Published online Aug 28, 2025. doi: 10.3748/wjg.v31.i32.108654
Revised: July 10, 2025
Accepted: August 4, 2025
Published online: August 28, 2025
Processing time: 77 Days and 19.9 Hours
Pancreatic cancer, characterized by aggressive proliferation and metastasis, is a lethal malignancy. The nightly hormone melatonin serves as a rhythm-regulating hormone, and is used to treat different cancers including pancreatic cancer.
To investigate how melatonin acts against human pancreatic cancer cell lines and analyze the biological processes that cause the observed effects.
Panc-1 and AsPC-1 cells were treated with melatonin. Cell viability was measured using the cell counting kit-8 assay. Western blotting and immunofluorescence were used to analyze protein expression levels. Ferroptosis was measured by analyzing lipid reactive oxygen species and malondialdehyde levels; apoptosis was assessed using flow cytometry.
Melatonin significantly inhibited the viability, colony formation, migration, and invasion of Panc-1 and AsPC-1 cells. Additionally, melatonin activated the endoplasmic reticulum (ER) stress pathway (protein kinase R-like ER kinase-eukaryotic initiation factor 2α-activating transcription factor 4), inhibited glutamine metabolism (alanine-serine-cysteine transporter 2-glutaminase 1-glutathione peroxidase 4, alanine-serine-cysteine transporter 2-glutathione peroxidase 4), and promoted ferroptosis in pancreatic cancer cells. Co-treatment with a high melatonin concentration and protein kinase R-like ER kinase agonist (CCT020312) enhanced melatonin-induced ferroptosis in pancreatic cancer cells. Melatonin demonstrated a variety of anticancer effects by inhibiting autophagy. This was achieved through the increased expression of sequestosome-1 and decreased expression of light chain 3. Additionally, melatonin facilitated the promotion of apoptosis.
Melatonin induces ferroptosis in pancreatic cancer cells by activating transcription factor 4-dependent ER stress and inhibiting glutamine metabolism, promotes apoptosis in pancreatic cancer cells, and inhibits autophagy, leading to synergistic anticancer effects.
Core Tip: In the present study, we demonstrate that melatonin activates endoplasmic reticulum stress-mediated-ferroptosis in the protein kinase R-like endoplasmic reticulum kinase-eukaryotic initiation factor 2α-activating transcription factor 4 axis via inhibition of the alanine-serine-cysteine transporter 2-glutathione peroxidase 4 signaling pathway, thereby exerting an anti-cancer effect on pancreatic cancer cells. These new findings suggest that melatonin may act as a potent anti-tumor agent and may have great potential as an adjuvant therapy in the future.
- Citation: Zhao Q, Zhang H, Wu HM, Yang QY, Zhao H, Kang L, Lv XY. Melatonin-induced ferroptosis in pancreatic cancer cells by stimulating endoplasmic reticulum stress and inhibiting alanine-serine-cysteine transporter 2-driven glutamine metabolism. World J Gastroenterol 2025; 31(32): 108654
- URL: https://www.wjgnet.com/1007-9327/full/v31/i32/108654.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i32.108654
Pancreatic cancer is one of the most aggressive and deadly forms of cancer, with its global prevalence steadily increasing in recent years. The World Health Organization predicts that by 2030, it will rank as the second most common cause of cancer fatalities. This is largely due to challenges in early detection and poor treatment outcomes. As of now, the 5-year survival rate for people diagnosed with pancreatic cancer is very low at around just 10%[1]. Therefore, developing novel therapeutic drugs for improving treatment outcomes and enhancing patient prognosis is essential. A recently identified form of regulated cell death, ferroptosis, is characterized by the presence of excessive amounts of iron dependent lipid peroxides. This phenomenon is closely related to oxidative damage and associated with several biochemistry pathways, in particular the control of glutathione (GSH) and activity of the enzyme GSH peroxidase 4 (GPX4)[2].
Targeting and inducing ferroptosis in tumor cells reverse their resistance to chemotherapy drugs[3]. Yamaguchi et al[4] demonstrated that the GSH level is lowered by S-transferase and concomitantly, while the levels of intracellular lipid reactive oxygen species (ROS) are increased. These ROS trigger ferroptosis in pancreatic cancer cells. Daher et al[5] showed that depletion of GSH by knocking out the solute carrier family 7 member 11 of the Xc-system of pancreatic cancer cell lines induced ferroptosis. Yet, the specific mechanisms whereby ferroptosis results in cell death in pancreatic cancer remain unresolved.
The solute carrier family 1 member 5 gene encodes alanine-serine-cysteine transporter 2 (ASCT2) and its critical function in transporting glutamine. Moreover, this transporter is essential for the metabolic functions in cancer cells. Glutamine contributes to cellular antioxidant defense by promoting the production of GSH, which reduces ROS formation and helps pancreatic cancer cells resist oxidative stress-induced damage[6]. Therefore, exploring the role and regulatory mechanisms of this metabolic pathway in pancreatic cancer is crucial to understanding its pathogenesis and acquiring new insights for developing therapeutic strategies aimed at improving the prognosis of pancreatic cancer.
The cells of pancreatic cancer are relatively sensitive to ferroptosis, particularly under nutrient-deprived conditions, such as glutamine deficiency, wherein ferroptosis induction is pronounced. Glutamine provides the essential carbon and nitrogen for pancreatic cancer cell growth and protects cells against ferroptosis. However, glutamine inhibitors, such as l-azetidine-2-carboxylic acid and 6-diazo-5-oxo-l-norleucine, promote ROS formation and ferroptosis in pancreatic cancer cells. In contrast, ferroptosis inhibitors rescue pancreatic cancer cells from ferroptosis[7].
Endoplasmic reticulum (ER) stress develops from defective or unfolded proteins accumulating inside this cellular structure[8]. Activating transcription factor 4 (ATF4) is a member of the ATF/cyclic adenosine monophosphate response element binding protein family, which is frequently activated when stress signals emerge from factors like ER stress, nutrient shortages, oxidative stress, and low oxygen levels. These stress signals are transduced by various eukaryotic initiation factor 2α (eIF2α) kinases, which promote eIF2α phosphorylation and ATF4 mRNA translation. The upregulated ATF4 enhances oxidative stress resistance by activating glutamine metabolism mediated by ASCT2[8-10]. Tumor cells usually exploit the ER stress response to promote their survival and proliferation, and to adapt to harsh microenvironments. However, when stress exceeds a certain threshold, ER dysfunction causes tumor cell death. Excessive ATF4 activation promotes tumor cell death under stress conditions[11], particularly in the absence of glutamine. Nevertheless, whether ATF4 signaling inhibits glutamine metabolism and induces ferroptosis in pancreatic cancer cells remains unclear.
Melatonin (N-acetyl-5-methoxytryptamine), a vital bioactive molecule, is crucial in circadian rhythm regulation and various other functions, including antioxidant defense, anti-apoptotic effects, anti-inflammatory properties, iron metabolism regulation, metal chelation, and mitochondrial quality control[12]. Melatonin exerts tumor-suppressive effects on pancreatic cancer cells[13]. Additionally, a recent study investigated the ability of high-dose melatonin to induce ferroptosis in oral squamous cell carcinoma by promoting lipid peroxidation and ROS while reducing GSH levels[14]. However, whether ghrelin influences glutamine metabolism in pancreatic cancer cells through the ER stress pathway to induce ferroptosis and exert its anticancer effects remains unclear.
In the present study, the potential anti-tumor mechanism of melatonin in human pancreatic cancer, including the Panc-1 and AsPC-1 cell lines, was explored. Further evidence demonstrated that melatonin exerts its anticancer effects by activating the protein kinase R-like ER kinase (PERK)-eIF2α-ATF4-mediated ER stress pathway, inhibiting ASCT2-mediated glutamine metabolism, and increasing the sensitivity of pancreatic cancer cells to ferroptosis. Additionally, melatonin exerts multifaceted effects on pancreatic cancer cell behavior by promoting apoptosis and inhibiting autophagy. This study provides an experimental foundation for the clinical application of melatonin in pancreatic cancer treatment.
Our team tested multiple chemicals and research tools to measure cells during this study. Our laboratory obtained all chemical supplies from MedChem Express based in the United States including ferrostatin-1 (Fer-1), CCT020312, and (5-phenylacetmido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Chemicals used for this study block ferroptosis death in cells and restrain glutaminase (GLS) activity. The tested compounds reached 99.96% purity for Fer-1, 99.28% purity for CCT020312, and 98.98% purity for BPTES. Higher product purity levels bring reliable and factual outcomes because small levels of contamination will affect cell behavior during experiments.
Multiple detection systems formed part of the experimental design. Beyotime Biotechnology (Shanghai, China) provided the malondialdehyde (MDA) assay kit (catalogue number: S0131S) and lipid peroxidation detection kit (BODIPY 581/591 C11; catalogue number: S0043S). These kits serve as essential tools for measuring lipid peroxidation because this parameter significantly defines ferroptosis progression. The experiments used both essential lab tools and laboratory reagents that help measure biological samples and determine how treatments affect cell health and oxidative harm.
Two pancreatic cancer cell lines known as Panc-1 (FH0286) and AsPC-1 (FH0295) were chosen for laboratory studies, and we obtained from the Shanghai FuHeng Cell Bank (Shanghai, China). Scientists favor Panc-1 and AsPC-1 cells in pancreatic cancer research owing to their similar behavior to human pancreatic tumors. The cells stayed healthy and active for experiments at 37 °C with 5% CO2 under specific conditions that matched human body environments. The Panc-1 cells grew best when cultured with high-glucose Dulbecco’s modified Eagle’s medium media provided by Gibco (MT, United States). This solution offers important nutrients needed for cellular growing and replicating. The AsPC-1 cells received growth support from Roswell Park Memorial Institute 1640 media (Gibco, MT, United States) alongside other basic materials. The culture media received 10% foetal bovine serum for necessary growth factors plus 100 μg/mL streptomycin and 100 units/mL penicillin to avoid bacterial contamination. The growing cells required fresh media every 2 days to continue thriving. We tested different concentrations of bioactive melatonin agent to monitor its influence on cell maintenance and growth patterns. We made melatonin stock solutions at different strength levels from 0.25 to 4 mmol/L using dimethyl sulfoxide (DMSO) solvent. We adjusted the final amount of melatonin in culture media just before beginning the experiments; this cell processing approach allowed us to deliver melatonin exactly according to set parameters.
Panc-1 and AsPC-1 cells received different melatonin doses (0.25 to 4 mmol/L) during a 24-72 hours treatment period to check cell viability. The dose and time were based on previous studies[15]. Panc-1 and AsPC-1 cells were tested for survival through a standard cell counting kit-8 test (CCK-8) during 24-72 hours exposure to varying levels of melatonin. Each well received 10 μL of the CCK-8 solution, making it feasible to obtain precise test results. The living cells received treatment for 2 hours before reactions took place with active cells. The Bio-Tek United States microplate reader determined 450 nm absorption levels, which directly shows the number of live cells per well. The higher the absorbance, the greater the number of viable cells, and vice versa. This method showed how chemicals impact cell life and death specifically to study melatonin effectiveness. We tested how Fer-1 protects cells while observing melatonin’s actions directly. Fer-1 blocked the effects of melatonin at 2.5, 5, and 10 μmol/L during 1-hour cell exposure. We repeated the same steps and used the CCK-8 assay to determine if Fer-1 blocked the cytotoxicity of melatonin treatment.
Colony formation assays reliably measure the survival and growth behavior of cells when exposed to various treatments. Each well of a six-well plate received 5 × 105 cells of both Panc-1 and AsPC-1 cell types. We let the cells grow in 37 °C conditions with 5% CO2 for 24 hours after they attached to the plate surfaces. The attached cells received different melatonin levels for testing purposes. To detect melatonin’s impact on cell survival and growth both cell lines received 8-day treatments at 0.5, 1, 2 and 4 mmol/L for Panc-1 cells, but treatment at only 0.25, 0.5, 1 and 2 mmol/L for AsPC-1 cells. The growth environment was replaced with fresh media every 2 days throughout the 8-day incubation to support cell health. The experiment was concluded after 8 days when we fixed each colony with crystal violet to stain their DNA and proteins so that they could be seen and counted. This test shows how well cells can multiply and create communities under different treatment settings. Our experiment shows that melatonin treatment can slow the growth of pancreatic cancer cells in cells.
Researchers commonly use a wound healing test to evaluate both cell movement and how medical treatments affect movement. The laboratory setup involved placing 3 × 104 cells of Panc-1 and AsPC-1 onto 12-well plates. We used a 100 μL pipette tip to create a sterile cut in the existing layer of Panc-1 and AsPC-1 cells when they had reached complete coverage of the well. Wells with phosphate-buffered saline (PBS) rinses were treated with fresh medium that contained melatonin at 0.25-4 mmol/L or 0.1% DMSO alone. The cell culture sections were photographed using an inverted phase-contrast microscope before and after specific observation points to assess cell movement. The process tested how melatonin affects cell movement, which is key for cancer spread.
During our research on cell invasion, we used 24-well chamber Transwells to analyze Panc-1 and AsPC-1 cell behavior. The chambers included 8 μm polycarbonate filters coated with 10 μg Matrigel to let cells pass through while providing an environment similar to the natural cell surroundings. We placed 3 × 104 cells per well into the upper chamber of Transwells during the logarithmic cell growth phase by trypsinization. Serum-free medium was added to the upper chamber to stop cells from migrating toward serum-based factors. The lower chamber received media with 20% foetal bovine serum to serve as a cell attracting platform.
We maintained cells at 37 °C for 48 hours before subjecting them to different melatonin concentrations between 0.25 to 4 mmol/L alongside a 0.1% DMSO control treatment. The treated cells that penetrated Matrigel and settled on the bottom membrane surface were stabilized using 4% paraformaldehyde at room temperature over 15 minutes. The fixation protected cell shape to make it possible for later staining. The cells went through a 20-minute crystal violet staining process for easy observation. The PBS rinse was used to clean chambers while a cotton swab removed cells from the top membrane surfaces. The inverted phase-contrast microscope examined the invasion cells that remained on the bottom side of the membrane. We measured invasive cells three times and returned to these tests for dependable outcomes.
We tested lipid ROS levels in cell internal space using a C11-BODIPY kit with Panc-1 and AsPC-1 cells. This testing kit helps measure lipid peroxides, which provide important information on ferroptosis. The cells received 5 micromolar C11-BODIPY 581/591 diluted in serum-free medium for 30 minutes at 37 °C for lipid ROS detection. This special probe attaches to lipid peroxides and turns on its fluorescence signal when the peroxides react with oxygen. The cells received three thorough washes with serum-free medium to eliminate any free dye from the process. The inverted fluorescence microscope from Leica enabled us to measure the accumulation of lipid ROS in our cells by taking fluorescence pictures. The strength of fluorescence light reveals how much lipid peroxidation occurred in the cells to show their oxidative stress level. The study ran through its methods three times to get dependable and exact measurement outcomes.
Cells of the Panc-1 and AsPC-1 type underwent tests to measure lipid peroxidation through MDA as a sign of oxidative cell damage. The scientists collected cells and added radio immunoprecipitation assay solution to obtain protein extracts from them. To detect MDA levels, the lysates were processed with thiobarbituric acid, which forms a pink color in response to MDA presence. The spectrophotometer measured the 532 nm light absorption of the thiobarbituric acid-oxidized lipid solution, which shows the color reaction. We prepared a measurement line with MDA standards to precisely identify the MDA content in our test samples. The MDA content in each sample was determined through values measured from the standard curve compared to sample readings. By applying this technique, we measured how much cell damage occurred because of peroxide damage. The experiment ran through three test runs to show accurate and reliable results.
We used immunofluorescence staining to study how important proteins related to ER stress, glutamine use, ferroptosis, and autophagy work in cells. We tested phosphorylated-PERK (P-PERK), ATF4, glutaminase 1 (GLS1), GPX4, microtubule-associated protein light chain 3 (LC3), and sequestosome-1 (p62) protein levels. These biological markers show how cells react to pressure and cell killing from autophagy processes, revealing key details about cancer growth and medicine. We placed 4 × 105 Panc-1 and AsPC-1 cells onto six-well plates with coverslips for cell culture. Panc-1 cells received 0.5 or 4 mmol/L melatonin treatment while AsPC-1 cells received 0.25 or 2 mmol/L melatonin, with 0.1% DMSO used as a control. The coverslips received PBS washes three times during the 24-hour treatment period. The cell structures remained intact when they underwent 20 minutes of 4% paraformaldehyde fixation. After removing the paraformaldehyde solution, the coverslips underwent treatment with 0.3% Triton X-100 to let antibodies permeate through the cells.
The cells needed 1 hour at 24-26 °C to block non-specific binding with goat serum at a 10% concentration. The cells required overnight (16-18 hours) incubation at 4 °C against specific antibodies that serve as targets for protein detection. Next, the cells received fluorescent dye-linked secondary rabbit antibodies at 24-26 °C for 1 hour. Finally, the samples were stored in a dark environment to protect them during DAPI staining of cell nuclei, and the slides were covered with a coverslip. A Leica upright fluorescence microscope took several random pictures of each sample to create valid results. We conducted the experiment in sets of three tests to verify the final findings.
Protein expressions of target molecules were studied through western blot testing in both Panc-1 and AsPC-1 cell samples. A mixture of radio immunoprecipitation assay buffer lysis buffer was used to extract total protein by adding phosphatase inhibitors and phenylmethylsulfonyl fluoride to avoid protein breakage. We measured protein concentrations using bicinchoninic acid analysis kits and 30 μg total protein samples. We used 10% or 12.5% sodium-dodecyl sulfate gel electrophoresis gels for protein separation based on the size of the target proteins. Following electrophoresis, the proteins passed through polyvinylidene fluoride membranes. A blocking buffer without proteins was applied to the membrane for 20 minutes to stop unspecific antibody reactions. The membrane was soaked in 4 °C with primary antibodies from our protein list (Table 1). Next, we cleaned those areas with tris-buffered saline with Tween to discard excess antibodies. The membrane required 1 hour of incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature. After additional washing steps, protein target bands became visible by the detergency of an e-blot system. The target protein measurement results were standardized to β-actin as the loading control protein. The experiment was repeated thrice to produce accurate results.
| Antibody | Clonality | Species | Dilutions WB/IF | Company |
| PERK | Polyclonal | Rabbit | 1:1000 | Affinity, China (AF5304) |
| P-PERK | Polyclonal | Rabbit | 1:1000 | Affinity, China (DF7576) |
| elF2α | Polyclonal | Rabbit | 1:1000 | Affinity, China (AF6087) |
| P-elF2α | Polyclonal | Rabbit | 1:1000 | Affinity, China (AF3087) |
| ATF4 | Polyclonal | Rabbit | 1:1000/1:200 | Proteintech Group, China (10835-1-AP) |
| ASCT2 | Polyclonal | Rabbit | 1:1000 | Cell Signaling Technology (CST), United States (D7C12) |
| GLS1 (KGA/GAC) | Polyclonal | Rabbit | 1:25000/1:200 | Proteintech Group, China (12855-1-AP) |
| GCLC | Polyclonal | Rabbit | 1:8000 | Proteintech Group, China (12601-1-AP) |
| GSS | Polyclonal | Rabbit | 1:4000 | Proteintech Group, China (15712-1-AP) |
| XCT | Monoclonal | Rabbit | 1:1000 | Abcam, United Kingdom (AB307601) |
| GPX4 | Monoclonal | Rabbit | 1:5000/1:400 | Abcam, United Kingdom (AB125066) |
| LC3 | Polyclonal | Rabbit | 1:5000/1:200 | Proteintech Group, China (14600-1-AP) |
| P62 | Monoclonal | Rabbit | 1:25000/1:1200 | Abcam, United Kingdom (AB109012) |
| β-actin | Monoclonal | Rabbit | 1:25000 | Abclonal Technology, China (AC038) |
We used the combination of annexin-V-fluorescein isothiocyanate (FITC) and propidium iodide to determine the amount of apoptosis and necrosis in Panc-1 and AsPC-1 cells during melatonin treatment at different dosages. The combination of FITC-labeled annexin-V and propidium iodide (PI) shows the locations where apoptosis begins in treated cells. The annexin-V binds external phosphatidylserine while PI enters cells with broken membranes of late apoptotic and necrotic cells. The dual stain method helps identify living cells separately from cells in the early and final stages of apoptosis or dying by necrosis.
We planted 4 × 105 cells in each well of six-well plates for cell culture of Panc-1 and AsPC-1. The laboratory used multiple cell samples treated with melatonin concentrations between 0.25 and 4 mmol/L across time intervals from 0 to 48 hours. Then, we used trypsinization to collect the cells, which were washed with PBS. The cell suspension underwent centrifuging at 1000 × g for 5 minutes before being placed in 1 × binding buffer for staining preparation. During the process, each cell sample received 500 μL suspension mixed with 5 μL of FITC-labeled annexin-V and 10 μL of PI. The stained solution was left undisturbed in a dark environment for 5 minutes to help the stains attach to their target molecules. The cells went through an incubation stage and were then moved for fluorescence-activated cell sorting analysis to measure their apoptosis and necrosis levels.
Flow cytometry measured the cells based on their fluorescent light output values. We used 3 hours incubation with plasma to separate cell populations into four areas on the dot plot: (1) Living cells (annexin-V-FITC negative and PI negative); (2) Early apoptotic cells (annexin-V-FITC positive and PI negative); (3) Late apoptotic/secondary necrotic cells (annexin-V-FITC positive and PI positive); and (4) Dead cells (annexin-V-FITC negative and PI positive). We counted cells in each section to show how melatonin treatment at varying times and doses causes cell destruction and death. The experiment was repeated thrice to produce accurate results.
We used standard deviation as a mean to summarize all experimental data collected from the study. Researchers used GraphPad Prism software version 10.3 as their standard program to analyze biological data. To examine multiple groups, we used one-way analysis of variance followed by the Tukey test; this approach prevents unintended type I errors by allowing focused group evaluations. We set a P value threshold of 0.05 to show that the results between groups did not occur by random chance.
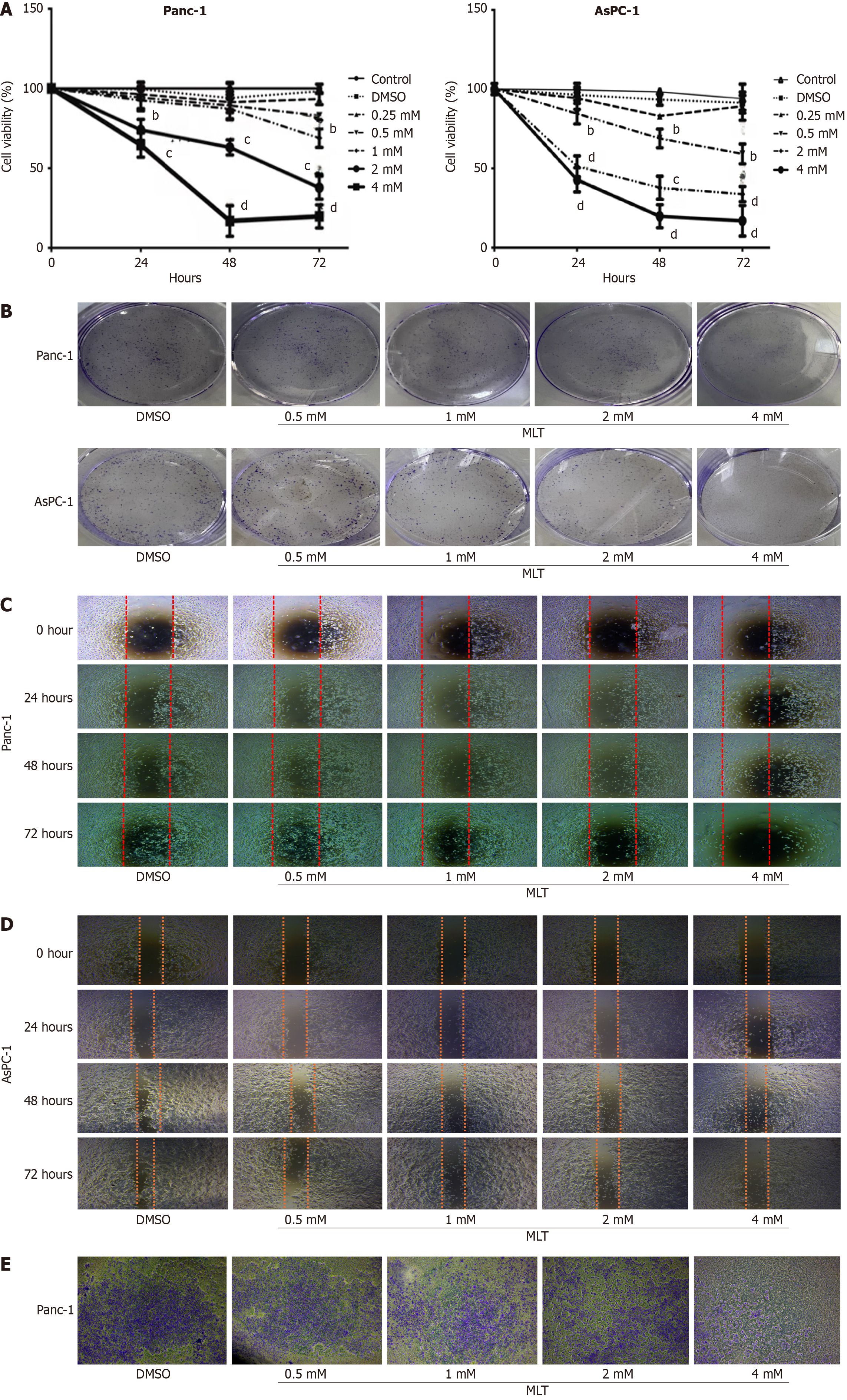
Human pancreatic cancer cell lines (Panc-1 and AsPC-1) were used to evaluate the anticancer effects of melatonin on pancreatic cancer cells. The effect of melatonin on pancreatic cancer cell viability was examined using the CCK-8 assay. Treatment with 4 mmol/L melatonin for 24 hours significantly reduced the viability of Panc-1 cells to 72%, whereas treatment with 2 mmol/L melatonin for 24 hours reduced the viability of AsPC-1 cells to 48% (Figure 1A). Cell viability in both cell lines declined in dose- and time-dependent manners. Similar results were observed in the colony formation assay for both pancreatic cancer cell lines. Melatonin treatment at 2 and 4 mmol/L significantly reduced colony numbers (Figure 1B).
To evaluate the effects of melatonin on cell migration and invasion, cells were treated with different melatonin concentrations (0, 0.25, 0.5, 1, 2, and 4 mmol/L) for different periods (0, 24, 48, and 72 hours). Melatonin (2 and 4 mmol/L) significantly reduced the migration of Panc-1 and AsPC-1 cells in dose- and time-dependent manners (Figure 1C and D). To further examine the inhibitory effects of melatonin on cell motility, its impact on cell invasion was investigated. Treatment with a high melatonin concentration (4 mmol/L) for 48 hours significantly inhibited the invasion of Panc-1 cells compared with that in the DMSO group (Figure 1E). Notably, AsPC-1 cells exhibited weaker migration than did Panc-1 cells and showed no significant invasive behavior.
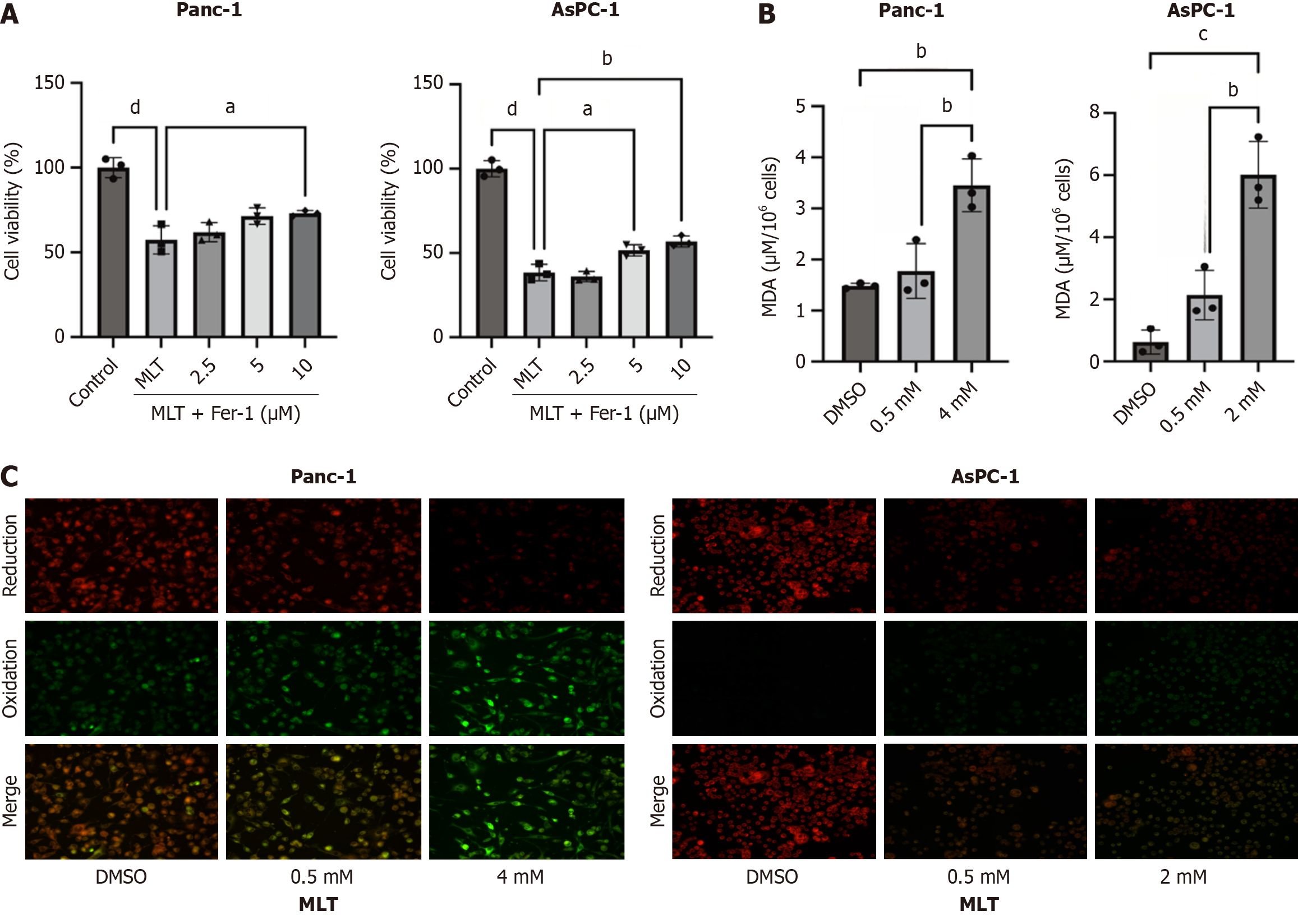
To investigate the effect of melatonin on ferroptosis in pancreatic cancer cells and its role in tumor progression, Panc-1 and AsPC-1 cells were treated with 2 and 4 mmol/L melatonin and different concentrations of the ferroptosis inhibitor Fer-1 (2.5, 5, and 10 μmol/L) for 24 hours. The results showed that high Fer-1 concentrations weakened the anticancer effects of melatonin (Figure 2A). In addition, 24 hours treatment with high melatonin concentrations significantly increased MDA (Figure 2B) and lipid ROS (Figure 2C) levels.
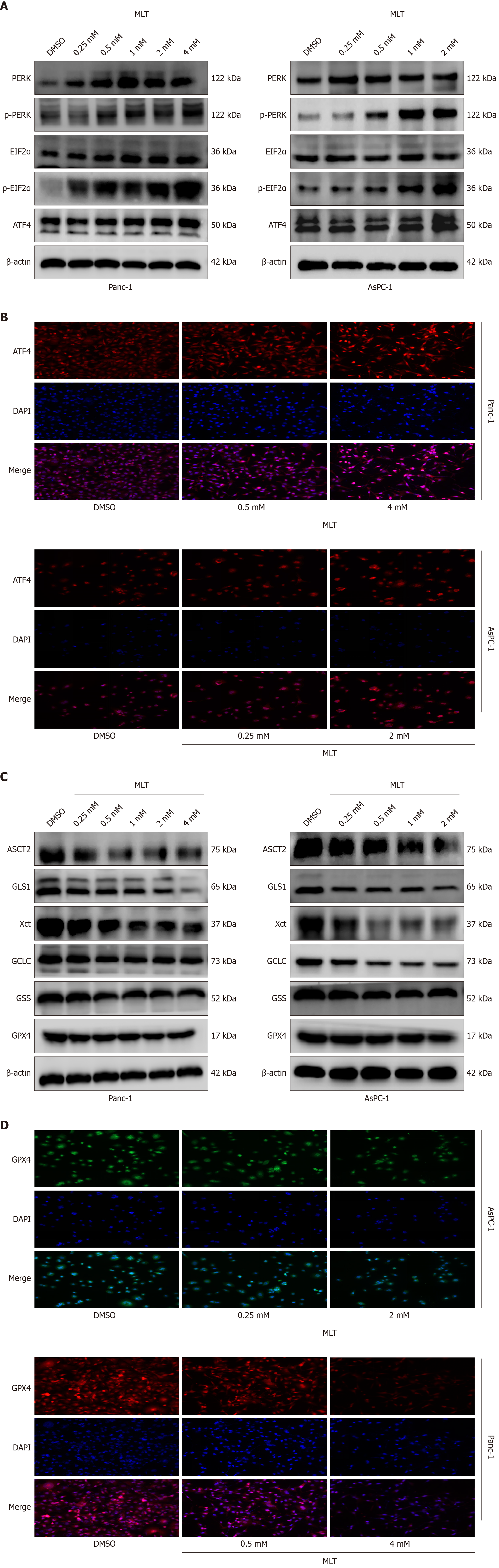
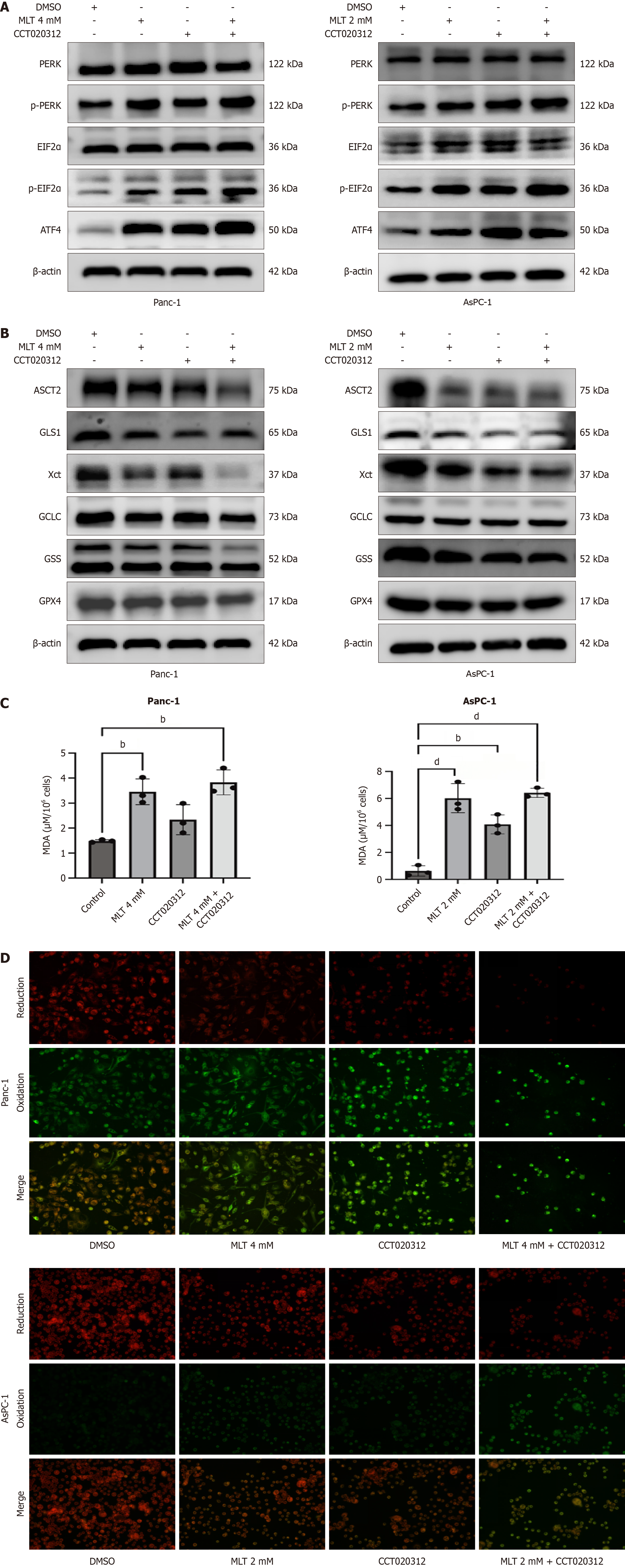
Given the significance of ER stress, particularly the PERK axis, in pancreatic tumor cell death[16], the effect of melatonin on the PERK-eIF2α-ATF4 axis in Panc-1 and AsPC-1 cells was further examined in vitro. As shown in Figure 3A, Western blotting indicated that, compared with those in the DMSO group, treatment with high concentrations of melatonin for 24 hours significantly increased P-PERK, phosphorylated-eIF2α, and ATF4 protein levels. Similarly, the immunofluorescence of ATF4 showed consistent results. These data indicate that melatonin enhances the activation of the PERK-eIF2α-ATF4 axis of ER stress in pancreatic cancer cells (Figure 3B).
Besides, the potential association between the anticancer effects of melatonin and inhibition of the glutamine metabolism pathway was investigated in vitro. In Panc-1 and AsPC-1 cells, western blotting and immunofluorescence showed that, after the 24-hour treatment, high melatonin concentrations significantly inhibited the expression levels of ASCT2, GLS1, X-cysteine transporter, glutamate-cysteine ligase catalytic subunit, GSH synthetase, and GPX4 proteins in pancreatic tumor cells compared with those in the DMSO group (Figure 3C). Similarly, the immunofluorescence of GPX4 showed consistent results (Figure 3D). These results suggest that melatonin exerts its anticancer effects on pancreatic cancer cells through the ASCT2/GLS1/GPX4 pathway.
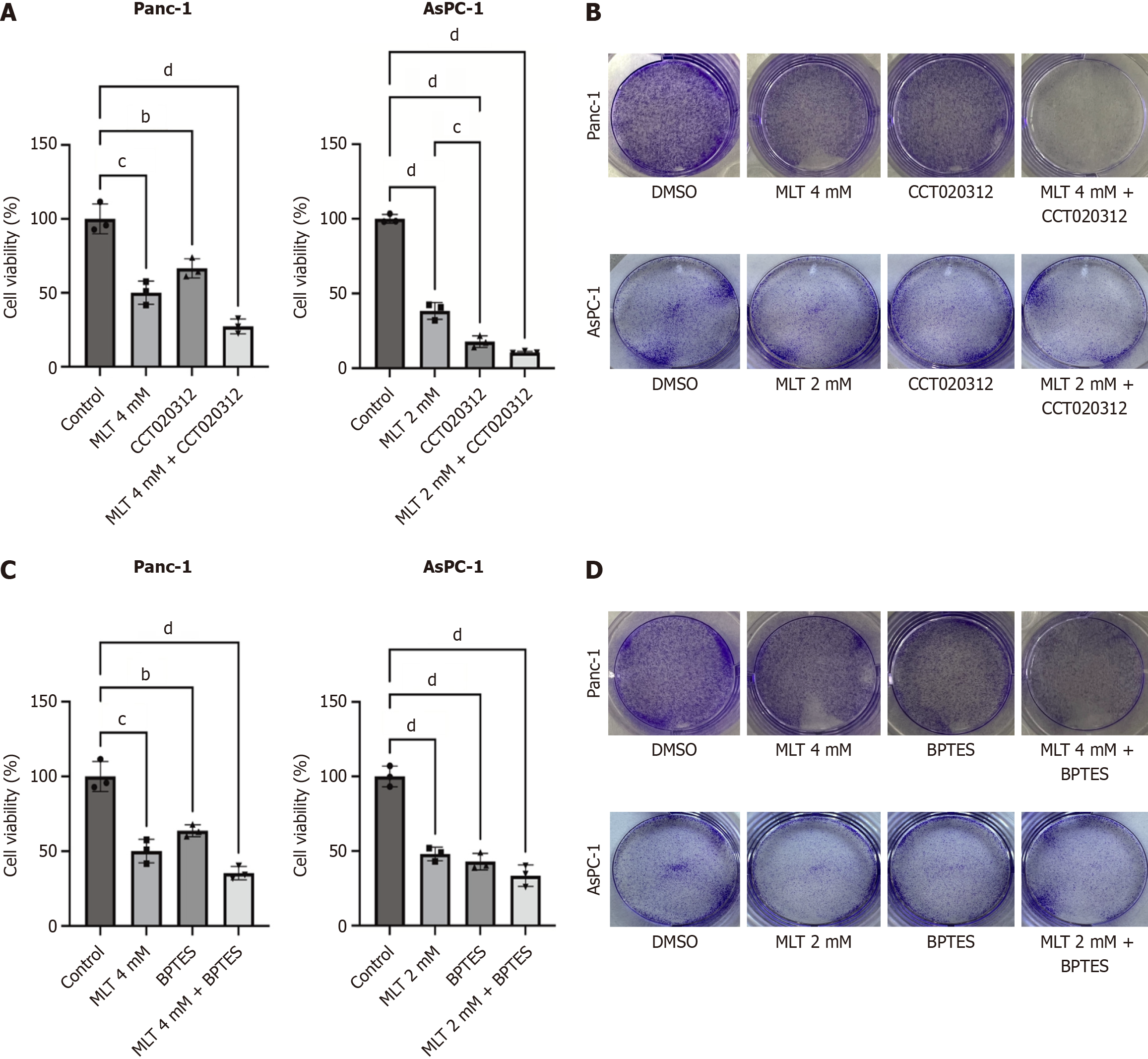
To evaluate the enhanced anticancer efficacy of melatonin combined with CCT020312 in pancreatic cancer cells, a CCK-8 assay was performed to determine cell viability. Panc-1 and AsPC-1 cells were treated with high melatonin concentrations (2 or 4 mmol/L) for 24 hours, followed by co-treatment with 5 μmol/L CCT020312, which significantly reduced cell viability (Figure 4A). To further investigate the inhibitory effect of melatonin combined with CCT020312 on pancreatic cancer cell proliferation, a colony formation assay was conducted (Figure 4B). Compared with that in the DMSO group, high melatonin concentrations significantly limited colony formation. This effect was enhanced following a combination with CCT020312 treatment. A similar effect was observed when melatonin was combined with 10 μmol/L BPTES, resulting in comparable outcomes (Figure 4C and D).
In Panc-1 and AsPC-1 pancreatic cancer cells, western blotting showed that 24-hour treatment with high melatonin concentrations and 5 μmol/L CCT020312 significantly increased P-PERK, phosphorylated-eIF2α, and ATF4 protein levels compared with those in the DMSO group. Among the four groups, the highest expression levels were observed in the melatonin + CCT020312-treated cells (Figure 5A). Therefore, the combination of melatonin and CCT020312 amplified the activation of the PERK-eIF2α-ATF4 axis in the pancreatic cancer cells.
The above results have confirmed that ATF4 overactivation promotes tumor cell death. We further investigated the role of the PERK-eIF2α-ATF4 pathway in melatonin-induced ferroptosis mediated by the glutamine metabolism pathway in pancreatic cancer cells. The melatonin and melatonin + CCT020312 treatment groups showed a significant decrease in the expression levels of glutamine metabolism-related proteins ASCT2, GLS1, X-cysteine transporter, glutamate-cysteine ligase catalytic subunit, GSH synthetase, and GPX4. Among the four groups, cells treated with melatonin + CCT020312 exhibited the lowest expression levels at 24 hours (Figure 5B). To investigate changes in ferroptosis levels in pancreatic cancer cells under combined anticancer treatment, Panc-1 and AsPC-1 cells were treated with high concentrations of melatonin and CCT020312. Compared with the effects of monotherapy, the combination treatment significantly increased the MDA (Figure 5C) and lipid ROS (Figure 5D) levels. Therefore, the combination of melatonin and CCT020312 more effectively inhibited glutamine metabolism in the pancreatic cancer cells and promoted ferroptosis.
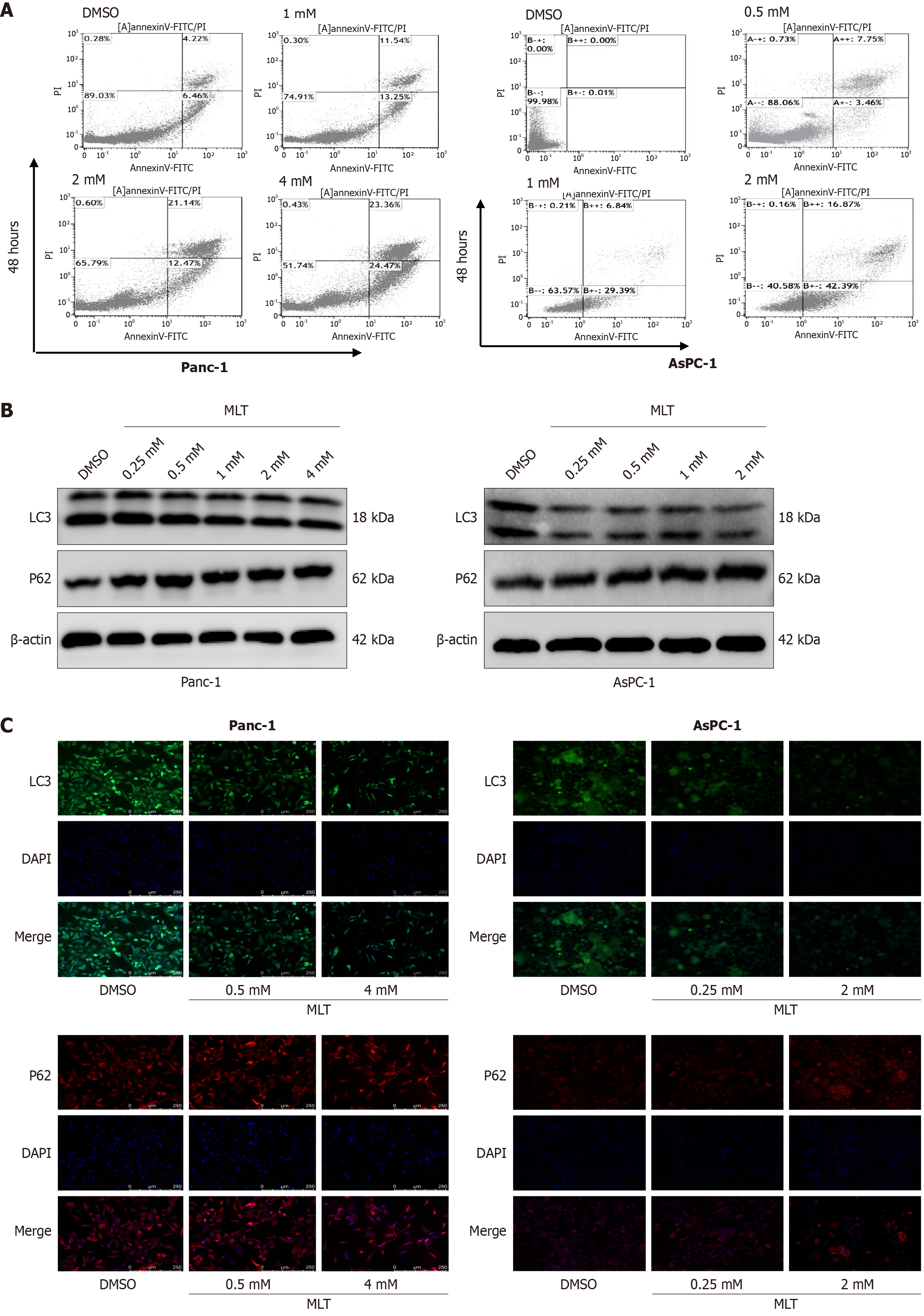
To further investigate the potential mechanisms through which melatonin exerts its anticancer effects in cells, cell apoptosis and autophagy were assessed through flow cytometry analysis. Annexin-V-FITC/PI staining and flow cytometry were used to identify early apoptotic cells and assess apoptosis in the human pancreatic cancer cells treated with different melatonin concentrations for 48 hours. A significant increase in the percentage of early apoptotic cells was observed after 48 hours of treatment with high melatonin concentrations, with a high early apoptosis rate (annexin-V-FITC positive and PI negative; Figure 6A). These results indicate that pancreatic cancer cells underwent increased apoptosis in response to melatonin treatment.
Western blotting revealed that after a 24 hours treatment, a significant decrease in the LC3B-II/LC3B-I ratio and increase in autophagy substrate p62 expression were observed in the high-concentration melatonin group compared with those in the DMSO group (Figure 6B). Immunofluorescence revealed a significant decrease in LC3 (green) fluorescence intensity in the high-concentration melatonin group compared with that in the DMSO group, with p62 showing a similar trend (Figure 6C). This indicates that the autophagy process in the pancreatic cancer cells was inhibited by high melatonin concentrations.
To the best of our knowledge, this study is the first to elucidate the upregulation of ferroptosis regulated by the glutamine metabolism pathway in melatonin-induced pancreatic cancer cell death. In addition, the activation of the PERK-eIF2α-ATF4 axis of ER stress in pancreatic cancer was observed, consistent with previous studies[17]. ER stress induces ferroptosis in different models[18]. The present study validates this concept, demonstrating that melatonin promotes ferroptosis in pancreatic cancer cells by activating the PERK-eIF2α-ATF4 ER stress pathway. In addition, several studies have demonstrated that inhibiting the glutamine metabolism pathway promotes ROS production and induces tumor cell death[19]. The present study demonstrated that melatonin activates the PERK-eIF2α-ATF4 signaling pathway, exerting its anti-tumor effects in pancreatic cancer cells by inhibiting glutamine metabolism and inducing ferroptosis via the ASCT2-GLS1-GPX4 axis. In addition, it identifies a potential molecular mechanism supporting the use of melatonin in pancreatic cancer treatment. This prospective anticancer mechanism shows potential for future drug-targeting applications.
Ferroptosis is a recently discovered iron-dependent, non-apoptotic form of cell death closely associated with the accumulation of intracellular iron ions and lipid ROS. Numerous recent studies have highlighted the therapeutic potential of inducing ferroptosis in pancreatic cancer cells[20]. As a crucial regulator of ferroptosis, GPX4 is directly and positively regulated by GSH, protecting cells from lipid peroxidation by limiting ROS levels and inhibiting ferroptosis[21]. In recent studies, ASCT2 has been reported as a glutamine transporter in various disease models, whereas GLS1, a GLS, specifically targets the degradation of glutamine, contributing to GSH production to counteract oxidative stress[22]. The present study is the first to demonstrate that melatonin promotes ferroptosis in pancreatic cancer cells by inhibiting glutamine metabolism, exerting its anti-tumor effects.
As a major contributor to ferroptosis, ROS primarily originate from the mitochondria. Mitochondrial fission and the opening of mitochondrial permeability transition pores cause ROS accumulation, exacerbating mitochondrial damage[23]. Genetic alterations and the aberrant proliferation of cancer cells are associated with elevated ROS-induced oxidative stress[24]. Melatonin has been shown to counteract ferroptosis by inhibiting ROS; however, notably, high pharmacological doses of melatonin may induce ROS production[25,26] in various tumor cells. Excessive ROS accumulation in cancer cells shifts its role from promoting proliferation and invasion to inducing regulated cell death programs, including apoptosis, necroptosis, and ferroptosis[14,27]. The present study showed that melatonin promotes ROS accumulation in pancreatic cancer cells, suggesting that its anticancer mechanism is related to mitochondrial dysfunction.
The role of glutamine in pancreatic cancer cell proliferation is crucial. Pancreatic cancer cells exhibit an “addiction” to glutamine, which enables them to sustain growth even under nutrient deprivation, hypoxia, and other stresses[6]. Previous studies have shown that targeting ASCT2-mediated glutamine metabolism disrupts cellular redox balance, inhibiting the proliferation of pancreatic cells[28]. The present study further elucidated the anticancer mechanism of melatonin by targeting the glutamine signaling pathway. In this study, melatonin inhibited the glutamine metabolism pathway in pancreatic cancer cells, exacerbated cellular oxidative stress, induced ferroptosis, and exerted anticancer effects.
Under pathological conditions, ATF4, as a downstream effector of PERK, activates ASCT2 at the transcriptional level, promoting GSH biosynthesis in tumor cells and helping to defend against oxidative stress[10]. In recent years, growing evidence has reported that excessive and severe ER stress, particularly the PERK-ATF4 axis, induces ferroptosis. One study indicated that the PERK axis promotes the expression of the p53 gene, which transcriptionally suppresses solute carrier family 7 member 11 and facilitates ferroptosis in HepG2 cells[29]. The anticancer effect of melatonin is speculated to be related to ER stress on the ATF4-ASCT2 axis, which suppresses glutamine metabolism. In our study, high melatonin concentrations were observed to enhance the PERK-eIF2α-ATF4 axis in pancreatic cancer cells, inhibit glutamine metabolism, and promote ferroptosis. This effect was reinforced when combined with the PERK activator CCT020312. Meanwhile, melatonin, as a multifunctional hormone, has long been renowned for its anti-tumor effects. Recently, numerous studies[30,31] have reported that the combination of melatonin and chemotherapy can produce a synergistic anti-tumor effect. After the application of melatonin combination therapy, melatonin reduces the vitality of cancer cells by regulating signals related to cancer progression, restoring the circadian rhythm, and disrupting the redox system of cancer cells, thereby reducing the dosage of chemotherapy drugs, improving the survival rate of patients, enhancing their quality of life, and alleviating the side effects on the body. This also provides further direction for subsequent in vivo and clinical research.
Additionally, under ER stress conditions, glutamine deficiency causes disruptions in cellular energy metabolism, increasing the risk of cell apoptosis. Conversely, glutamine supplementation alleviates cell damage caused by ER stress, promotes autophagy, and repairs cellular processes[32]. Gillson et al[33] revealed that the development of pancreatic cancer depends on autophagy, and that using autophagy inhibitors reverses cancer progression and increases its sensitivity to chemotherapy. The present study also confirmed that melatonin induces apoptosis and inhibits autophagy. It is speculated that melatonin exerts a synergistic anticancer effect against pancreatic cancer by regulating oxidative stress to activate various cell death forms, including ferroptosis, apoptosis, and autophagy.
Both in vivo and in vitro studies have shown that melatonin supplementation is a suitable approach for treating pancreatic cancer. Melatonin may effectively induce cancer tumor cell death by regulating multiple molecular pathways, including oxidative stress, heat shock proteins, and vascular endothelial growth factor[34,35]. However, this study has some limitations. The anti-cancer mechanism of melatonin on pancreatic cancer is a complex pathological process, but our team only explored some of the mechanism. For example, two other ER stress pathways other than the PERK branch have not been detected. In addition, we have not determined the impact of melatonin on animal models of pancreatic cancer. We will conduct animal experiments in the future to further verify the efficacy and safety of melatonin as a drug.
Melatonin significantly inhibits the viability, colony formation, migration, and invasion of Panc-1 and AsPC-1 cells. Similarly, melatonin activates the ER stress pathway, inhibits glutamine metabolism, and promotes ferroptosis in pancreatic cancer cells. Co-treatment with a high melatonin concentration and PERK agonist enhances melatonin-induced ferroptosis in pancreatic cancer cells. Moreover, melatonin inhibits autophagy by upregulating p62 expression and downregulating light chain 3 expression while promoting apoptosis. We believe that our study significantly contributes to the literature because it comprehensively demonstrates the mechanisms underlying the antitumor effect of melatonin. Furthermore, because it is the first study to reveal the upregulation of ferroptosis regulated by the glutamine metabolism pathway in melatonin-induced pancreatic cancer cell death, we expect that our results will have significant utility for researchers interested in novel therapeutic targets.
| 1. | Gupta N, Yelamanchi R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J Gastroenterol. 2021;27:3158-3181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (5)] |
| 2. | Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 2902] [Article Influence: 483.7] [Reference Citation Analysis (0)] |
| 3. | Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 1498] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 4. | Yamaguchi Y, Kasukabe T, Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 5. | Daher B, Parks SK, Durivault J, Cormerais Y, Baidarjad H, Tambutte E, Pouysségur J, Vučetić M. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 2019;79:3877-3890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 6. | Wang X, Ding B, Liu W, Qi L, Li J, Zheng X, Song Y, Li Q, Wu J, Zhang M, Chen H, Wang Y, Li Y, Sun B, Ma P. Dual Starvations Induce Pyroptosis for Orthotopic Pancreatic Cancer Therapy through Simultaneous Deprivation of Glucose and Glutamine. J Am Chem Soc. 2024;146:17854-17865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 7. | Xiao Z, Deng S, Liu H, Wang R, Liu Y, Dai Z, Gu W, Ni Q, Yu X, Liu C, Luo G. Glutamine deprivation induces ferroptosis in pancreatic cancer cells. Acta Biochim Biophys Sin (Shanghai). 2023;55:1288-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752-1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 493] [Article Influence: 29.0] [Reference Citation Analysis (9)] |
| 10. | Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2380] [Cited by in RCA: 2639] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 11. | Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, Hogarty MD, Simon MC. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (3)] |
| 12. | Romero A, Ramos E, de Los Ríos C, Egea J, Del Pino J, Reiter RJ. A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res. 2014;56:343-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Oh BS, Im E, Lee HJ, Sim DY, Park JE, Park WY, Park Y, Koo J, Pak JN, Kim DH, Shim BS, Kim SH. Inhibition of TMPRSS4 mediated epithelial-mesenchymal transition is critically involved in antimetastatic effect of melatonin in colorectal cancers. Phytother Res. 2021;35:4538-4546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Wang L, Wang C, Li X, Tao Z, Zhu W, Su Y, Choi WS. Melatonin and erastin emerge synergistic anti-tumor effects on oral squamous cell carcinoma by inducing apoptosis, ferroptosis, and inhibiting autophagy through promoting ROS. Cell Mol Biol Lett. 2023;28:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 15. | Li W, Fan M, Chen Y, Zhao Q, Song C, Yan Y, Jin Y, Huang Z, Lin C, Wu J. Melatonin Induces Cell Apoptosis in AGS Cells Through the Activation of JNK and P38 MAPK and the Suppression of Nuclear Factor-Kappa B: a Novel Therapeutic Implication for Gastric Cancer. Cell Physiol Biochem. 2015;37:2323-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Carracedo A, Gironella M, Lorente M, Garcia S, Guzmán M, Velasco G, Iovanna JL. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006;66:6748-6755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Mukherjee D, Chakraborty S, Bercz L, D'Alesio L, Wedig J, Torok MA, Pfau T, Lathrop H, Jasani S, Guenther A, McGue J, Adu-Ampratwum D, Fuchs JR, Frankel TL, Pietrzak M, Culp S, Strohecker AM, Skardal A, Mace TA. Tomatidine targets ATF4-dependent signaling and induces ferroptosis to limit pancreatic cancer progression. iScience. 2023;26:107408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Wang Z, Li M, Liu Y, Qiao Z, Bai T, Yang L, Liu B. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein responseinduced upregulation of CHAC1 expression. Oncol Rep. 2021;46:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Wang L, Liu Y, Zhao TL, Li ZZ, He JY, Zhang BJ, Du HZ, Jiang JW, Yuan ST, Sun L. Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomedicine. 2019;57:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Kuang Y, Sechi M, Nurra S, Ljungman M, Neamati N. Design and Synthesis of Novel Reactive Oxygen Species Inducers for the Treatment of Pancreatic Ductal Adenocarcinoma. J Med Chem. 2018;61:1576-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Yu L, Lv Z, Li S, Jiang H, Han B, Zheng X, Liu Y, Zhang Z. Chronic arsenic exposure induces ferroptosis via enhancing ferritinophagy in chicken livers. Sci Total Environ. 2023;890:164172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 22. | Abu Aboud O, Habib SL, Trott J, Stewart B, Liang S, Chaudhari AJ, Sutcliffe J, Weiss RH. Glutamine Addiction in Kidney Cancer Suppresses Oxidative Stress and Can Be Exploited for Real-Time Imaging. Cancer Res. 2017;77:6746-6758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Han B, Lv Z, Han X, Li S, Han B, Yang Q, Wang X, Wu P, Li J, Deng N, Zhang Z. Harmful Effects of Inorganic Mercury Exposure on Kidney Cells: Mitochondrial Dynamics Disorder and Excessive Oxidative Stress. Biol Trace Elem Res. 2022;200:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, Zhang YJ, Keating MJ, Huang P, DiGiovanni J, Georgiou G, Stone E. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23:120-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 25. | Radogna F, Sestili P, Martinelli C, Paolillo M, Paternoster L, Albertini MC, Accorsi A, Gualandi G, Ghibelli L. Lipoxygenase-mediated pro-radical effect of melatonin via stimulation of arachidonic acid metabolism. Toxicol Appl Pharmacol. 2009;238:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Florido J, Rodriguez-Santana C, Martinez-Ruiz L, López-Rodríguez A, Acuña-Castroviejo D, Rusanova I, Escames G. Understanding the Mechanism of Action of Melatonin, Which Induces ROS Production in Cancer Cells. Antioxidants (Basel). 2022;11:1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, Chen D, Piao HL, Liu HX. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839-4857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 28. | Wang W, Pan H, Ren F, Chen H, Ren P. Targeting ASCT2-mediated glutamine metabolism inhibits proliferation and promotes apoptosis of pancreatic cancer cells. Biosci Rep. 2022;42:BSR20212171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Zheng X, Liu B, Liu X, Li P, Zhang P, Ye F, Zhao T, Kuang Y, Chen W, Jin X, Li Q. PERK Regulates the Sensitivity of Hepatocellular Carcinoma Cells to High-LET Carbon Ions via either Apoptosis or Ferroptosis. J Cancer. 2022;13:669-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Mafi A, Rezaee M, Hedayati N, Hogan SD, Reiter RJ, Aarabi MH, Asemi Z. Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Commun Signal. 2023;21:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 31. | Najafi M, Salehi E, Farhood B, Nashtaei MS, Hashemi Goradel N, Khanlarkhani N, Namjoo Z, Mortezaee K. Adjuvant chemotherapy with melatonin for targeting human cancers: A review. J Cell Physiol. 2019;234:2356-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Wu YZ, Chen YH, Cheng CT, Ann DK, Kuo CY. Amino acid restriction induces a long non-coding RNA UBA6-AS1 to regulate GCN2-mediated integrated stress response in breast cancer. FASEB J. 2022;36:e22201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Gillson J, Abd El-Aziz YS, Leck LYW, Jansson PJ, Pavlakis N, Samra JS, Mittal A, Sahni S. Autophagy: A Key Player in Pancreatic Cancer Progression and a Potential Drug Target. Cancers (Basel). 2022;14:3528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z. Melatonin and pancreatic cancer: Current knowledge and future perspectives. J Cell Physiol. 2019;234:5372-5378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Jaworek J, Leja-Szpak A, Nawrot-Porąbka K, Szklarczyk J, Kot M, Pierzchalski P, Góralska M, Ceranowicz P, Warzecha Z, Dembinski A, Bonior J. Effects of Melatonin and Its Analogues on Pancreatic Inflammation, Enzyme Secretion, and Tumorigenesis. Int J Mol Sci. 2017;18:1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |