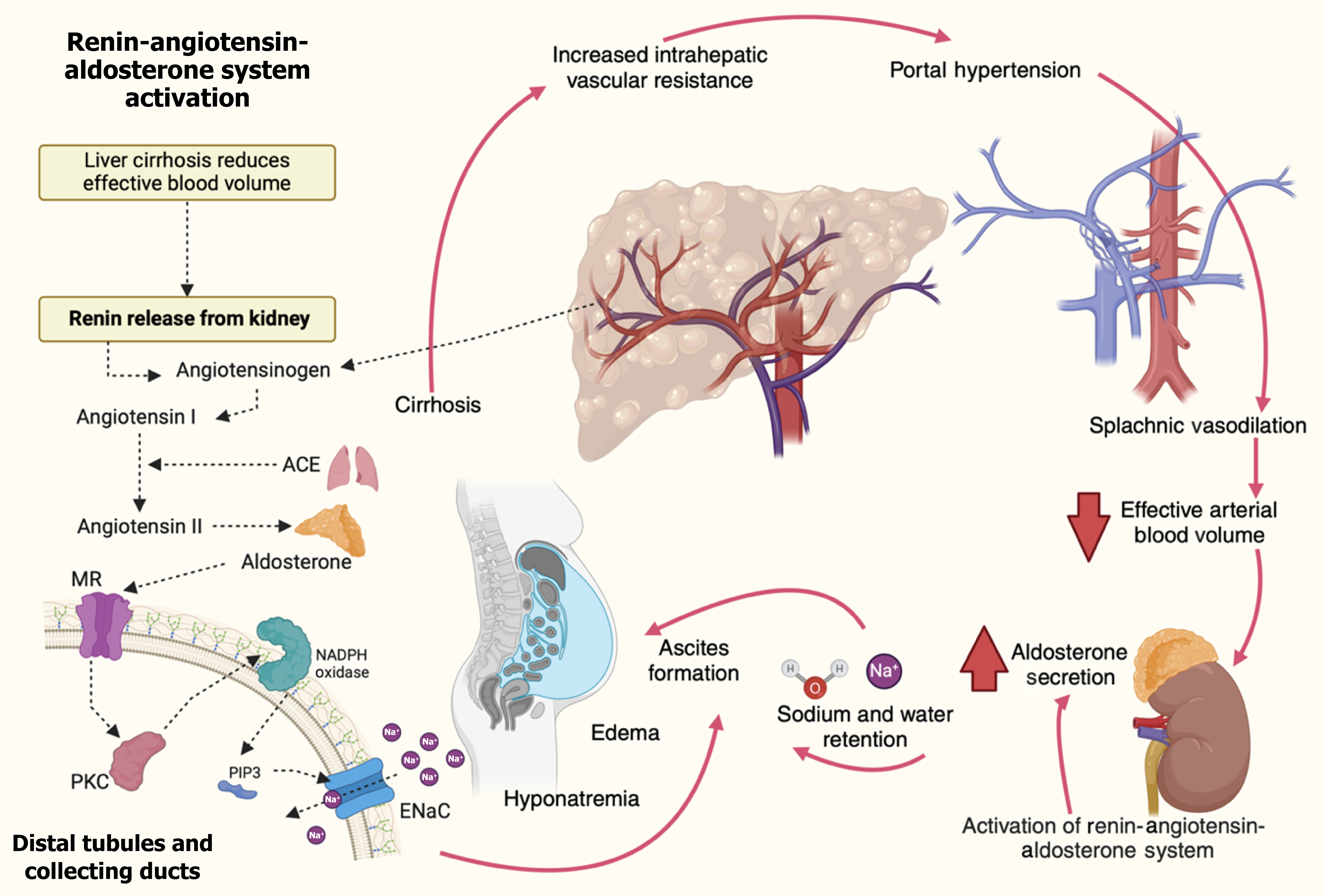
Figure 4 Pathophysiology of secondary hyperaldosteronism in cirrhosis.
Molecular mechanisms drive secondary hyperaldosteronism in cirrhosis. Liver fibrosis increases intrahepatic vascular resistance, causing portal hypertension and splanchnic vasodilation. This reduces adequate arterial blood volume despite normal or increased total blood volume. The kidneys respond by activating the renin-angiotensin-aldosterone system (RAAS), triggering renin release, which converts angiotensinogen to angiotensin I. Angiotensin-converting enzyme converts angiotensin I to angiotensin II, stimulating the adrenal glands to secrete aldosterone. Aldosterone promotes sodium and water retention by activating mineralocorticoid receptors in the distal tubules and collecting ducts. This leads to upregulating the epithelial sodium channels (ENaC) and the sodium ion/potassium ion adenosine triphosphate pump. At the molecular level, aldosterone-induced activation of protein kinase C and phosphatidylinositol-3,4,5-trisphosphate (PIP3) enhances ENaC activity. PIP3 serves as a secondary messenger that facilitates the insertion of ENaC into the cell membrane, promoting sodium reabsorption. Oxidative stress via nicotinamide adenine dinucleotide phosphate oxidase also potentiates ENaC activation and fluid retention. This cascade leads to ascites formation, edema, and hyponatremia. The cycle of portal hypertension, RAAS activation, and sodium retention perpetuates the development of ascites and exacerbates the complications of cirrhosis. ACE: Angiotensin-converting enzyme; ENaC: Epithelial sodium channels; Na: Sodium; MR: Mineralocorticoid receptor; PIP3: Phosphatidylinositol-3,4,5-trisphosphate; PKC: Protein kinase C.
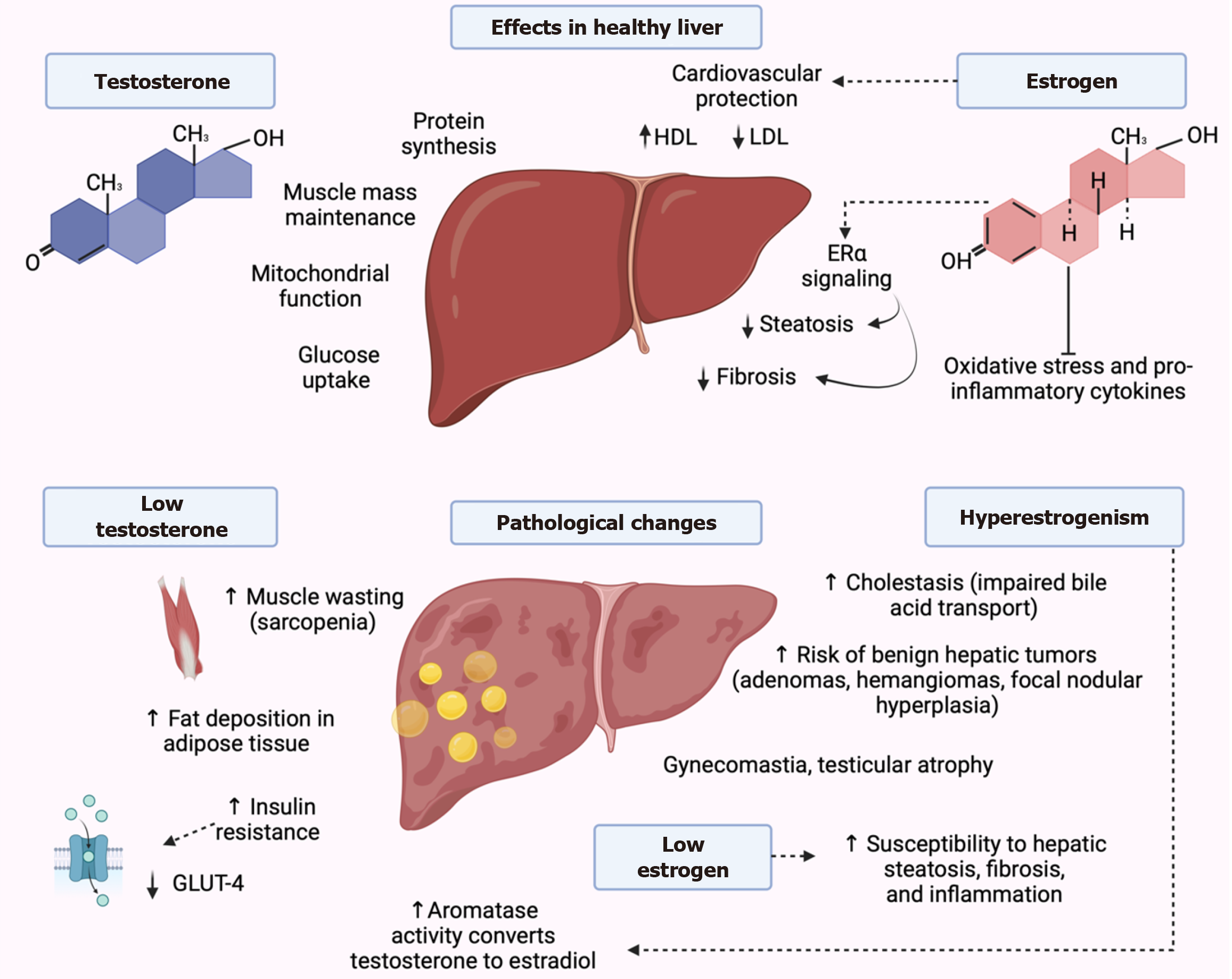
Figure 5 Molecular interplay between sex hormones and liver pathology.
Testosterone promotes protein synthesis, mitochondrial function, and glucose uptake via androgen receptor activation, supporting muscle mass and metabolic homeostasis. In cirrhosis, reduced testosterone levels lead to sarcopenia, insulin resistance, and increased adiposity. Additionally, elevated aromatase activity enhances the conversion of testosterone to estradiol, contributing to hyperestrogenism. Estrogens, through estrogen receptors (ER) α, ERβ, and G-protein-coupled ER, regulate lipid metabolism, enhance high-density lipoprotein, reduce low-density lipoprotein, and activate antioxidant and anti-inflammatory pathways, protecting against steatosis and fibrosis. However, excess estrogen in advanced liver disease is linked to cholestasis, benign hepatic tumors, and feminizing features such as gynecomastia and testicular atrophy. Low estrogen levels, conversely, are associated with increased hepatic fat accumulation, fibrosis, and inflammation. These interactions emphasize the importance of hormonal balance in maintaining liver health and mitigating progression of liver disease. GLUT-4: Glucose transporter type 4; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; ERα: Estrogen receptor α.
- Citation: Vargas-Beltran AM, Armendariz-Pineda SM, Martínez-Sánchez FD, Martinez-Perez C, Torre A, Cordova-Gallardo J. Interplay between endocrine disorders and liver dysfunction: Mechanisms of damage and therapeutic approaches. World J Gastroenterol 2025; 31(32): 108827
- URL: https://www.wjgnet.com/1007-9327/full/v31/i32/108827.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i32.108827