©The Author(s) 2021.
World J Gastroenterol. Oct 28, 2021; 27(40): 6861-6873
Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6861
Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6861
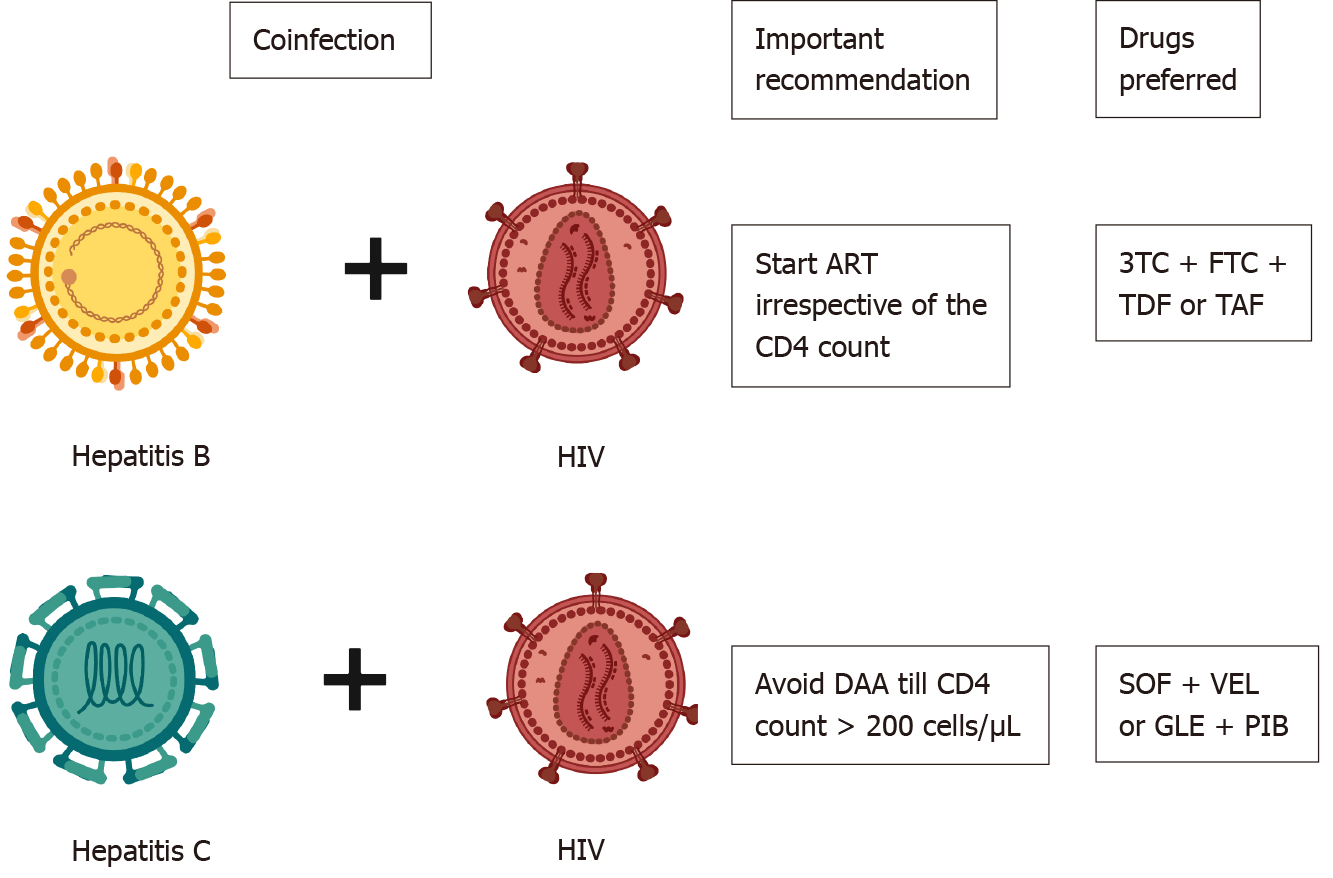
Figure 1 Management of coinfection.
Created with biorender.com. HIV: Human immunodeficiency virus; ART: Antiretroviral therapy; 3TC: Lamivudine; FTC: Emtricitabine; TDF: Tenofovir disoproxil fumarate; TAF: Tenofovir alafenamide fumarate; DAA: Directly acting antiviral agents; SOF: Sofosbuvir; VEL: Velpatasvir; GLE: Glecaprevir; PIB: Pibrentasvir.
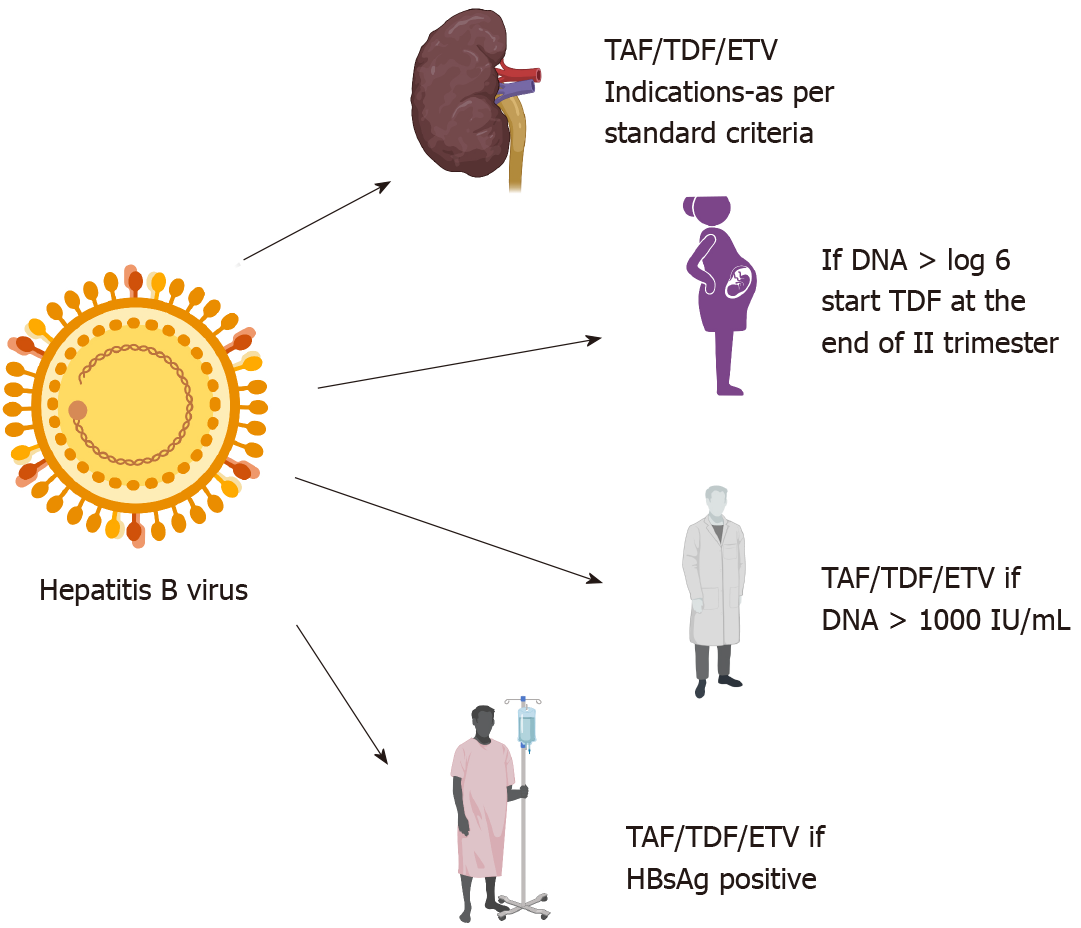
Figure 2 Indications and safety of drugs in special circumstances for hepatitis B.
Created with biorender.com. TAF: Tenofovir alafenamide fumarate; TDF: Tenofovir disoproxil fumarate; ETV: Entecavir.
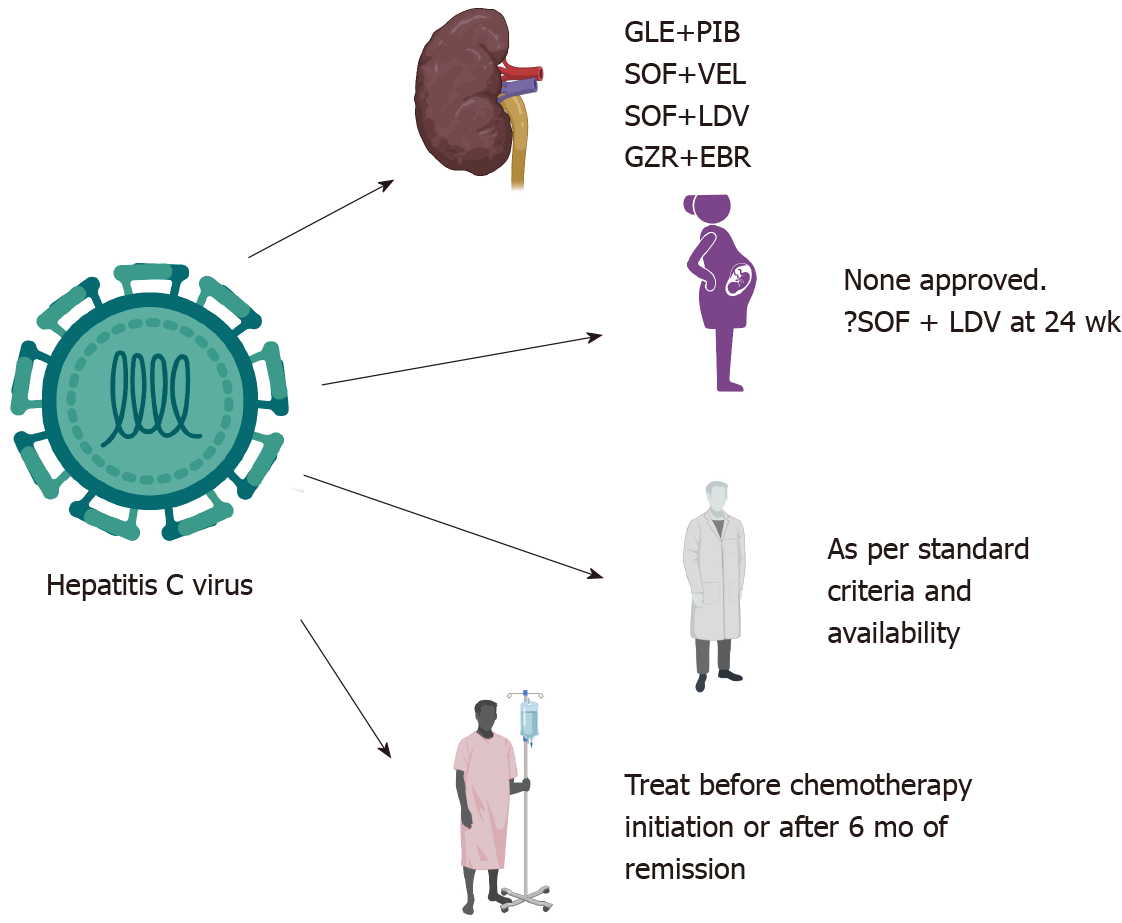
Figure 3 Indications and safety of drugs in special circumstances for hepatitis C.
Created with biorender.com. GLE: Glecaprevir; PIB: Pibrentasvir; SOF: Sofosbuvir; VEL: Velpatasvir; LDV: Ledipasvir; GZR: Grazoprevir; EBR: Elbasvir.
- Citation: Kulkarni AV, Duvvuru NR. Management of hepatitis B and C in special population. World J Gastroenterol 2021; 27(40): 6861-6873
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6861.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6861