Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6844
Peer-review started: February 17, 2021
First decision: April 5, 2021
Revised: April 19, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 28, 2021
Processing time: 252 Days and 4.3 Hours
The prognosis of pancreatic cancer is poor with the overall 5-year survival rate of less than 5% changing minimally over the past decades and future projections predicting it developing into the second leading cause of cancer related mortality within the next decade. Investigations into the mechanisms of pancreatic cancer development, progression and acquired chemoresistance have been constant for the past few decades, thus resulting in the identification of human nucleoside transporters and factors affecting cytotoxic uptake via said transporters. This review summaries the aberrant expression and role of human nucleoside transports in pancreatic cancer, more specifically human equilibrative nucleoside transporter 1/2 (hENT1, hENT2), and human concentrative nucleoside transporter 1/3 (hCNT1, hCNT3), while briefly discussing the connection and importance between these nucleoside transporters and mucins that have also been identified as being aberrantly expressed in pancreatic cancer. The review also discusses the incidence, current diagnostic techniques as well as the current therapeutic treatments for pancreatic cancer. Furthermore, we address the importance of chemoresistance in nucleoside analogue drugs, in particular, gemcitabine and we discuss prospective therapeutic treatments and strategies for overcoming acquired chemoresistance in pancreatic cancer by the enhancement of human nucleoside transporters as well as the potential targeting of mucins using a combination of mucolytic compounds with cytotoxic agents.
Core Tip: Pancreatic cancer continues to be one of the leading causes of cancer-related mortality worldwide, with prevalence expected to increase drastically within the next decade primarily due to difficulties in diagnosis and acquired resistance to chemotherapeutic drugs. Due to this, in-depth work is extremely important for the future diagnosis and treatment of the disease. Human Nucleoside Transporters have been identified as a key target in not only diagnosis but also overcoming chemoresistance. Here, we summarize the information currently available on Pancreatic Cancer and the role of Nucleoside Transporters in chemoresistance.
- Citation: Carter CJ, Mekkawy AH, Morris DL. Role of human nucleoside transporters in pancreatic cancer and chemoresistance. World J Gastroenterol 2021; 27(40): 6844-6860
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6844.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6844
Pancreatic cancer is the seventh leading cause of cancer-related mortality[1], and is ranked as the 14th most common cancer worldwide[2]. The prognosis of pancreatic cancer is often poor with an average five-year survival rate of less than 5%[3]. This poor prognosis has been attributed, in part, to the difficulties in early-stage detection, advancement of disease at the time of diagnosis, frequent recurrence and lack of effective treatment therapies specifically targeting tumour cells[4,5].
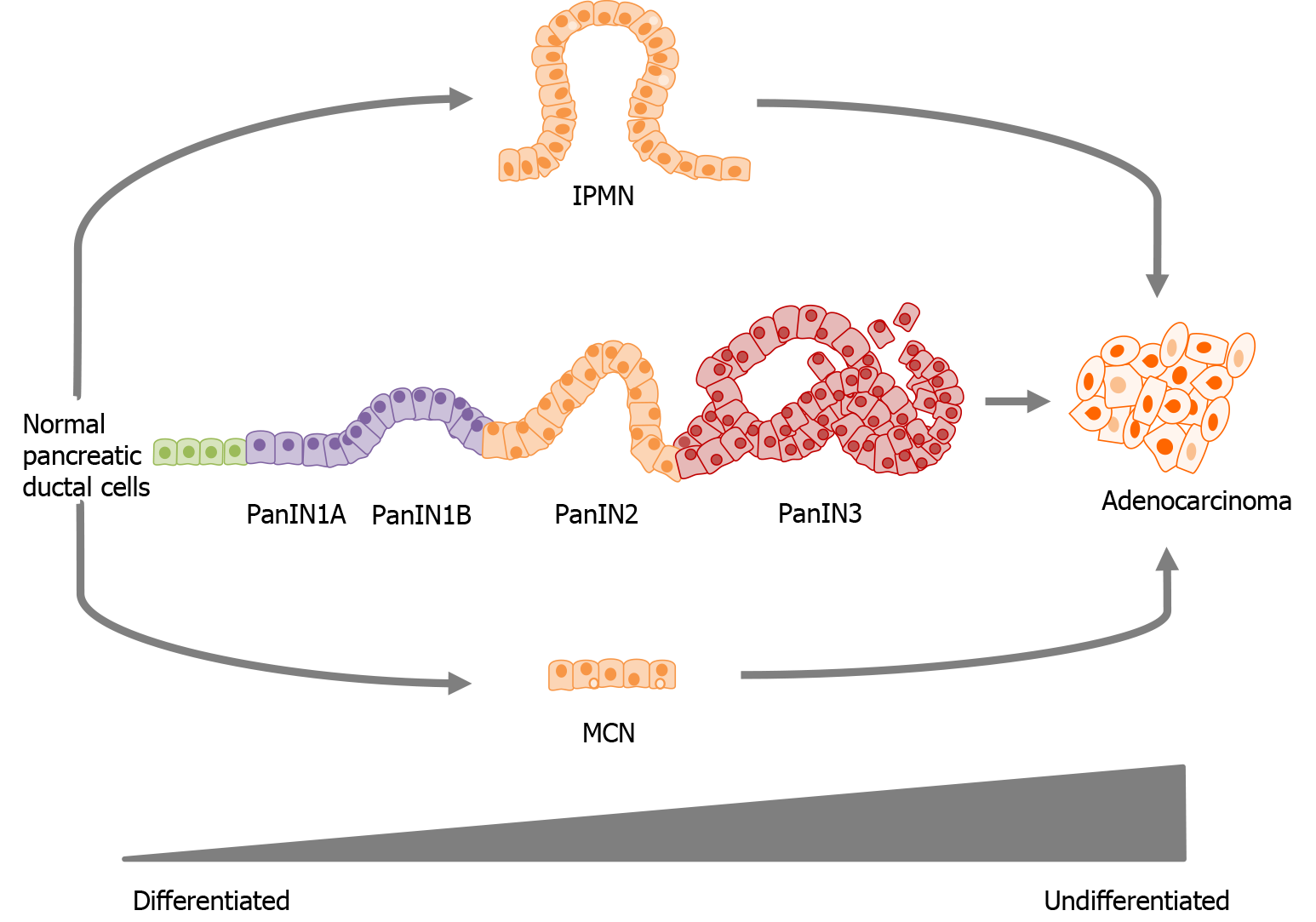
Pancreatic ductal adenocarcinoma (PDAC), which accounts for > 90% of all pancreatic cancer cases is characterized by dense desmoplastic stromal development proximal to cancerous tissue, as well as the aberrant expression of mucins and nucleoside transporters[6,7]. Three different preneoplastic lesions of the pancreatic duct have been identified as precursors of PDAC those being, pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN)[3].
PanIN accounts for 85% of PDAC cases and is categorised into PanIN1A, PanIN1B, PanIN2 and PanIN3[8]. Prior to PanIN progression, normal pancreatic ductal cells express mucin proteins MUC1/5AB/6/17 which have been identified in assisting cancer cell in evading immune systems[3]. PanIN1A/1B is described as hyperplasia without dysplasia, followed by PanIN2 with dysplasia at variable rates and a loss of polarity and finally PanIN3, representative of an in-situ carcinoma. For all PanIN, increased expression can be seen for MUC1/6/16 and neoexpression is observed for MUC3/4/5AC (Figure 1)[4,9].
MCN and IPMN account for the remainder 15% of PDAC cases, with MCN characterized as cyst forming pancreatic epithelial neoplasms that produce mucin, have a distinct stroma type, and have a broad spectrum of dysplasia. Importantly, MCN’s are also representative of early Kras mutations as well as late oncogene SMAD4 and proto-oncogene TP53 inactivation and lastly, increased MUC1 expression and neoexpression of MUC2/5AC [2-4].
IPMN which originates from the main pancreatic duct is characterized by the increased secretion of select mucins (MUC1/6), neoexpression of MUC2/4/5AC, and a large dilation of the ducts or cysts formation. Much like MCN, IPMN presents with a Kras mutation and the inactivation of TP53 and P16/CDKN2A. MCN and IPMN may further progress into adenocarcinoma where an increased expression of MUC1/6 and neoexpression of MUC3/4/5AC/5AB/7/13/16/17 is observed (Figure 1)[3,4].
The aim of this review is to outline the role of nucleoside transporters in pancreatic cancer progression and their contribution to chemotherapeutic drug resistance. This review will also examine the current treatments and therapies to overcome cancer progression and acquired resistance to nucleoside analogue drugs, specifically, gemcitabine.
The incidence rates of pancreatic cancer have significant variation between countries; however, the higher rates can be seen in developed countries (North America and Europe) compared to developing countries (South Central Asia and Africa). World
Despite the cause of pancreatic cancer being multifactorial and complex, several factors whether modifiable or non-modifiable have been identified as having a direct association. Non-modifiable factors include age, sex, ethnicity, blood group, family history, genetic susceptibility, gut microbiota, and diabetes. The modifiable risk factors that have been identified are smoking, alcohol consumption, obesity, chronic pancreatitis, dietary factors, and helicobacter pylori infections[1,2,10].
There are significant challenges with the diagnosis of pancreatic cancer with most patients presenting with late-stage tumour development at the time of diagnosis. The reasons of late-stage progression diagnosis are multi-factorial and therefore, several factors must be assessed[1,10]. Firstly, the initial stage of pancreatic cancer is, for the most part, clinically silent. This results in patients not presenting with symptoms until being in advanced stages of disease. Presenting symptoms are non-specific and can include jaundice, dark urine, abdominal pain, acholic stools and pruritus. Due to the broad range of non-specific symptoms, there are several diseases that require differentiation, including but not limited to: pancreatitis, gastritis, abdominal aortic aneurysm, and pancreas. As a result, diagnosis can be missed or delayed which results in pancreatic cancer being the most commonly detected tumour during autopsy studies[2,10,11].
Currently, several diagnostic tools are available for the diagnosis of pancreatic cancer, such as magnetic resonance imaging (MRI), abdominal ultrasonography, endoscopic ultrasound-guided fine-needle aspiration and the standard for staging and diagnosis tri-phase pancreatic protocol CT. Additionally, the cancer biomarker serum cancer antigen 19-9 (CA 19-9) is the only approved marker for pancreatic cancer and is used to assist in diagnosis and to predict patient prognosis and recurrence post resection. Unfortunately, the CA19-9 biomarker is not tumour-specific and therefore is not capable for use as a screening tool for the patients that are asymptomatic[1,2,10].
Recently, biomarkers for early diagnosis have been investigated, including plasma-based metabolite panels, increased concentrations of volatile organic compounds in exhaled air, DNA mutations such as p53 in pancreatic juice, and tumour markers such as CA125, CA242, CEA, NSE[2,12]. Despite continued investigation into diagnostic biomarkers, the lack of a specific and validated biomarker as well as sensitive screening programs remains a major issue.
The treatment strategy for pancreatic cancer is dependent on the primary tumour resectability, which is defined by the absence of distance metastasis as well as the locoregional anatomical contacts allowing for a R0 resection. For patients eligible, the primary treatment that presents a possible cure for pancreatic cancer is surgical resection in addition to chemotherapy[2,13].
The surgical options for achieving surgical resection are open Pacnreatico-duodenectomy and total or distal pancreatectomy, either open or laparoscopic. The surgical method employed is dependent on the tumour or tumour’s anatomical location. It is important to mention that the survival rates following surgical resection alone are low with approximately 10% after 5 years. Despite observed benefits when adjuvant therapies are utilised, the recurrence rates remain high, with a 2-year relapse of approximately 70%[2,13,14].
Unfortunately, as previously mentioned, at the time of diagnosis approximately 90% of patients present with tumours that are locally advanced or metastatic, making them ineligible for surgical resection. The only options for these patients are the management of jaundice, system control, palliative radiotherapy, and chemotherapy. A variety of chemotherapeutic drugs are used to treat pancreatic cancer including Oxaliplatin, Paclitaxel, Leucovorin, Irinotecan, Doxorubicin, and 5-fluorouracil (5-FU) (Table 1)[15,16]. Currently, the standard of care for patient’s ineligible for surgical resection remains FOLFIRINOX (Fluorouracil, Oxaliplatin, Leucovorin and Irinotecan) or Gemcitabine (2’,2’-diflurodeoxyxytidine) either in combination with Paclitaxel or as a single agent[3,4,14,17,18].
| Drug | Classification | Action |
| Gemcitabine (Gemzar) | Antimetabolite | Pyrimidine antagonist |
| Doxorubicin (Rubex) | Anthracycline Antibiotic | Cell-cycle specific antagonist |
| Fluorouracil (5-FU) | Antimetabolite | Pyrimidine antagonist |
| Paclitaxel (Abraxane) | Plant alkaloid, Taxane, Antimircotubule | Inhibition of cell microtubule structures |
| Oxaliplatin (Eloxatin) | Alkylating agent | Cell-cycle non-specific antagonist |
| Leucovorin (Folinic acid) | Chemoprotectant | Enhancement anti-cancer effects |
| Irinotecan (Camptosar) | Plant alkaloid, Topoisomerase I inhibitor | Camptothecan analogs |
| Erlotinib (Tarceva) | Tyrosine kinase inhibitor | Ant-EGFR |
| Cetuximab (Erbitux) | Biologic | Anti-EGFR |
| Bevacizumab (Avastin) | Biologic | Anti-VEGF |
The most effective treatment regimen is guided by patient fitness, FOLFIRNOX is administered to fit patients with tumours in the tail, body, and head of the pancreas, with treatment resulting in significant longer cancer-specific, metastasis-free, disease-free, and overall survival (11.1 mo) when compared to Gemcitabine (6.8 mo). However, a significantly increased risk of adverse effects and complications is seen in patients that receive FOLFIRNOX when compared to Gemcitabine. Gemcitabine +/- Capecitabine is relative safe compared to other chemotherapy options and is therefore the standard treatment for patients that are ineligible for FOLFIRNOX treatment regimens. It has been found to provide improved system control, a higher response rate and a greater overall survival rate compared to 5-FU (4.41 mo)[2,14,19]. Despite the poor survival outcomes, 5-FU is used for periampullary tumours primarily due to insufficient evidence for other chemotherapeutic agents specifically targeting said tumour types[2]. Due to the clinical benefits of Gemcitabine compared to other treatment regimes, it has become the standard reference agent in the first line treatment choice of pancreatic cancer[20,21]. This shows that the patient benefit to current treatments is minimal, hence identifying better targets and more efficient treatments is required.
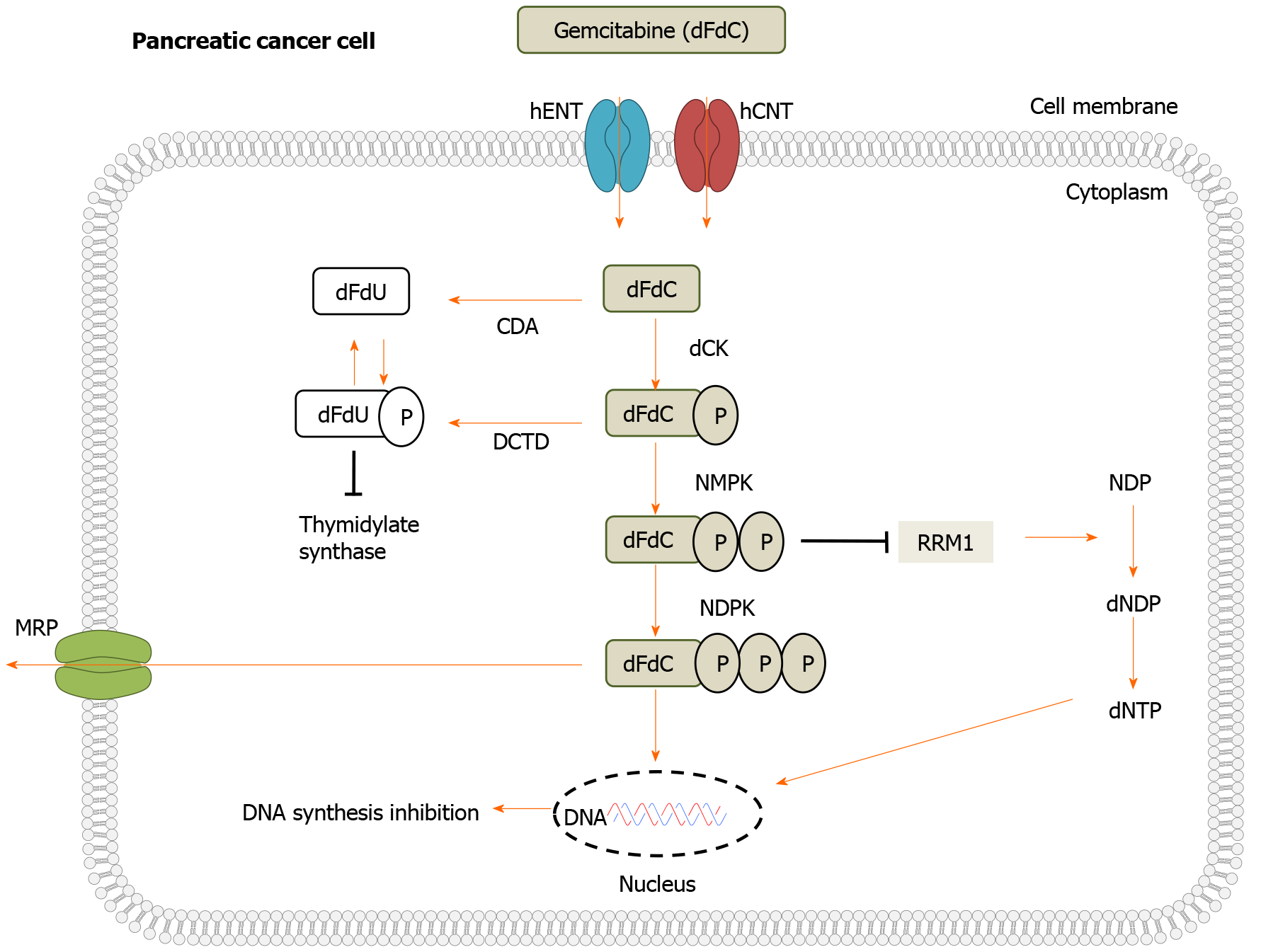
Gemcitabine is a cytotoxic pyrimidine nucleoside analogue that has been utilized as a chemotherapeutic agent for not only pancreatic cancer but for a variety of solid tumours and more recently in certain lymphomas[22]. Due to the hydrophilic nature of gemcitabine, passive diffusion is slow through cellular membranes and therefore requires transportation into cells by specialized integral membrane proteins known as human nucleotide transporters (NTs)[16,23,24].
To exert clinical action, gemcitabine needs to be metabolized and requires multiple kinases to initiate serial phosphorylation. Once transported intracellularly, gemci
Although gemcitabine and other chemotherapeutic drugs have been proven effective among patients with various stages of pancreatic cancer, the effectiveness of gemcitabine is severely limited due to the development of chemoresistance[19]. Chemoresistance is simply the ability of cancer cells to survive or evade therapeutics and can be intrinsic or acquired during treatment[28]. Chemotherapeutic drug resistance is widely common in pancreatic cancer with less than 30% of patients showing improvement post treatment and previous studies have identifying a heightened resistance to gemcitabine in comparison to other chemotherapeutic drugs in pancreatic cancer cells[19,29,30].
To date, several mechanisms have been identified as contributors to acquired gemcitabine resistance in pancreatic cancer due to the drugs rapid metabolism and hydrophilic nature including increased levels of ribonucleotide reductase subunit II and II (RRM1/2), mucins, deoxycytidine kinase (dCK), as well as concentrative and equilibrative nucleoside transporters (hCNT/hENT). Regrettably, many of these mechanisms are complex and the identification of their specific roles and conse
Nucleoside transporters (NTs) are a group of membrane proteins that transport the analogs of both natural and synthetic nucleosides that act as anticancer agents across the membranes of vesicles and cells. There are two classes of transporter proteins that are encoded by two different solute carrier families (SLC), SLC28 known as human concentrative nucleoside transports and SLC29 also known as human equilibrative nucleoside transporters[33].
There are three members of the human concentrative nucleoside transporter family including, hCNT1, hCNT2 and hCNT3. These transporters mediate the unidirectional co-transport of Na+ and/or H+ and nucleosides into cells at a 1:1 coupling ratio for hCNT1 and hCNT2 and a 2:1 ratio for hCNT3. Initially, hCNTs were believed to be expressed only in polarized epithelia, however, recent studies have identified the expression to be considerably broader. These proteins favour nucleoside transe
Human equilibrative nucleoside transporters include four members, hENT1, hENT2, hENT3 and hENT4. These proteins are bio-directional and mediate the facilitative transport of nucleosides and select hENTs also facilitate the transportation of nucleobases dependent on their concentration gradient. Except for hENT4, hENT proteins can transport both pyrimidines and purines, they have a broad permeant selectivity, and are widely distributed in a variety of cell types. Despite their similarities, hENT1, 2 and 3 differ in in nucleoside specificity, however, they all present with a lower substrate affinity then the members of the hCNT family[16,23,33].
Due to the importance of gemcitabine in pancreatic cancer treatment and the requirement of transporters for drug uptake, extensive research has been undertaken on transports and their role in tumorigenesis. Alterations in the expression of transporters have been identified in the progression of normal pancreatic cells to malignant pancreatic cancer cells. Despite significant efforts of researchers to identify the roles transporters undertake in substrate translocation and their functions in cell physiology, more research evaluating the clinical application of transporters as potential biomarkers or therapeutical targets is required. The primary transporter that efficiently mediates the cellular uptake of gemcitabine is hENT1 (SLC29A1), however, evidence suggests minor cellular uptake of gemcitabine via hENT2 (SLC29A2), hCNT1 (SLC28A1) and hCNT3 (SLC28A3)[15,16,23,26]. Each transporter undertakes specific functions (Table 2), and each will be discussed as follows:
| Transporter | Mechanisms of action | Efficacy, toxicity, and affinity | Known disadvantages | Ref. |
| hENT1 (SLC29A1) | Nucleoside-derived anticancer drug update. Antiviral drug uptake. Cellular uptake of nucleosides for RNA or DNA synthesis. Adenosine signalling | Clear correlation between the efficacy of nucleoside analog chemotherapeutic treatment and expression of hENT1. Increase in chemotherapeutic drug efficacy with an increase in transporter expression. Highly sensitive to NBMPR, draflazine, dilazep, and dipyridamole.Increase in cell toxicity with an increase in transporter expression. Highest affinity for gemcitabine uptake | Mutations in the SLC29A1 gene associated with Faisalabad histiocytosis, pigmented hypertrichosis including insulin dependent diabetes, and H syndrome. Select variants of the gene highly immunogenic, may result in haemolytic disease of newborns and foetus as well as acute haemolytic transfusion reaction | [72-77] |
| hENT2 (SLC29A2) | Nucleoside-derived anticancer drug update. Antiviral drug uptake Mediates the influx and efflux of pyrimidine and purine nucleosides as well as the purine base hypoxanthine. Transport’s bile salts, metal ions, organic acids, amine compounds, glucose and other sugars | Although identified to transport gemcitabine into cells, it is less effective than hENT1 at cytotoxic drug uptake. Minimally sensitive to inhibition via NBMPR, draflazine, dilazep, and dipyridamole. Higher affinity for nucleobases when compared to hENT1 | Mutations in the SLC29A2 gene associated with familial and Histiocytosis-Lymphadenopathy Plus Syndrome and hepatic Adenomas. Dysregulation may result in have adverse effects on cell homeostasis and possibly contribute to tumour progression | [33,37,38,40,78] |
| hCNT1 (SLC28A1) | Transport’s bile salts, metal ions, organic acids, amine compounds, glucose and other sugars. Transport’s nucleosides, related molecules, and vitamins. Nucleoside binding. Nucleoside sodium symporter activity. Nucleoside-derived anticancer drug update. Antiviral pyrimidine nucleoside analog drug uptake | Minimally effective at cytotoxic drug uptake. Expression is negatively regulated by MUC4 in gemcitabine resistant pancreatic cancer cells. Has a selective inhibition for thymidine, adenosine, uridine, inosine, guanosine, and cytidine | Mutations in the SLC28A1 gene are associated with Uridine-Cytidineuria disease. Overexpression of protein may result in translocation of pyrimidine alone, resulting in modifications of cell physiology | [16,45,51,79] |
| hCNT3 (SLC28A3) | Regulates cellular processes, such as vascular tone, neurotransmission, surface receptors, the concentration of adenosine in the vicinity of cell as well as transport, and finally the metabolism of nucleoside drugs. hCNT3 has a broad specificity for pyrimidine and purine nucleosides | Clear correlation between hCNT3 downregulation and gemcitabine toxicity | Mutations in the SLC28A3 gene results in the altered sensitivity to the compound BMS-536924 | [80-82] |
The hENT1 protein is a transmembrane glycoprotein that is localized to plasma membranes and is widely expressed with a broad selectivity for pyrimidines and purines[16]. In a healthy pancreas, hENT1 has medium expression in exocrine glandular cells and low expression in islets of Langerhans. In comparison, pancreatic tumours present with a decrease in hENT1 expression[34].
Previous clinical studies on pancreatic cancer patients and various cell lines have identified that that low levels of hENT1 expression on pancreatic tumour cells correlates with a significant reduction in progression free survival (PFS) and disease-free survival (DFS) when compared to patients with medium to high levels of hENT1 expression[7,35]. If gemcitabine is incapable of cellular uptake due to a lack of transportation into the cell, it is unable to inhibit cell growth and therefore the abundance of hENT1 may contribute to gemcitabine resistance as a result of reduced intracellular build-up. In fact, previous studies reported the inhibition of hENT1 in cancer cell lines with NBMPR (nucleoside analogue nitrobenzylmercaptopurine ribonucleoside - a potent inhibitor of hENT1) resulted in cell chemoresistance to gemcitabine[34,36,37]. Thus, further supporting the importance of hENT1 in gemcitabine sensitivity.
This research indicates that hENT1 Levels are correlated with gemcitabine response as the upregulation of hENT1 proteins results in the enhancement of gemcitabine’s cytotoxic effects, while the loss of hENT1 protein expression results in the development of gemcitabine resistance. Due to this, hENT1 is an attractive potential prognostic biomarker in pancreatic cancer patients for gemcitabine treated patients[7,19].
It is important to note that the hENT1 gene itself is not reduced in the development of chemoresistance to gemcitabine, nor does it correlate with gemcitabine's IC50 values for several pancreatic cancer cell lines. This suggests that hENT1 expression is not solely alone in patients acquired and inherent chemoresistance to gemcitabine[34].
The hENT2 protein is encoded by the gene SLC29A2 and transports a range purine and pyrimidine nucleosides as well as select nucleobases across cells membranes with broad affinity. The protein is present in a wide range of cell types though its gene expression is greatest in the skeletal muscle. The hENT2 protein is highly expressed in the pancreatic cells of healthy individuals but is shown to be reduced in pancreatic cancer patients and pancreatic cancer cell lines[38,39].
hENT2 has recently been identified as being a key element in the regulation of nucleotide and nucleoside pool for effective cell cycle progression and synthesis of DNA. This evidence indicates that hENT2 is partly responsible for transporting, into the nucleus, the supply of nucleotides required for DNA replication. Therefore, the dysregulation of this transporter protein may have adverse effects on cell homeostasis and possibly contribute to tumour progression[33].
hENT2 has also been the subject of several investigations due to its ability to transport gemcitabine into cells. It is however proven to transport the chemotherapeutic drug with a much lower affinity when compared to hENT1[38]. Unlike the extensive research undertaken on hENT1 which has proven a link between the protein and chemoresistance, there are few studies investigating the importance of hENT2[22]. hENT2 protein expression has been identified in various pancreatic cancer cell lines, although less than the expression of hENT1 transporters[25].
Previous studies aimed at investigating hENT2 Levels in chemoresistance pancreatic cancer cell lines identified the expressed protein had a lower expression after gemcitabine treatment[37,40]. In cohesion with the reduction in protein expression, hENT2 mRNA levels are also reduced following gemcitabine treatment. Furthering these studies, the inactivation of hENT2 via exposure to dilazep (a hENT2 inhibitor), in conjunction with the inhibition of hENT1 via NBMPR, results in a reduction of gemcitabine uptake and sensitivity. However, the decrease in hENT2 expression may not provide an explanation for the limited uptake of gemcitabine into cells and therefore chemoresistance, as the expression of other gemcitabine transporters are not affected by hENT2 downregulation[37,40].
These results provide evidence that hENT2 when aberrantly expressed may contribute to gemcitabine resistance, however, more extensive research is required to understand the exact impact hENT2 expression has on other mechanisms of chemoresistance in addition to pancreatic cancer cells.
The hCNT1 protein, encoded by the SLAC28A1 gene, is a sodium dependant transporter with a high affinity for pyrimidines[25]. It is broadly detected in epithelia with a moderate to high expression in healthy pancreatic tissue. The expression of hCNT1 is decreased to an almost negligible in undifferentiated states such as fetal hepatocytes, intestinal crypt cells and human tumours, namely pancreatic and breast tumours[41].
The exact role hCNT1 undertakes in the regulation of gemcitabine resistance and cytotoxicity in pancreatic cancer cells is yet to be described, however, data obtained from multiple studies have indicated that the hCNT1 expression is frequently reduced in pancreatic cancer cell lines and tumours when compared to unaffected pancreatic cells[42-44]. In chemoresistant pancreatic cancer cells, hCNT1 expression is limited and can be correlated with limited influx of gemcitabine into target cells. However, investigations into the pharmacological inhibition of the degradation of hCNT1 results in a moderate increase of the cellular transport of gemcitabine thus suggesting the transporter as a possible target mechanism for increasing chemosensitivity and drug transport[42].
Due to the correlation between decreased hCNT1 expression levels and the increased chemoresistance of pancreatic cancer cells, recent research has investigated the mechanisms that decrease transporter expression. As a result, it has been identified that the transmembrane mucin MUC4 utilises the NF-KB pathway to inhibit hCNT1[45]. The increase of MUC4 expression is commonly observed in pancreatic cancer cells, which results in a higher rate of inhibition of hCNT1 transporters and therefore an increase in chemoresistance. Importantly, studies have shown that the inhibition of MUC4 oncogenic receptor Erb2 resulted in the increase of hCNT1 expression and an increased uptake and sensitivity to gemcitabine[42]. Although more extensive work is needed to determine the processes affecting hCNT1 expression, mucins are an interesting contributing factor.
The hCNT3 transporter belongs to the SLC28 family and is characterized by the broadest tissue specificity and tissue distribution. It is a symporter that couples the transportation of a nucleoside to the symport of one proton or two Na+ ions. In healthy individuals the transporter is expressed at high levels in the pancreas, mammary glands and in bone marrow. HCNT3 is also expressed in low levels in the prostate, liver, testis, lungs, and intestines[46].
The importance of hCNT3 in pancreatic cancer is of clinical and pharmacological significance due to its ability to transport a large variety of nucleoside-derived drugs such as valacyclovir for viral infections and more importantly gemcitabine for solid tumors. Due to this, it is identified as a crucial mediator of drug response and the development of resistance to anti-cancer drugs[46].
The aberrant expression of hCNT3 has been observed in various pancreatic tumors and pancreatic cancer cell lines. More specifically, a decrease in expression is observed which has been correlated with increased gemcitabine cytotoxicity[45]. Furthermore, uncharacterized polymorphisms of hCHT3 such as the nonsynonymous A25G mutation, have been associate with tumor response to drug toxicity and therapy in pancreatic cancer patients[24]. It has also been shown that hCNT3 expression in pancreatic tumors correlates with overall patient survival, with an increased expression of the transporter associated with a longer overall patient survival[45]. Recent studies investigating the mechanisms that regulate hCNT3 expression in tumor cells suggest that ErbB2 expression and epithelial to mesenchymal transition (EMT) have a negative impact on the regulation of transporter expression[47].
hCNT3 has been observed to have multiple advantages over other NTs capable of gemcitabine uptake, primarily as a potential therapeutic target for reducing resistance of toxic nucleoside analog treatments. Despite extensive research aimed at hENT1 as a therapeutic target for overcoming gemcitabine resistance in pancreatic cancer patients, Paproski et al[23] identified an 8-fold uptake of gemcitabine in hCNT3 transfected cells with functional hENTs and 142-434-fold uptake in cells without hENT activity. The evidence suggesting that functional hENTs present in cells result in a decrease in gemcitabine uptake. However, another study undertaken by Maréchal et al[26] identified pancreatic cancer patients that presented with both high hENT1 and high hCNT3 expression had a 5-fold longer overall survival than those with one favorable prognostic factor and a 7-fold overall survival length than individuals with no favorable prognostic factors.
To date, several strategies aim at effectively treating pancreatic cancer and overcoming chemoresistance commonly seen in patients undertaking chemotherapeutic treatments. A variety of techniques and agents are currently or have previously been investigated for their effectiveness against not only pancreatic cancer, but also gastrointestinal, peritoneal, and respiratory mucous tumours[3,48].
Numerous target treatments have been investigated and evaluated either alone or in combination with chemotherapeutic agents for pancreatic cancer. Currently, the only targeted therapeutic agent to have statistically significant benefit for patient survival is the tyrosine kinase inhibitor (TKI) erlotinib in combination with gemcitabine with a 2-week mean survival benefit over gemcitabine as a single agent. Though promising, erlotinib is clinically marginal and further investigations are required[48,49].
Other investigated drugs include multikinase inhibitors exhibiting antiangiogenic activity like axitinib, sunitinib and sorafenib and antiangiogenic drugs including vascular endothelial growth factor (VEGF) inhibitors aflibercept and bevacizumab. Recently, compounds targeting signalling cascades such as the multikinase inhibitor masitinib67, phosphoinositide 3-kinase inhibitor rigosertib, and anti-insulin-like growth factor 1 receptor antibodies cixutumumab65,66 and ganitumab have been investigated in combination with gemcitabine. Regrettably, these agents have shown no improvement in patient survival, they have however, provided insight into the cause of futility, with the dense stroma surrounding pancreatic cancer cells speculated to be a key contributor[48,50].
The microenvironment of pancreatic tumours is composed of fibrotic stroma including inflammatory cells, fibroblasts, nerve cells, blood vessels, hyaluronan, fibronectin, and collagen. The theory of targeting the stroma of pancreatic tumours to enhance the effectiveness of chemotherapeutic drugs is of great interest. It is hypothesised that due to the poor vascularization and deposition of extracellular matrix component causes increased hydrostatic pressure and stiffness resulting in a barrier of drug uptake. Currently investigated treatments included calcipotriol for the inactivation of pancreatic stellate cells (PSCs), smoothened homologue inhibitor IPI-926 for the inhibition of collagen deposition and myofibroblast growth and finally evofosfamide which inhibits DNA replication. As seen with other therapeutic treatments, agents attacking the stroma are still in the early stages of investigation and more work is required[48,50].
To date, no viable agents specifically targeting nucleoside transporters in relation to chemoresistance has been developed. Though in vivo investigations into the upregulation of hCNT1 expression have shown promising results, the clinical application of these techniques is not yet applicable[51,52]. Although, it has been hypothesised that the overexpression of selected mucins in pancreatic cancer is a contributing factor to nucleoside transporter efficacy and therefore chemoresistance[53]. This mucin overexpression results in the production of a thick, mucinous physical barrier between cancer cells and the extracellular matrix, thus inhibiting the ability of intracellular drug uptake via nucleoside transporters and affecting the drugs cytotoxicity. Due to this, several agents have been investigated for their mucolytic properties[42,53].
Much of this research has focused on agents that involve the immune system, such as adoptive immunotherapy, oncolytic viral therapy, peptide vaccines to enhance the cytotoxic T cell or T helper cell response, or specific antibodies. These techniques have shown promising results for patients with metastasised disease, when implemented in pre-clinical as well as early-stage clinical trials. However, many of these approaches are limited in their targeting abilities and have only been examined in small patient cohorts[2,3].
Another avenue readily investigated is gene editing technology specifically targeting the mechanisms that have been identified as contributors of disease progression. These methods can include transfection of cells, silencing, and upregulating genes via gene promotors, and at the transcriptional level, targeting epigenetic regulation. It is important to note, despite the promising results obtained in vitro and in early clinical stages for these treatments, there are issues surrounding the effectiveness of treatment delivery in vivo. Due to this, further research is required on the feasibilities of implementing these treatments to patients[2,3,54].
A variety of micro RNAs (miRs), pharmacological and natural agents have also been investigated for their ability to limit pancreatic cancer progression by specifically targeting and downregulating well known mucins such as MUC1 and MUC4[54,55].
miRs are small noncoding RNAs that occur naturally, functioning in gene regulation and have been identified as possible regulators of mucin expression in pancreatic cancer. Four key miRs have been investigated and provided positive results, those being, miR-Let-7b, miR-150, miR-219-1-3p, and miR-200c. All mentioned miRs have been found to downregulate MUC4 expression, miR-150 directly targets MUC4 resulting in inhibition and is therefore a MUC4-regulating miR. Despite the promising effect of miRs in regulating mucins, there are minimal studies aimed at assessing their viability against established pancreatic tumors in vivo and further studies are required prior to clinical application[36,56-58].
Regarding pharmacological agents, the modulation of mucins, specifically MUC4, is achieved through anti-inflammatory drugs, corticosteroids. Key investigated corticosteroids include dexamethasone, as well as afatinib and canertinib, two pan-EGFR family inhibitors. These agents had various levels of effectiveness with dexamethasone providing variable modulation results, while afatinib and canertinib resulted in the downregulation of MUC4 expression. Interestingly, canertinib, unlike afatinib, is an irreversible inhibitor and treatment resulted in not only downregulation of MUC4, but also reduction in tumor growth. Though promising, the variable effects due to corticosteroids not only on mucins, but other mechanisms of disease have limited the possibility of clinical application[54,59,60].
Natural agents are of great significance in the treatment of pancreatic cancer due to their ability to target inflammation signaling pathways and regulate the expression of mucins. Several of these agents have been identified as possible pancreatic cancer treatments, including, Thymoquinone, Guggulsterone, Graviola extract, Bromelain and N-acetylcysteine.
Guggulsterone is a phytosteroid derived from the resin of Commiphora Mukul plants. Historically, it has been utilized for the treatment of atherosclerosis, hyperlipidemia, diabetes, hypertension, and obesity. More recently, it has been investigated for its anti-proliferative activity, ability to induce apoptosis and inhibit cell invasion and motility in various tumor types whether in vitro or vivo. Furthermore, Guggulsterone has shown a synergistic effect with gemcitabine against pancreatic cancer, as well as the reversal of multidrug resistance in breast cancer cell lines. In addition to its anti-tumor properties, Guggulsterone has been identified to decrease the activation of STAT/JAK pathways, downregulating MUC4 as a result. Accounting for all the possible benefits of Guggulsterone it is an attractive agent for clinical application in combination with Gemcitabine against pancreatic cancer[54,61].
Thymoquinone is a bioactive constitute derived from black seed oil (Nigella sativa). It has previously been identified as having several promising pharmacological properties against diseases due to its anti-inflammatory, antioxidant, and anticancer activities. More recently, it has been shown to exhibit antineoplastic properties against pancreatic cancer cell lines FG/ COLO357 and CD18/HPAF. Treatment with Thymoquinone against these cell lines resulted in the downregulation of MUC4 resulting in the increased apoptosis and decrease of migration of tumor cells. The downregulation of MUC4 results in the activation of c-Jun-(NH2)-terminal kinase and p38 MAP kinase, thereby implicating MUC4 as a key target of Thymoquinone. As seen with other natural agents, synergistic effects have been observed both in vitro and vivo with Thymoquinone and chemotherapeutic agents, specifically, Gemcitabine and Oxaliplatin[54,62,63].
Annona muricata (Graviola) evergreen trees are the source of medicinal treatment for inflammatory conditions such as insomnia, hypertension, rheumatism, diabetes, and neuralgia. Within the last decade, investigations into the effect of extracted graviola on pancreatic cancer cell lines have been undertaken in vitro and vivo. It has been identified that graviola downregulated genes associated with glycolysis and hypoxia, and more importantly, downregulated MUC4, while exhibiting antimetastatic and antiproliferative effects[54,64]. Interestingly, graviola has been shown effective in destroying chemoresistant breast cancer cells[65]. This study indicating graviola to be an attractive agent of investigation for chemoresistant cancer cells.
Bromelain is a cysteine proteolytic enzyme extracted from the stem of immature pineapple plants (Ananas comosus). The enzyme is well known for its anti-inflammatory and mucolytic properties as well as its uses for relief of indigestion, thrombosis, fevers, wound debridement, and edema. Bromelain was originally utilised for its anti-cancer properties in ovarian and breast cancer, with clinical trials of administration resulting in a decrease of metastasis and the resolution of tumour masses. When administered in combination treatments with chemotherapeutic agents such as 5-FU greater patient outcomes and further tumour regression has been observed[66]. Interestingly, bromelain has been recently investigated for its proteolytic properties, specifically its ability to target and disrupt glycosidic linkages in mucins resulting in the disruption of the protective mucolytic layer. The disruption of mucolytic layers produced by mucins on cancerous cells enables the effective uptake of cytotoxic drugs into cells resulting in better patient survival[3,53,66].
N-acetyl cystine (NAC) is a sulphur containing amino acid that is commonly used for the treatment of acetaminophen toxicity and to liquify the thick mucus formed in respiratory diseases such as chronic obstructive pulmonary disorder (COPD) and cystic fibrosis[66]. Due to its chemical composition, NAC holds the ability to reduce disulphide bonds (S-S) to sulphydryl (S-H) bonds in glycoproteins and therefore convert purulent and viscous mucin into an aqueous state resulting in ciliary elimination in the respiratory system[66].
A further strategy for overcoming chemoresistance and increasing patient survival is combination therapy with chemotherapeutic and mucolytic agent. Two natural agents have been identified as effective against mucous cancers when combined, those being bromelain and NAC[67].
Recently, studies into the efficiency of these agents as single agents, in combination with each other and in combination with gemcitabine and 5-FU against pancreatic cancer cell lines has been investigate with promising results[53]. This can present beneficial outcomes for clinical application due to the ease of application as well as a reduction in required chemotherapeutic doses therefore reduced toxicity when compared to currently utilised chemotherapeutic drugs[3,53].
The combination of bromelain and NAC has been shown to have a synergistic cytotoxic effect against several tumour types. It is known that late-stage pancreatic tumours express high amounts of MUC1, MUC4, MUC16 which have been associated with invasiveness, cellular proliferation, metastasis and chemoresistance. Previous studies have shown that a bromelain and NAC combination treatment resulted in the reduction of mucin expression both in vitro and vivo[53,68]. Recent in vivo investigations into combination therapy of bromelain and NAC with the addition of cytotoxic agents such as gemcitabine resulted in observed synergism and introduce the possibility of reducing the clinical cytotoxic dosage required for treatment and therefore reducing side effects that commonly present with high dose treatments[53]. It is thought that the combination of bromelain and NAC will disrupt the glycosylic linkages and disulphate bonds within mucins allowing for gemcitabine to effectively enter the cells via nucleoside transporters resulting in its ability to incite its cytotoxic effect[53].
Pancreatic cancer is a deadly disease with a high mortality rate attributed to late tumour detection, disease aggressiveness and chemoresistance. Although predicted to be the second leading cause of cancer associated mortality within the next 10 years, steady progress has been achieved in determining the mechanisms that contributes to the poor progression of the disease, which, in turn contributes to the development of diagnostic and therapeutic targets.
One of these mechanisms has been identified as human nucleoside transporters, namely for their importance in the transportation of chemotherapeutic drugs into diseased cells along with their contribution to acquired chemoresistance. Primarily, investigations have been undertaken on the nucleoside transporters hENT1, hENT2, hCNT1, and hCNT3, namely due to their ability to transport the nucleoside analogue gemcitabine, however, more research is needed to evaluate their use not only as diagnostic markers but as therapeutic targets.
The expression of nucleoside transporter proteins has been shown to be adverse in pancreatic cancer cells, with decreased protein expression correlating with not only reductions in progression and disease-free patient survival but also an increase in acquired chemoresistance. The lack or inability of nucleoside transporter proteins to facilitate the uptake of chemotherapeutic drugs has been identified as a contributor of gemcitabine chemoresistance and although the majority of research has been focused on hENT1, all examine nucleoside transporters have exhibited this correlation.
There have been several suggestions on overcoming the factors contributing to nucleoside transport related chemoresistance, with treatment options such as mucins being of great interest. Specific mucins have been shown to be overexpressed in pancreatic cancer cells and new combination treatment therapies, such as bromelain + NAC + gemcitabine, targeting the linkages seen in mucins expose the encased nucleoside transporters, allowing for the uptake of cytotoxic drugs which is advantageous for several reasons. The two main benefits being increased drug uptake, and reduction of required cytotoxic dose although further research of this strategy for not only the treatment of pancreatic cancer but also overcoming chemoresistance is required.
We would like to thank the team at the Department of Surgery at St George Hospital, UNSW for all of their support.
| 1. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1613] [Article Influence: 230.4] [Reference Citation Analysis (1)] |
| 2. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1345] [Article Influence: 168.1] [Reference Citation Analysis (47)] |
| 3. | Suh H, Pillai K, Morris DL. Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am J Cancer Res. 2017;7:1372-1383. [PubMed] |
| 4. | Jonckheere N, Skrypek N, Van Seuningen I. Mucins and pancreatic cancer. Cancers (Basel). 2010;2:1794-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Murthy D, Attri KS, Singh PK. Phosphoinositide 3-Kinase Signaling Pathway in Pancreatic Ductal Adenocarcinoma Progression, Pathogenesis, and Therapeutics. Front Physiol. 2018;9:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Attia F, Fathy S, Anani M, Hassan A, Attia F, Ibrahim G, Elazab M. Human equilibrative nucleoside transporter-1 and deoxycytidine kinase can predict gemcitabine effectiveness in Egyptian patients with Hepatocellular carcinoma. J Clin Lab Anal. 2020;34:e23457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Ringel J, Löhr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:9. [PubMed] |
| 9. | Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24:2047-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 401] [Article Influence: 50.1] [Reference Citation Analysis (12)] |
| 11. | Mohammed S, Van Buren G 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20:9354-9360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 95] [Reference Citation Analysis (1)] |
| 12. | Liu F, Du F, Chen X. Multiple tumor marker protein chip detection system in diagnosis of pancreatic cancer. World J Surg Oncol. 2014;12:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Yamamoto T, Uchida Y, Terajima H. Clinical impact of margin status on survival and recurrence pattern after curative-intent surgery for pancreatic cancer. Asian J Surg. 2019;42:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 2103] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 15. | Tsujie M, Nakamori S, Nakahira S, Takahashi Y, Hayashi N, Okami J, Nagano H, Dono K, Umeshita K, Sakon M, Monden M. Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res. 2007;27:2241-2249. [PubMed] |
| 16. | Spratlin JL, Mackey JR. Human Equilibrative Nucleoside Transporter 1 (hENT1) in Pancreatic Adenocarcinoma: Towards Individualized Treatment Decisions. Cancers (Basel). 2010;2:2044-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5900] [Article Influence: 393.3] [Reference Citation Analysis (23)] |
| 18. | Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in Pancreatic Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 448] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 20. | Manji GA, Olive KP, Saenger YM, Oberstein P. Current and Emerging Therapies in Metastatic Pancreatic Cancer. Clin Cancer Res. 2017;23:1670-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Alvarellos ML, Lamba J, Sangkuhl K, Thorn CF, Wang L, Klein DJ, Altman RB, Klein TE. PharmGKB summary: gemcitabine pathway. Pharmacogenet Genomics. 2014;24:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Paproski RJ, Yao SY, Favis N, Evans D, Young JD, Cass CE, Zemp RJ. Human concentrative nucleoside transporter 3 transfection with ultrasound and microbubbles in nucleoside transport deficient HEK293 cells greatly increases gemcitabine uptake. PLoS One. 2013;8:e56423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 24. | Maréchal R, Bachet JB, Mackey JR, Dalban C, Demetter P, Graham K, Couvelard A, Svrcek M, Bardier-Dupas A, Hammel P, Sauvanet A, Louvet C, Paye F, Rougier P, Penna C, André T, Dumontet C, Cass CE, Jordheim LP, Matera EL, Closset J, Salmon I, Devière J, Emile JF, Van Laethem JL. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012;143:664-674.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | García-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2',2'-difluorodeoxycytidine- induced cytotoxicity. Clin Cancer Res. 2003;9:5000-5008. [PubMed] |
| 26. | Maréchal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, Cass CE, Young J, Salmon I, Devière J, Van Laethem JL. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009;15:2913-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Hagmann W, Jesnowski R, Löhr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Yeldag G, Rice A, Del Río Hernández A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 29. | Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, Illies AL, Gebregiworgis T, Dai B, Augustine JJ, Murthy D, Attri KS, Mashadova O, Grandgenett PM, Powers R, Ly QP, Lazenby AJ, Grem JL, Yu F, Matés JM, Asara JM, Kim JW, Hankins JH, Weekes C, Hollingsworth MA, Serkova NJ, Sasson AR, Fleming JB, Oliveto JM, Lyssiotis CA, Cantley LC, Berim L, Singh PK. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell. 2017;32:71-87.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 393] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 30. | Dauer P, Nomura A, Saluja A, Banerjee S. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology. 2017;17:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | Mittal A, Chitkara D, Behrman SW, Mahato RI. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35:7077-7087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Pastor-Anglada M, Pérez-Torras S. Emerging Roles of Nucleoside Transporters. Front Pharmacol. 2018;9:606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, Dicker AP, Mackey JR. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 354] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 36. | Wang C, Lin W, Playa H, Sun S, Cameron K, Buolamwini JK. Dipyridamole analogs as pharmacological inhibitors of equilibrative nucleoside transporters. Identification of novel potent and selective inhibitors of the adenosine transporter function of human equilibrative nucleoside transporter 4 (hENT4). Biochem Pharmacol. 2013;86:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Paproski RJ, Young JD, Cass CE. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3'-deoxy-3'-fluorothymidine. Biochem Pharmacol. 2010;79:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Liu Y, Zuo T, Zhu X, Ahuja N, Fu T. Differential expression of hENT1 and hENT2 in colon cancer cell lines. Genet Mol Res. 2017;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Griffiths M, Yao SY, Abidi F, Phillips SE, Cass CE, Young JD, Baldwin SA. Molecular cloning and characterization of a nitrobenzylthioinosine-insensitive (ei) equilibrative nucleoside transporter from human placenta. Biochem J. 1997;328 ( Pt 3):739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 202] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Zhang S, Furugawa T, Takahashi H, Che X, Zhao H, Akiyama S, Li Y. Analysis of gene expression in gemcitabine resistant cells derived from human pancreatic cancer cell. Proceedings of the Proceedings 2011 International Conference on Human Health and Biomedical Engineering. 2011: 182-185. |
| 41. | Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 42. | Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956-6961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 43. | Bhutia YD, Hung SW, Patel B, Lovin D, Govindarajan R. CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Res. 2011;71:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Skrypek N, Duchêne B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (4)] |
| 45. | Stecula A, Schlessinger A, Giacomini KM, Sali A. Human Concentrative Nucleoside Transporter 3 (hCNT3, SLC28A3) Forms a Cyclic Homotrimer. Biochemistry. 2017;56:3475-3483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Hesler RA, Huang JJ, Starr MD, Treboschi VM, Bernanke AG, Nixon AB, McCall SJ, White RR, Blobe GC. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis. 2016;37:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 849] [Article Influence: 106.1] [Reference Citation Analysis (0)] |
| 48. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2792] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 49. | Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Pérez-Torras S, Vidal-Pla A, Cano-Soldado P, Huber-Ruano I, Mazo A, Pastor-Anglada M. Concentrative nucleoside transporter 1 (hCNT1) promotes phenotypic changes relevant to tumor biology in a translocation-independent manner. Cell Death Dis. 2013;4:e648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Pastor-Anglada M, Pérez-Torras S. Nucleoside transporter proteins as biomarkers of drug responsiveness and drug targets. Front Pharmacol. 2015;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Pillai K, Mekkawy AH, Akhter J, Badar S, Dong L, Liu AI, Morris DL. Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine - an in vitro study with pancreatic and hepatic cancer cells. Am J Transl Res. 2020;12:7404-7419. [PubMed] |
| 53. | Gautam SK, Kumar S, Cannon A, Hall B, Bhatia R, Nasser MW, Mahapatra S, Batra SK, Jain M. MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opin Ther Targets. 2017;21:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Srivastava SK, Bhardwaj A, Singh S, Arora S, Wang B, Grizzle WE, Singh AP. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Arora S, Swaminathan SK, Kirtane A, Srivastava SK, Bhardwaj A, Singh S, Panyam J, Singh AP. Synthesis, characterization, and evaluation of poly (D,L-lactide-co-glycolide)-based nanoformulation of miRNA-150: potential implications for pancreatic cancer therapy. Int J Nanomedicine. 2014;9:2933-2942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Lahdaoui F, Delpu Y, Vincent A, Renaud F, Messager M, Duchêne B, Leteurtre E, Mariette C, Torrisani J, Jonckheere N, Van Seuningen I. miR-219-1-3p is a negative regulator of the mucin MUC4 expression and is a tumor suppressor in pancreatic cancer. Oncogene. 2015;34:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, Ganti AK, Batra SK. Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6:5164-5181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Gollub EG, Waksman H, Goswami S, Marom Z. Mucin genes are regulated by estrogen and dexamethasone. Biochem Biophys Res Commun. 1995;217:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, Jain M. Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett. 2013;341:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, Batra SK. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9:1419-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 63. | Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, Johansson SL, Singh PK, Ganti AK, Batra SK. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 64. | Syed Najmuddin SU, Romli MF, Hamid M, Alitheen NB, Nik Abd Rahman NM. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement Altern Med. 2016;16:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Pillai K. Anti-Tumour and Chemosensitising Effect of a Combination of Bromelain + N-Acetyl Cysteine with Cisplatin or 5-Fu on Malignant Peritoneal Mesothelioma Cells. J Glycobiol. 2013;s1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 66. | Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: sequential and combination therapy of gastrointestinal cancer cells. Am J Cancer Res. 2016;6:350-369. [PubMed] |
| 67. | Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950-59964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 495] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 68. | Goral V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac J Cancer Prev. 2015;16:5619-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 69. | Maher DM, Gupta BK, Nagata S, Jaggi M, Chauhan SC. Mucin 13: structure, function, and potential roles in cancer pathogenesis. Mol Cancer Res. 2011;9:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Chemocare. Chemotherapy Drugs and Drugs Often Used During Chemotherapy. 2020 [cited 12 April 2021] Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Gemcitabine. |
| 71. | National Center for Biotechnology Information. PubChem Compound Summary for CID 60750, Gemcitabine. 2020 [cited 12 April 2021] Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Gemcitabine. |
| 72. | Wright NJ, Lee SY. Structures of human ENT1 in complex with adenosine reuptake inhibitors. Nat Struct Mol Biol. 2019;26:599-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 73. | Rehan S, Paavilainen VO, Jaakola VP. Functional reconstitution of human equilibrative nucleoside transporter-1 into styrene maleic acid co-polymer lipid particles. Biochim Biophys Acta Biomembr. 2017;1859:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Vincenzi B, Stacchiotti S, Collini P, Pantano F, Rabitti C, Perrone G, Iuliani M, Baldi A, Badalamenti G, Sanfilippo R, Santini D, Muda AO, Gronchi A, Casali P, Dei Tos AP, Tonini G. Human equilibrative nucleoside transporter 1 gene expression is associated with gemcitabine efficacy in advanced leiomyosarcoma and angiosarcoma. Br J Cancer. 2017;117:340-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Kim J, Kim H, Lee JC, Kim JW, Paik WH, Lee SH, Hwang JH, Ryu JK, Kim YT. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS One. 2018;13:e0209104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Bolze A, Abhyankar A, Grant AV, Patel B, Yadav R, Byun M, Caillez D, Emile JF, Pastor-Anglada M, Abel L, Puel A, Govindarajan R, de Pontual L, Casanova JL. A mild form of SLC29A3 disorder: a frameshift deletion leads to the paradoxical translation of an otherwise noncoding mRNA splice variant. PLoS One. 2012;7:e29708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Daniels G. An update on the Augustine blood group system. Immunohematology. 2019;35:1-2. [PubMed] |
| 78. | Owen RP, Lagpacan LL, Taylor TR, De La Cruz M, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, Giacomini KM. Functional characterization and haplotype analysis of polymorphisms in the human equilibrative nucleoside transporter, ENT2. Drug Metab Dispos. 2006;34:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Cano-Soldado P, Gorraitz E, Errasti-Murugarren E, Casado FJ, Lostao MP, Pastor-Anglada M. Functional analysis of the human concentrative nucleoside transporter-1 variant hCNT1S546P provides insight into the sodium-binding pocket. Am J Physiol Cell Physiol. 2012;302:C257-C266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Koczor CA, Torres RA, Lewis W. The role of transporters in the toxicity of nucleoside and nucleotide analogs. Expert Opin Drug Metab Toxicol. 2012;8:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Yao SYM, Young JD. Inward- and outward-facing homology modeling of human concentrative nucleoside transporter 3 (hCNT3) predicts an elevator-type transport mechanism. Channels (Austin). 2018;12:291-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, Ritzel RG, Mowles DA, Carpenter P, Chen XZ, Karpinski E, Hyde RJ, Baldwin SA, Cass CE, Young JD. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). J Biol Chem. 2001;276:2914-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernández-Placencia RM, Jin Y S-Editor: Gong ZM L-Editor: A P-Editor: Ma YJ