Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6874
Peer-review started: March 16, 2021
First decision: May 1, 2021
Revised: May 3, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 28, 2021
Processing time: 224 Days and 15.5 Hours
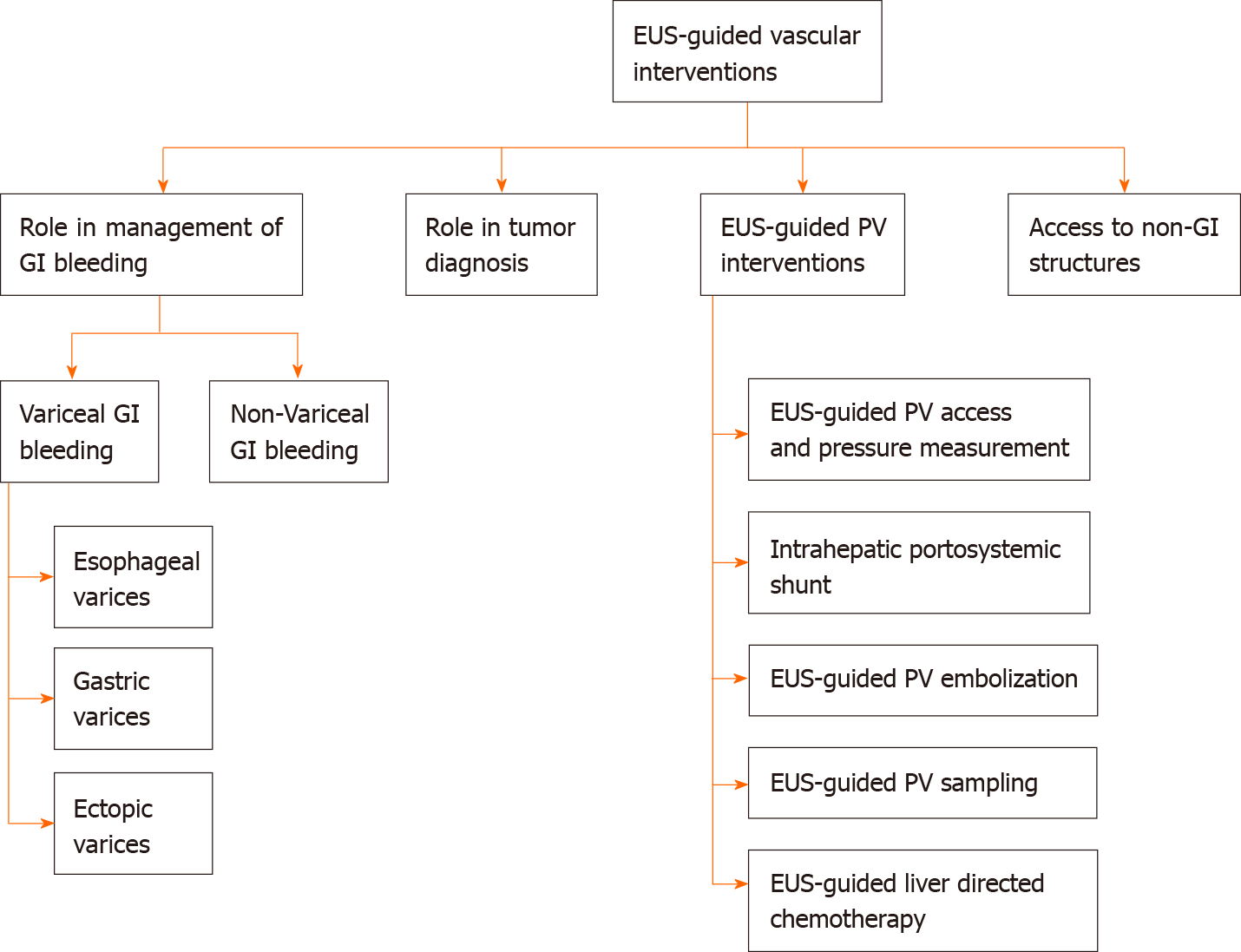
Endoscopic ultrasound (EUS) is one of the significant breakthroughs in the field of advanced endoscopy. In the last two decades, EUS has evolved from a diagnostic tool to a real-time therapeutic modality. The luminal gastrointestinal (GI) tract provides a unique opportunity to access multiple vascular structures, especially in the mediastinum and abdomen, thus permitting a variety of EUS-guided vascular interventions. The addition of the doppler and contrast-enhanced capability to EUS has further helped provide real-time visualization of blood flow in vessels through the GI tract. EUS-guided vascular interventions rely on standard endoscopic accessories and interventional tools such as fine-needle aspiration needles and fine-needle biopsy. EUS allows the visualization of various structures in real-time by differentiating tissue densities and vascularity, thus, avoiding radiation exposure. EUS-guided techniques also allow real-time microscopic examination after target biopsy. Furthermore, many necessary interventions can be done during the same procedure after diagnosis. This article provides an overview of EUS-guided vascular interventions such as variceal, non-variceal bleeding interventions, EUSguided portal vein (PV) access with the formation of an intrahepatic portosystemic shunt, and techniques related to diagnosis of GI malignancies. Furthermore, we discuss current insights and future outlook of therapeutic modalities like PV embolization, PV sampling, angiography, drug administration, and portal pressure measurement.
Core Tip: Endoscopic ultrasound (EUS) technology has evolved rapidly in clinical practice, first as merely a diagnostic tool and now a therapeutic modality. EUS-guided interventions involve combining real-time imaging capability with invasive therapeutic interventions. The gastrointestinal (GI) tract has proximity to various vascular structures in the abdomen and mediastinum and thus provides a unique window to access these structures with EUS to render EUS-guided vascular interventions. Herein, this article provides an overview of EUS-guided vascular interventions for GI bleeding, portal vein access, and therapeutic implications, tumor diagnosis, and access to non-GI structures.
- Citation: Mann R, Goyal H, Perisetti A, Chandan S, Inamdar S, Tharian B. Endoscopic ultrasound-guided vascular interventions: Current insights and emerging techniques. World J Gastroenterol 2021; 27(40): 6874-6887
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6874
Endoscopic ultrasound (EUS) is a minimally invasive specialized procedure that blends endoscopy with ultrasound. The endoscope allows visualizing the gastrointestinal (GI) tract lining, and ultrasound allows visualization of the GI tract walls, surrounding organs, and blood vessels with high-frequency waves. Three different types of echoendoscopes are available: Linear, radial, and mini-probes. Linear echoendoscopes are preferred for pancreaticobiliary interventions such as acquiring tissue, drainage of collections, and injections. Radial echoendoscopes are preferred for the staging of esophageal and gastric cancers[1,2]. Mini probes are more useful in diagnosing mucosal malignancy, pancreaticobiliary diseases (such as malignancy or stricture) because of their high frequency[1]. EUS can visualize both solid and fluid structures in the GI lumen and extraluminal. Various structures accessible with EUS include the luminal wall of the GI tract (esophagus, stomach, duodenum, and rectum), liver, pancreas, gallbladder, biliary tree, mediastinum, and lymph nodes. In addition, EUS can identify various arterial and venous vascular structures in proximity to the GI tract are accessible. Feeding vessels from small branches of vessels and aberrant vascular shunts can also be visualized by EUS[2,3].
Since the introduction of EUS in 1980 for diagnostic purposes, significant evolution has occurred (Figures 1 and 2). Its application on humans dates back to 1982[4]. This evolved in 1991 when EUS-guided fine-needle aspiration (FNA) was first introduced, and it allowed to do therapeutic interventions outside the lumen of the GI tract[5]. In 1996, EUS-guided cholangiography and EUS-guided biliary drainage were introduced[6]. Furthermore, the role of EUS expanded rapidly over the last 20 years from the diagnostic to the therapeutic tool as it provides direct visualization, access to structures within and outside the GI tract. Several new advancements in EUS-guided vascular procedures have emerged due to the proximity of many blood vessels to the GI tract and the ability to deliver precise real-time interventions. It has been further expanded to target gastric variceal bleeding by cyanoacrylate (CYA) injection[7]. In 2008, EUS-guided glue injection and micro coil embolization were used to treat a patient with refractory gastric variceal bleeding. Additionally, EUS can obtain real-time portal pressures in patients suspected of portal hypertension (PH) and obtain liver core biopsies to evaluate for fibrosis in one setting. Given its ability to access the portal vein (PV), interventions such as PV thrombectomy have been possible. Given these advances, we aim to discuss updates and emerging trends in EUS related vascular interventions.
Esophageal varices: Endoscopic band ligation (EBL) is the preferred treatment for bleeding and non-bleeding esophageal varices. However, endoscopic sclerotherapy is an additional treatment that is still used, especially when EBL is not feasible[8]. Although these therapies are successful, the rate of recurrent bleeding can range up to 15%-65%[9]. The higher recurrence is most likely due to failure to treat perforating and collateral vessels. Echoendoscope provides benefits over endoscope due to its ability to visualize and target these high-risk vessels under direct visualization[10,11]. EUS-guided sclerotherapy for esophageal varices was first described in 2000 by Lahoti et al[12] in a study of 5 patients. A sclerosing agent such as sodium morrhuate was injected into target perforating vessels under EUS guidance until complete blood flow cessation was confirmed with the doppler. A mean of 2.2 sessions (range 2-3 sessions) were required to achieve the complete eradication of varices. No recurrence of bleeding or death was reported during 15 mo follow-up. Only one patient developed esophageal stricture, which was managed with balloon dilation[12].
The above study showed comparable results of EUS-guided sclerotherapy for esophageal varices. Therefore, EBL is still a preferred treatment for esophageal varices. Large, randomized trials are needed to show the clinical benefits of EUS-guided sclerotherapy compared to EBL to consider it as one of the first-line treatments.
Gastric varices: Although gastric varices (GV) are less common than esophageal varices, it affects around 20% of patients with PH[13,14]. GV can occasionally cause bleeding, leading to iron deficiency anemia, and the risk of rebleeding (34%-89%) is much higher than compared to esophageal varices (15%-65%)[13]. There are no well-established treatment guidelines for the management of GV compared to esophageal varices[15]. EBL is not recommended for managing GV as they are larger in size, with thick overlying gastric mucosa making it difficult to band. EBL of GV can lead to life-threatening bleeding due to post-band ulcerations, developed due to a failure to capture the contralateral wall of varices during the procedure[16]. In a study of 22 patients with bleeding GV treated with EBL, 18.2% developed early rebleeding even after complete hemostasis was achieved in all cases on EBL[17]. Endoscopic sclerosing therapy should also be avoided in bleeding GV as it provides only temporary control of bleed and a higher incidence of adverse events like gastric ulcerations, perforations, and rebleeding in 37%–53% of cases[18 ].
Glue therapy with CYA is the primary treatment of choice for GV. It was first described in 1986 as it has higher rates (> 90%) of achieving hemostasis and a lower rate of rebleeding (0%-40%) compared to other therapies (EBL and sclerotherapy)[19,20]. However, there are reports of significant adverse effects associated with CYA injections like systemic embolization (cardiac embolism, pulmonary embolism, splenic vein thrombosis, splenic artery embolism, renal vein thrombosis, and cerebral infarct), which is thought to be related to the volume of CYA injection[21]. EUS assists in both the diagnosis and treatment of GV. It helps in diagnosis and allows a precise evaluation of pathological vessels, improving therapeutic targeting. Color doppler also permits to differentiation of GV from other structures and can help confirm eradication of varices. EUS has provided us a new array of treatment options, inclu
Glue therapy. Endoscopic-CYA injection has been shown to control bleeding, but there is a high recurrence of bleeding, probably due to incomplete obliteration of varices. EUS-guided CYA glue injection can minimize recurrent bleeding and decrease CYA volume by directly visualizing the perforating vessels, thus more precise obliteration of varices. Theoretically, the risk of embolization also decreases as it allows precise CYA injection into the target vessel[7].
A total of six patients with GV, including four for secondary prophylaxis, were treated with EUS-guided coil embolization followed by CYA glue injection in a single-center retrospective study. Complete eradication of GV was achieved in 3 patients. One patient had pulmonary embolism as a complication of CYA glue injection[22]. In a large single-center study, 40 patients underwent EUS-guided n-butyl-2-CYA for GV. Out of 40 patients, 13 patients were treated during active bleeding and another 23 within 24 h of bleeding. Thus, bleeding was acutely controlled in 100% of cases after treatment with EUS-guided n-butyl-2-CYA therapy. Only six patients required additional intervention for long-term management (Table 1)[23].
| Type of Intervention | Year | Ref. | Number of patients, n (%) | Follow up (mo) | Obliteration of varices/Clinical success % | Recurrent bleeding rate % | Adverse events % |
| CYA glue | 2019 | Lôbo et al[13] | 16 | 9.9 | 75% | - | 50% |
| CYA glue | 2014 | Gubler and Bauerfeind[23] | 40 | - | 100% | 15% | 5% |
| CYA glue | 2019 | Bick et al[24] | 64 | 6.6 | 96.9% | 8.8% | 17.5% |
| CYA glue | 2013 | Romero-Castro et al[26] | 19 | 6 | 94.7% | 0% | 57.9% |
| CYA glue | 2018 | Krill et al[62] | 10 | 4 | 100% | - | 0% |
| Coil | 2013 | Romero-Castro et al[26] | 11 | 6 | 90.9% | 0% | 9.1% |
| Coil | 2018 | Krill et al[62] | 6 | 4 | 100% | - | 0% |
| Coil | 2010 | Romero-Castro et al[63] | 4 | 5 (1-3) | 75% | 0% | 0% |
| Coil and CYA Glue | 2019 | Lôbo et al[13] | 16 | 9.9 | 73.3% | - | 25% |
| Coil and CYA Glue | 2019 | Kozieł et al[14] | 16 | 10.9 | 100% | - | 37.5% |
| Coil and CYA Glue | 2018 | Krill et al[62] | 12 | 4 | 100% | - | 8% |
In a single-center study, 40 patients with actively/recently bleeding or high-risk GV treated with direct endoscopic injection of CYA were compared with 64 patients treated prospectively with EUS-guided fine needle injection CYA. Gastroesophageal varices type 2 was the most common type of varices seen in both groups. During the procedure, a greater number of varices were obliterated in EUS-guided fine needle injection of the CYA group (1.6 ± 0.7) than the direct endoscopic injection of the CYA group (1.1 ± 0.4, P < 0.001). Whereas the mean volume of CYA was injected more in the direct endoscopic injection-CYA group (3.3 ± 1.3 mL) compared to EUS-guided fine needle injection of the CYA group (2.0 ± 0.8 mL, P < 0.001). Overall post-procedure GV rebleeding (23.7% vs 8.8%, P = 0.045) and non-GV-related GI bleeding (GIB) (27.5% vs 10.9%, P = 0.030) were found to be higher in the direct endoscopic injection of CYA group compared to EUS-guided fine needle injection of CYA group. No significant difference was found in the overall rate of adverse events in the direct endoscopic injection of CYA group (17.5%) and EUS-guided fine needle injection of CYA (20.3%, P = 0.361)[24]. EUS-guided glue injection appears to be safe and effective in decreasing the risk of rebleeding in patients with active or recent bleeding GV when compared to the endoscopic injection of CYA.
Coil embolization. EUS-guided coil application is another treatment modality for GV. Coils are commonly used for various interventional radiology procedures (Table 1). These micro coils can obliterate GV and avoids adverse effects associated with CYA use, such as embolization coils are made up of light metal alloy and are covered with synthetic fibers to induce clot formation and subsequent hemostasis. Furthermore, fibers can act as a scaffold for CYA if injected during the same procedure. Varices are identified and punctured through standard FNA size needles like 19G (0.035-inch coil) or a 22G (0.018-inch coil). These coils are then advanced from the needle into varix using the stylet as a pusher. The coil sizes are selected based on the size of varix[25,26].
A retrospective multicenter study compared EUS-guided CYA injection (n = 19) to EUS-guided coil embolization (n = 11) patients with GV. There was no statistically significant difference in obliteration rate, the mean number of sessions required, and recurrence noted during follow-up. However, adverse events were reported to be higher in the CYA group in [11/19 (57%)] when compared to EUS-guided coil embolization [1/11 (9.1%), P < 0.01]. A post-procedure computed tomography (CT) scan was performed in all patients, and nine patients in the CYA group were found to have asymptomatic glue embolism on imaging[26] (Table 1). This was the first study to compare coil embolization and CYA directly. Both procedures showed comparable results in terms of GV obliteration, but fewer adverse events were noted with coil embolization.
In another study, ten patients with GV underwent EUS-guided coil embolization and then reinforcement by gelatin sponges. Nine patients had either active bleeding or recently bled GV. A 100% obliteration of GV was achieved. Patients were followed for six mo, and only 1/10 patients developed severe abdominal pain as a complication. Further large prospective studies are needed describing its direct comparison with other treatment modalities like CYA or CYA and coil embolization combined[27].
Combined coiling and glue therapy. Although endoscopic CYA injection is considered primary therapy for GV, it is associated with systemic glue embolization. Synthetic fibers in the covering of coils function as a scaffold to keep CYA in varices; thus, a decrease of CYA reagent is needed to eradicate gastric fundal varices (GFV) and also may reduce the risk of glue embolization. In another study by Kozieł et al[14], four patients were treated with coils, and 12 patients were treated with EUS-guided coil and CYA injection. These patients were followed for an average of 327 d. The technical success rate was 94%, and the mean number of CYA volume and coils needed per procedure was 2 mL and 1.7, respectively. No serious complications like embolization or death were noted (Table 1).
A randomized pilot trial was conducted comparing the safety and efficacy of EUS-guided coil and CYA (n = 16) to EUS-guided CYA in GV patients, and these patients were followed for an average of 9.9 mo. EUS doppler was done in all cases to determine flow within varix after the procedure, and thoracic and abdomen CT was done in all cases 1 wk after the procedure. Repeat CT scans were done in symptomatic cases only afterward. In the EUS-guided coil and CYA vs EUS-guided CYA group, the total reduction inflow in the treated vessel was 37.5% vs 50% (P = 0.476) and 73% vs 80% (P = 1) immediately and at 30 d after the procedure, respectively. In addition, asymptomatic pulmonary embolism was found to be in 25%, and 50% cases (P = 0.144) in EUS-guided combined coil and CYA and EUS-guided CYA, respectively[13] (Table 1).
A metanalysis and systematic review was conducted comparing EUS-guided CYA injection, EUS-guided coil embolization and CYA injection combined, and EUS-guided coil injection alone. Combined EUS-guided CYA and coiling were found to have better technical and clinical success rate compared coil embolization alone (99% vs 97%; P < 0.001 and 96% vs 90%; P < 0.001) and CYA alone (100% vs 97%; P < 0.001 and 98% vs 96%; P < 0.001). Similarly, lower adverse events were found to be with EUS-guided CYA and coil combined compared to coil embolization (10% vs 3%; P = 0.057) and CYA alone (10% vs 21%; P < 0.001)[15]. Given the above results from various studies, EUS-guided coil embolization and CYA injection combined therapy could be preferred compared to EUS-guided monotherapy with either coil or CYA injection alone.
Thrombin injection. Recently, a study of eight patients (three with active bleeding and five as elective prevention) with GFV treated with EUS-guided thrombin injection was published. About 2/3 of patients with active bleeding had successful hemostasis and obliteration of varices. All five patients who underwent the procedure for prevention of future bleeding had complete obliteration of varices. No direct procedure-related complications were observed. Although this series showed positive results, further large prospective studies are needed[19].
Ectopic varices: Ectopic varices can develop at any site, including duodenal, small bowel, colon, rectum, common bile duct, and peristomal, with duodenum ectopic varices are being the most frequent to bleed[25,28]. Many case reports have described the use of EUS for variceal bleeding at ectopic sites. Cases of duodenal variceal bleed treated with EUS-guided coil and CYA have been described without any complications[29].
Rectal varies are reported in 44%-89% of cirrhosis patients because of PH[30]. It can also be seen in patients with vascular anomalies, mesenteric vein obstruction, adhesions, and heart failure. However, rectal varices are a lower bleeding risk than esophageal and GV[11]. EUS can help detect and treat the presence of rectal varices better than the endoscopy (Table 2). Although most of the data for EUS-guided intervention for bleeding ectopic varices is based on case reports/series, it is emerging as a viable option.
| Ref. | Presenting symptom | Number of patients, age, sex | Rectal varices size | Therapy | Results | Follow up | Results on follow up |
| Philips and Augustine[64], 2017 | Rectal bleeding | 1, 48 yr, M | Large rectal varix | EUS-guided embolization coil and glue | No further bleeding | 1 mo | No rebleeding |
| Bazarbashi et al[65], 2020 | Rectal bleeding | 1, 71 yr, M | Large rectal varices (4 mm in diameter) | EUS-guided coil embolization | No further bleeding | 6 mo | No bleeding |
| Mukkada et al[66], 2017 | Rectal bleeding | 1, 65 yr, M | Large rectal varices | First EUS-guided sclerotherapy, but unable to achieve hemostasis, EUS guided glue | No further bleeding | 1 wk | Rebleeding and then required EUS-guided coil embolization |
GIB can be due to variceal or non-variceal causes[31]. Standard endoscopic treatments for non-variceal bleeding include injection (epinephrine), thermal (argon plasma coagulation, electrosurgical coagulation), and mechanical therapy (such as clipping). Despite these interventions, rebleeding or refractory bleeding is reported as high as 10%-24% in these patients[31]. EUS-guided therapies are advantageous over endoscopic therapy due to the ability to directly visualize target vessels buried in the walls of the organ along with real-time doppler. A literature review of 35 patients who underwent EUS-guided treatment of non-variceal GIB (NVGIB) showed a favorable clinical outcome in 32/35 (91.4%) patients, with recurrent bleeding in only three patients. Moreover, bleeding eventually stopped in all cases. The median follow-up time was 11 mo, and no complications or adverse events were reported during or after the procedure[32]. EUS-guided treatment can also be used as an adjunct treatment in refractory and recurrent disease.
The first use of EUS-guided therapy for Dieulafoy’s lesion was described in 1996 when EUS was used to detect and treat eight patients referred for suspicion of Dieulafoy’s lesion. A large vessel was identified in the stomach wall in all eight patients, which was treated with adrenaline/polidocanol injection using a sclerotherapy needle. Two patients had rebleeding during follow-up, one with recurrent bleeding from Dieulafoy’s lesion and the other from duodenal ulcer. EUS-guided angiotherapy was described by Levy et al[33] in a case series of five patients with refractory bleeding due to hemosuccus pancreaticus, Dieulafoy lesion, duodenal ulcer, GI stromal tumor (GIST), and occult GIB. These patients had failed at least two conventional treatment options and received an average of 18 units of packed red blood cell transfusions. Patients were treated by injecting CYA (3-5 mL) or 99% alcohol into a feeding vessel using a 22G FNA needle under EUS-guidance. Doppler was used to ensuring the absence of blood flow after treatment. These patients were followed up for a mean 12-mo period (range 0.4-23 mo), no rebleeding or complication was reported[33].
Law et al[34] performed EUS-guided hemostatic interventions between June 2003 to May 2014 for 17 patients with refractory NVGIB. Causes of GIB were GIST, colorectal, vascular malformations, Dieulafoy lesions, duodenal ulcers, masses or polyps, rectally invasive prostate cancer, pancreatic pseudoaneurysms, ulcerated esophageal cancer, and ulceration after Roux-en-Y gastric bypass. These patients were treated with epinephrine, 99% ethanol, coil embolization, band ligation, hyaluronate, and CYA using a therapeutic curvilinear echoendoscope with a 22G standard FNA needle. On median follow-up of 12 mo (range 3 wk-120 mo), 15/17 (88%) patients didn’t have any recurrence. However, one patient required repeat EUS-guided band ligation for gastric Dieulafoy lesion, and another patient with rectally invasive prostate cancer experienced ongoing bleeding despite a decrease in vessel flow after treatment with 99% ethanol injection.
Pseudoaneurysms are a known complication of pancreatitis with a risk of rupture and life-threatening bleeding. The risk of rupture is as high as 50%, with 15%-40% of mortality after rupture. Gamanagatti et al[35] described a case series of three patients with pancreatitis-related pseudoaneurysm, which were technically challenging to treat by the endovascular route. These cases were managed with EUS-guided thrombin injection, which resulted in complete thrombosis of the pseudoaneurysms. There were no immediate or late complications on follow-up. In a prospective study, eight patients with symptomatic visceral artery pseudoaneurysm who were unable to undergo angioembolization underwent EUS-guided thrombin injection for pseudoaneurysm. Out of eight patients, 5 had pseudoaneurysm of the splenic artery, 2 had pseudoaneurysm of the hepatic artery, and one patient had pseudoaneurysm of the gastroduodenal artery. Five patients with splenic artery and gastroduodenal artery aneurysms had chronic pancreatitis due to alcohol abuse. The pseudoaneurysm's median size was 2.9 cm × 2.6 cm (range 1.8 cm × 1.9-4 cm × 5 cm), and the median dose of thrombin injected was 400 IU (200-500 IU). Thrombin was injected under EUS guidance with 100% technical success. Repeat EUS after 72 h and 4 wk showed obliteration of pseudoaneurysm in all patients. Whereas on median six mo (1-9 mo) follow up, EUS showed obliterated pseudoaneurysm in 7 patients, and one patient had recurrence requiring recanalization after 6 wk[36]. There are few case reports describing the use of EUS-guided intervention for pseudoaneurysms (Table 3).
| Ref. | Presenting symptom | Number of patients, age and sex | Pseudoaneurysm artery | Therapy | Results | Follow up | Results on follow up |
| Gamanagatti et al[35], 2015 | Pancreatitis with upper GI bleed in all three cases | 3; 56, 45 and 30 yr; M | Gastroduodenal artery-1, splenic artery for 2 patients | EUS-guided thrombin injection | Bleeding stopped, Obliteration of pseudoaneurysm | 1 mo | No bleeding |
| Robb et al[67], 2012 | Infected pseudoaneurysm | 1, 54 yr, M | Superior mesenteric artery branch | EUS-guided embolization | Obliteration of pseudoaneurysm | 5 mo | Asymptomatic |
| Somani et al[68], 2017 | Melena | 1, 50 yr, M | Gastroduodenal artery | EUS-guided coil embolization and thrombin injection | Obliteration of pseudoaneurysm | 2 wk | No further bleeding |
| Jhajharia et al[69], 2018 | Chronic pancreatitis, GI bleed | 3; 43, 25 and 55 yr; M | Gastroduodenal artery, hepatic artery, splenic artery | EUS-guided thrombin injection | Obliteration of pseudoaneurysm | 14 d | No rebleeding |
EUS is traditionally used to evaluate GI luminal tumors and obtain a tissue diagnosis. Bleeding GISTs are traditionally managed by either surgical resection, radiologic embolization, or rarely with endoscopic therapies like hemoclips and endoloop® ligation. In an elderly patient with a bleeding ulcer due to GIST who was not a candidate for surgery due to comorbidities, EUS-guided angiotherapy was done. A deep vessel was identified to bleeding GIST ulcer via echoendoscope, and the target vessel was treated with CYA, which stopped bleeding. Doppler confirmed the absence of vascularity, and the patient had no further bleeding at 6 mo follow-up[37]. In a study, 32 consecutive patients with submucosal tumors of the upper GI tracts underwent EUS examination with either radial or linear echoendoscope with color and Doppler capabilities: 51.4% had a discrepancy between suspected endoscopic and EUS diagnosis, 83.3% of malignant GISTs had significant intratumoral vessels seen on doppler or color EUS compared to 28% in benign GIST. Three patients were found to have vascular lesions, hemangioma on color Doppler EUS. In two patients, these lesions were treated with EUS-directed therapy consisted of ligation, coagulation, injection of sclerosing agents, or histoacryl resulting in complete eradication of lesions. One patient required surgery due to the severity of the bleeding. The patients remained asymptomatic on a mean follow-up of 48 mo[38].
Although the results of EUS-guided therapies in non-variceal bleeding are encouraging, most of the data is based on case reports and case series. No studies have compared EUS treatment with other management therapies such as endoscopic, surgical, and interventional radiology. Further large-scale studies comparing the standard treatment are needed. At present, EUS-guided therapies are available only at high-level care centers when other treatment options fail.
PV access can help to manage patients with the hepatobiliary diseases and PH. PV is not easy to access with traditional routes. Nevertheless, PV can easily be seen from the stomach and duodenum with EUS and accessed using a standard FNA needle. EUS, along with doppler, is used for needle puncture and withdrawal without hemorrhage[39]. Initial studies of EUS-guided PV access were performed in animals.
The measurement of PH can help to stage cirrhosis and thus prognosis. Portal pressure gradient (PPG) reflects the degree of PH. PPG ≥ 10 mmHg is associated with esophageal varices, and ≥ 12 mmHg is associated with variceal bleeding[40]. Currently, PH is evaluated by indirect measurement of the hepatic venous pressure gradient (HVPG), which poorly correlates with directly measured portal pressure in presinusoidal PH. Presinusoidal PH can be seen in the case of PV thrombosis, schistosomiases, and non-cirrhotic portal fibrosis[41-43].
The first human pilot study conducted to measure EUS-guided PPG measurement included 28 patients with a history of liver disease or suspected cirrhosis. The PV and hepatic vein (or inferior vena cava) were punctured with a 25G FNA needle under EUS guidance either through a transgastric or transduodenal approach. A 100% technical success with no complications was reported. PPG measurement showed a correlation with clinical and endoscopic parameters of PH, including the presence of varices (P = 0.0002), portal hypertensive gastropathy (P = 0.007), and thrombocytopenia (P = 0.036). PPG was shown to increase in patients with high clinical evidence of cirrhosis (P = 0.005)[40]. This study showed that the EUS-guided PPG measurement is safe in humans and further large clinical trials to evaluate safety and efficacy, especially compared to standard HVPG measurement methods, as intrahepatic portosystemic shunt (IPSS).
Transjugular IPSS (TIPS) is performed by interventional radiologists to reduce the PPG. TIPS decompresses the portal system and reduces complications due to PH, such as recurrent variceal bleeding and refractory ascites. It is usually performed in patients with refractory variceal bleed. TIPS involves catheter advancement and guidewire through the right heart and then inferior venacava (IVC) via transjugular route. It can expose patients to unintentional carotid or tracheal puncture, cardiac arrhythmias, and pneumothorax. Also, the transjugular approach for TIPS can be technically challenging in patients with IVC and hepatic vein obstruction, including Budd-Chiari syndrome. EUS-guided IPSS offer benefit as it does not involve heart catheterization, avoiding the related complications[44-48].
A study was conducted to create IPSS using lumen opposing metal stent (LAMS) in a porcine model. PV was accessed by puncturing through the stomach wall and IVC with a 19G needle under EUS guidance. A distal flange of the stent was deployed in the PV and the proximal flange in IVC. Gross necropsy of all five animals showed the correct placement of a stent and no tissue injury or hematoma[49]. Although IPSS has shown promising results in animal models, more studies are needed to evaluate the efficacy and safety of this technique.
Preoperative PV embolization induces the atrophy of the embolized liver lobe to be resected and compensatory hypertrophy of non-embolized remnant liver to increase future liver volume to prevent postoperative liver dysfunction. It is performed in patients with hepatocellular carcinoma (HCC), intrahepatic or hilar cholangiocarcinoma receiving extensive liver resection[25,50]. Liver resection should be performed 2 to 6 wk after PV embolization as compensatory hypertrophy of non-embolized remnant liver occurs in 6 wk with a maximum in the first 2 wk after the procedure. Proceduralists should have meticulous knowledge of liver and portal venous system anatomy before performing this procedure[51]. Presently it is performed through a percutaneous transhepatic approach by vascular interventional radiologist[50]. In a live porcine model to study EUS-guided selective intrahepatic PV embolization, PV was punctured with a 19G FNA needle under EUS guidance, the first coil, and then CYA was injected through the same FNA needle. Doppler was used to evaluating the blood flow. Coil and CYA delivery had a success rate of 88.9% and 87.5%, respectively. In one case, the embolized coil migrated to hepatic parenchyma, and CYA injection failed one case due to the early clogging of CYA in the FNA needle. One wk later, postoperative necropsy showed total occlusion of selected PV with embolus and no evidence of damage to any other organ[50]. Further studies are needed comparing the EUS-guided PV embolization to the percutaneous approach and evaluate the long-term effects.
Pancreaticobiliary cancers (PBCs) are usually at advance to late stages at the time of diagnosis. Circulating tumor cells (CTCs) circulate from the primary tumor to distant sites through vascular supply, and their number is usually low in peripheral blood. In the case of PBCs, CTCs can be detected in the portal circulation before the peripheral blood and can be used to detect metastasis[25,52,53].
In a single-center cohort study, 18 patients with suspected PBCs had blood aspirated from PV via a 19G FNA needle through a EUS-guided transhepatic approach. Paired peripheral blood samples were also collected. Epithelial-derived CTCs were isolated. CTCs were detected from PV samples in all 18 patients (100%), whereas only in peripheral blood samples of four patients (22.2%). These CTCs isolated from PV can also provide sufficient cells to do genomic and proteomic tumor profiling[52].
In another study performed on patients undergoing pancreaticoduodenectomy for presumed periampullary or pancreatic adenocarcinoma without metastatic disease, PV and peripheral venous samples were collected simultaneously at the time of surgery. Sixty patients were monitored postoperatively every three months for one year with imaging for liver metastasis. CTCs were detected in 58% of cases in PV blood compared to 40% in a peripheral blood sample (P = 0.0098). CTCs count was also high PV sample than peripheral blood sample (mean, 230.1 vs 71.7, P = 0.0002). Liver metastasis was detected in 11 of 13 patients with high portal CTCs count (> 112 CMx Platform estimated CTCs in 2 mL blood) compared to 6 of 47 patients with low portal CTCs count (P < 0.0001) at 6-mo follow-up after surgery. The results of this study concluded that the CTCs can be used as a predictor for liver metastases within six months after surgery in patients undergoing pancreaticoduodenectomy for presumed periampullary or pancreatic adenocarcinoma[53]. Unfortunately, PV blood sampling with an evaluation of CTCs is only available in the limited number of specialized tertiary care centers. Further larger studies are needed to ensure the efficacy and safety of the procedure before making it standard of care to predict hepatic metastasis in patients with pancreatic adenocarcinoma.
PV thrombosis due to tumor invasion by direct venous extension or metastasis is seen in up to 70% of cases of HCC[54]. Patients with HCC can also have nontumor (bland) thrombosis of PV. It could be challenging to differentiate PV tumor thrombosis (PVTT) from bland PV thrombosis based on routine radiographic imaging. It is essential to diagnose PVTT as it is a poor prognostic sign, and curative resection or liver transplantation is contraindicated if the patient has PVTT. Transabdominal ultrasound-guided FNA has limited utility due to the difficulty of sample thrombus in the central main PV without contaminating with normal hepatocytes or liver mass, which can affect results. Furthermore, this procedure can have several complications, including vascular injury, pseudoaneurysm formation, and bile duct injury. EUS-guided FNA can directly access the extrahepatic PV without passing the needle through liver tissue[54,55]. Several case reports of EUS-guided FNA of PV thrombosis for diagnosis and staging of HCC even in patients when imaging did not show any liver mass[54-56].
The liver is a common site of metastasis from other primary tumors. Patients with diffuse liver metastasis usually resort to palliative systemic chemotherapy given limited options available. It is hypothesized that the direct injection of chemotherapy into PV may increase the drug level in hepatic tissue while decreasing systemic side effects[25,57]. In a study, EUS-guided portal injection chemotherapy (EPIC) was performed by injecting irinotecan (100 mg) loaded microbeads by using a 22G FNA needle, and the control group had saline injected to PV and irinotecan (100 mg) injected into the jugular vein. EPIC resulted in twice a level of irinotecan in hepatic tissue after 1 h. and half the irinotecan level in plasma after 15 min[57]. These animal studies showed that EPIC could be an option for patients with diffuse liver metastasis; however, no human studies are available currently.
Invasion of the vascular structures by tumor impacts staging and therapeutic options, including tumor resectability. Vascular invasion can often be diagnosed based on radiographic imaging like CT, magnetic resonance imaging (MRI), or positron emission tomography scans. However, sometimes it is difficult to judge based on the imaging as in pancreatic ductal adenocarcinoma. Similarly, tumor thrombi can be challenging to differentiate from bland thrombi based on imaging. EUS-guided FNA and elastography can be helpful in these cases.
In a retrospective study, forty-four patients with pancreatic ductal adenocarcinoma underwent dynamic CT and EUS, EUS-B mode imaging was taken, and also EUS elastography was done in all cases. Sensitivity, specificity and accuracy (95%CI) for vascular invasion were 0.733, 0.697 and 0.708 on dynamic CT; 0.733, 0.606 and 0.646 in EUS B-mode; and 0.917, 0.900 and 0.906 in EUS elastography. EUS-B mode and EUS elastography should be considered in patients with pancreatic ductal adenocarcinoma, where dynamic cannot detect vascular invasion[58].
The heart and pulmonary vascular systems can be easily accessed via EUS because of their proximity to the esophagus. Transesophageal echocardiography is routinely done in cardiac patients for various conditions[59]. Fritscher-Ravens et al[60] conducted a study where they performed EUS-guided puncture of the heart in a porcine model using a linear array echoendoscope followed by three clinical cases. In the porcine study, 22- and 19-gauge EUS needles were used to access the left atrium, left ventricle, coronary arteries, and aortic valve. In the porcine group, procedures performed included needle biopsy of cardiac muscle; contrast injection into the left atrium, ventricle, coronary arteries; radiofrequency ablation of aortic and mitral valves; and passage of a guidewire. No arrhythmias were reported during the procedures. During necropsy, penetration sites were identified, but they were unremarkable in appearance; no bleeding or hematoma was noticed. Subsequently, EUS-guided cardiac access was performed in three patients. In two patients, pericardial fluid was aspirated for diagnostic purposes using a 22-gauge EUS needle, and the third patient had the FNA of atrial mass. No adverse events were observed after procedures in these patients.
Somani et al[61] described EUS-guided thrombolysis of pulmonary artery thrombus in a 57-year-old patient who presented with shortness of breath, shock, and acute abdominal pain. The patient had a superior mesenteric vein and right pulmonary artery thrombus. Given the shock state and history of recent hemorrhagic stroke, EUS-guided thrombolysis of pulmonary artery thrombus was done with Tenecteplase using 25G needle. Repeat EUS after 48 h and one before 15 d showed a reduction in the volume of thrombus.
The GI tract provides access to several vascular structures in the mediastinum, abdominal, and pelvis. Currently, most vascular interventions are done by an interventional radiologist through a percutaneous route. EUS provides access to most of the vasculature through the GI tract and the ability to do real-time interventions. Various studies have shown promising results for the safety, clinical and technical success of EUS-guided vascular intervention concluding that it should be considered either as first-line therapy or when conventional treatment fails. Although results are promising, it is based on case reports and series except in GV management, for which relatively more extensive studies are available.
Given that most data is available from case studies and series, it increases the risk of selection bias. Furthermore, these studies are available from tertiary care centers due to the limited availability of EUS and specialist trained in echoendoscope. EUS will eventually offer a less invasive and safer approach to various vascular interventions as this field expands further.
The GI tract provides a unique window to vascular structures in the mediastinum and abdomen, which can be accessed through FNA needle. Various studies have shown promising results for the safety, clinical and technical success of EUS-guided vascular intervention concluding that it should be considered either as first-line therapy or when conventional treatment fails. Although results are promising, it is based on case reports and series except in GV management for which relatively more extensive studies are available. Given that most data is available from case studies and series, it increases the risk of selection bias. Furthermore, these studies are available from tertiary care centers due to the limited availability of EUS and specialist trained in echoendoscope. As this field advances, EUS will offer a less invasive and safer approach to various vascular interventions.
| 1. | Deprez PH. Choice of endosonographic equipment and normal endosonographic anatomy. Best Pract Res Clin Gastroenterol. 2009;23:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Burmester E, Tiede U. Longitudinal Endoscopic Ultrasound–Anatomical Guiding Structures in the Upper Abdomen (Cranial–Right). J Enc GI End. 2013;1:501-504. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Snady H. Identification of major retroperitoneal vascular anatomy with endoscopic ultrasonography. Acta Endosc. 1995;25:491-498. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | DiMagno EP, DiMagno MJ. Endoscopic Ultrasonography: From the Origins to Routine EUS. Dig Dis Sci. 2016;61:342-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Friedberg SR, Lachter J. Endoscopic ultrasound: Current roles and future directions. World J Gastrointest Endosc. 2017;9:499-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Prachayakul V, Aswakul P. Endoscopic ultrasound-guided interventions in special situations. World J Gastrointest Endosc. 2016;8:104-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Oleas R, Robles-Medranda C. Insights into the role of endoscopic ultrasound-guided vascular therapy. Ther Adv Gastrointest Endosc. 2019;12:2631774519878282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Krige JE, Bornman PC, Goldberg PA, Terblanche J. Variceal rebleeding and recurrence after endoscopic injection sclerotherapy: a prospective evaluation in 204 patients. Arch Surg. 2000;135:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Hou MC, Lin HC, Lee FY, Chang FY, Lee SD. Recurrence of esophageal varices following endoscopic treatment and its impact on rebleeding: comparison of sclerotherapy and ligation. J Hepatol. 2000;32:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Bokun T, Grgurevic I, Kujundzic M, Banic M. EUS-Guided Vascular Procedures: A Literature Review. Gastroenterol Res Pract. 2013;2013:865945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Artifon ELA, Marson FP, Khan MA. Endoscopic Ultrasonography-Guided Hemostasis Techniques. Gastrointest Endosc Clin N Am. 2017;27:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Lahoti S, Catalano MF, Alcocer E, Hogan WJ, Geenen JE. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc. 2000;51:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Lôbo MRA, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: A randomized controlled trial. Arq Gastroenterol. 2019;56:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Kozieł S, Pawlak K, Błaszczyk Ł, Jagielski M, Wiechowska-Kozłowska A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Ryou M. Combination therapy versus monotherapy for EUS-guided management of gastric varices: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 16. | Ríos Castellanos E, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev. 2015;CD010180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Lee TH, Shih LN. Clinical experience of endoscopic banding ligation for bleeding gastric varices. Hepatogastroenterology. 2008;55:766-769. [PubMed] |
| 18. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 872] [Article Influence: 25.6] [Reference Citation Analysis (42)] |
| 19. | Frost JW, Hebbar S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc Int Open. 2018;6:E664-E668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Wang X, Yu S, Chen X, Duan L. Endoscopic ultrasound-guided injection of coils and cyanoacrylate glue for the treatment of gastric fundal varices with abnormal shunts: a series of case reports. J Int Med Res. 2019;47:1802-1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Hwang SS, Kim HH, Park SH, Kim SE, Jung JI, Ahn BY, Kim SH, Chung SK, Park YH, Choi KH. N-butyl-2-cyanoacrylate pulmonary embolism after endoscopic injection sclerotherapy for gastric variceal bleeding. J Comput Assist Tomogr. 2001;25:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | George N, Perisetti A, Banerjee D, Raghavapuram S, Garcia-Saenz-de-Sicilia M, Tharian B. EUS-Guided Treatment of Gastric Varices: A Single Center Experience: 783. Am J Gastroenterol. 2017;112. [DOI] [Full Text] |
| 23. | Gubler C, Bauerfeind P. Safe and successful endoscopic initial treatment and long-term eradication of gastric varices by endoscopic ultrasound-guided Histoacryl (N-butyl-2-cyanoacrylate) injection. Scand J Gastroenterol. 2014;49:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Bick BL, Al-Haddad M, Liangpunsakul S, Ghabril MS, DeWitt JM. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc. 2019;33:1837-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Anastasiou J, Berzin TM. Endoscopic Ultrasound-Guided Vascular Interventions: From Diagnosis to Treatment. Saudi J Med Med Sci. 2018;6:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, Subtil-Inigo JC, Junquera-Florez F, Gornals JB, Repiso-Ortega A, Vila-Costas J, Marcos-Sanchez F, Muñoz-Navas M, Romero-Gomez M, Brullet-Benedi E, Romero-Vazquez J, Caunedo-Alvarez A, Pellicer-Bautista F, Herrerias-Gutierrez JM, Fritscher-Ravens A. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc. 2013;78:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Bazarbashi AN, Wang TJ, Thompson CC, Ryou M. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable hemostatic gelatin sponge: a novel alternative to cyanoacrylate. Endosc Int Open. 2020;8:E221-E227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Grgurevic I, Kujundzic M, Banic M, Kusec R, Bokun T, Vukelic-Markovic M, Bogdanovic Z, Lukic A, Tsochatzis E, Brkljacic B. Subtypes and clinical significance of common bile duct varices in portal vein thrombosis: diagnosis and follow-up by Doppler US and EUS. Abdom Radiol (NY). 2016;41:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Kinzel J, Pichetshote N, Dredar S, Aslanian H, Nagar A. Bleeding from a duodenal varix: a unique case of variceal hemostasis achieved using EUS-guided placement of an embolization coil and cyanoacrylate. J Clin Gastroenterol. 2014;48:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Sharma M, Rai P, Bansal R. EUS-Assisted Evaluation of Rectal Varices before Banding. Gastroenterol Res Pract. 2013;2013:619187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Maggio D, Barkun AN, Martel M, Elouali S, Gralnek IM; Reason Investigators. Predictors of early rebleeding after endoscopic therapy in patients with nonvariceal upper gastrointestinal bleeding secondary to high-risk lesions. Can J Gastroenterol. 2013;27:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | De Angelis CG, Cortegoso Valdivia P, Rizza S, Venezia L, Rizzi F, Gesualdo M, Saracco GM, Pellicano R. Endoscopic Ultrasound-Guided Treatments for Non-Variceal Upper GI Bleeding: A Review of the Literature. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Levy MJ, Wong Kee Song LM, Farnell MB, Misra S, Sarr MG, Gostout CJ. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008;103:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Law R, Fujii-Lau L, Wong Kee Song LM, Gostout CJ, Kamath PS, Abu Dayyeh BK, Gleeson FC, Rajan E, Topazian MD, Levy MJ. Efficacy of endoscopic ultrasound-guided hemostatic interventions for resistant nonvariceal bleeding. Clin Gastroenterol Hepatol. 2015;13:808-12.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Gamanagatti S, Thingujam U, Garg P, Nongthombam S, Dash NR. Endoscopic ultrasound guided thrombin injection of angiographically occult pancreatitis associated visceral artery pseudoaneurysms: Case series. World J Gastrointest Endosc. 2015;7:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Maharshi S, Sharma SS, Sharma D, Sapra B, Nijhawan S. Endoscopic ultrasound-guided thrombin injection, a management approach for visceral artery pseudoaneurysms. Endosc Int Open. 2020;8:E407-E412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Kumbhari V, Gondal B, Okolo Iii PI, Lennon AM, Law JK, Singh VK, Saxena P, Shin EJ, Canto MI, Kalloo AN, Khashab MA. Endoscopic ultrasound-guided angiotherapy of a large bleeding gastrointestinal stromal tumor. Endoscopy. 2013;45 Suppl 2 UCTN:E326-E327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Săftoiu A, Vilmann P, Hassan H, Krag Jacobsen G. Utility of colour Doppler endoscopic ultrasound evaluation and guided therapy of submucosal tumours of the upper gastrointestinal tract. Ultraschall Med. 2005;26:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Crowe DR, Eloubeidi MA, Chhieng DC, Jhala NC, Jhala D, Eltoum IA. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, Chen WP, Chang KJ. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | Sarin SK, Khanna R. Non-cirrhotic portal hypertension. Clin Liver Dis. 2014;18:451-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 43. | Pomier-Layrargues G, Kusielewicz D, Willems B, Villeneuve JP, Marleau D, Côté J, Huet PM. Presinusoidal portal hypertension in non-alcoholic cirrhosis. Hepatology. 1985;5:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41 Suppl 3:S344-S351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Wong F. The use of TIPS in chronic liver disease. Ann Hepatol. 2006;5:5-15. [PubMed] |
| 46. | Quateen A, Pech M, Berg T, Bergk A, Podrabsky P, Felix R, Ricke J. Percutaneous transjugular direct porto-caval shunt in patients with Budd-Chiari syndrome. Cardiovasc Intervent Radiol. 2006;29:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Perry BC, Kwan SW. Portosystemic Shunts: Stable Utilization and Improved Outcomes, Two Decades After the Transjugular Intrahepatic Portosystemic Shunt. J Am Coll Radiol. 2015;12:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Silva RF, Arroyo PC Jr, Duca WJ, Silva AA, Reis LF, Cabral CM, Sgnolf A, Domingues RB, Barao GT, Coelho DJ, Deberaldini M, Felício HC, Silva RC. Complications following transjugular intrahepatic portosystemic shunt: a retrospective analysis. Transplant Proc. 2004;36:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Kenneth F. Janak NS. Sa1428 EUS-Guided Transgastric Intrahepatic Portosystemic Shunt Using the Axios Stent. Gastro Endos. 2011;73. [DOI] [Full Text] |
| 50. | Park TY, Seo DW, Kang HJ, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Feasibility and safety of EUS-guided selective portal vein embolization with a coil and cyanoacrylate in a live porcine model. Endosc Ultrasound. 2018;7:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Loffroy R, Favelier S, Chevallier O, Estivalet L, Genson PY, Pottecher P, Gehin S, Krausé D, Cercueil JP. Preoperative portal vein embolization in liver cancer: indications, techniques and outcomes. Quant Imaging Med Surg. 2015;5:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 52. | Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015;149:1794-1803.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 53. | Tien YW, Kuo HC, Ho BI, Chang MC, Chang YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, Shew JY, Chang YC, Lee EY, Lee WH. A High Circulating Tumor Cell Count in Portal Vein Predicts Liver Metastasis From Periampullary or Pancreatic Cancer: A High Portal Venous CTC Count Predicts Liver Metastases. Medicine (Baltimore). 2016;95:e3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Kayar Y, Turkdogan KA, Baysal B, Unver N, Danalioglu A, Senturk H. EUS-guided FNA of a portal vein thrombus in hepatocellular carcinoma. Pan Afr Med J. 2015;21:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Michael H, Lenza C, Gupta M, Katz DS. Endoscopic Ultrasound -guided Fine-Needle Aspiration of a Portal Vein Thrombus to Aid in the Diagnosis and Staging of Hepatocellular Carcinoma. Gastroenterol Hepatol (N Y). 2011;7:124-129. [PubMed] |
| 56. | Storch I, Gomez C, Contreras F, Schiff E, Ribeiro A. Hepatocellular carcinoma (HCC) with portal vein invasion, masquerading as pancreatic mass, diagnosed by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Dig Dis Sci. 2007;52:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Faigel D, Lake D, Landreth T, Kelman C, Marler R. Endoscopic ultrasonography-guided portal injection chemotherapy for hepatic metastases. Endosc Ultrasound. 2014;3:S1. [PubMed] |
| 58. | Yamada K, Kawashima H, Ohno E, Ishikawa T, Tanaka H, Nakamura M, Miyahara R, Ishigami M, Hirooka Y, Fujishiro M. Diagnosis of vascular invasion in pancreatic ductal adenocarcinoma using endoscopic ultrasound elastography. BMC Gastroenterol. 2020;20:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Kühl HP, Hanrath P. The impact of transesophageal echocardiography on daily clinical practice. Eur J Echocardiogr. 2004;5:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Fritscher-Ravens A, Ganbari A, Mosse CA, Swain P, Koehler P, Patel K. Transesophageal endoscopic ultrasound-guided access to the heart. Endoscopy. 2007;39:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Somani P, Talele R, Sharma M. Endoscopic Ultrasound-Guided Thrombolysis of Pulmonary Artery Thrombus and Mesenteric Vein Thrombus. Am J Gastroenterol. 2019;114:379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Krill JT, Othman MO, Munot K, Jalal PK, Patel K. Su1411 EUS-Guided coil application with glue injection for gastric variceal obliteration is associated with less endoscopic reintervention compared to glue injection alone. Gastro Endos. 2018;87:AB345. [DOI] [Full Text] |
| 63. | Romero-Castro R, Pellicer-Bautista F, Giovannini M, Marcos-Sánchez F, Caparros-Escudero C, Jiménez-Sáenz M, Gomez-Parra M, Arenzana-Seisdedos A, Leria-Yebenes V, Herrerias-Gutiérrez JM. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy. 2010;42 Suppl 2:E35-E36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Philips CA, Augustine P. Endoscopic Ultrasound-Guided Management of Bleeding Rectal Varices. ACG Case Rep J. 2017;4:e101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Bazarbashi AN, Thompson CC, Ryou M. Targeting the perforator vein: EUS-guided coil embolization for the treatment of bleeding rectal varices. VideoGIE. 2020;5:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Mukkada RJ, Mathew PG, Francis Jose V, Paul Chettupuzha A, Antony R, Koshy A. EUS-guided coiling of rectal varices. VideoGIE. 2017;2:208-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Robb PM, Yeaton P, Bishop T, Wessinger J. Endoscopic Ultrasound Guided Embolization of a Pancreatic Pseudoaneurysm. Gastroenterology Res. 2012;5:239-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Somani P, Sharma M, Jindal S. EUS-Guided Coil Embolization and Thrombin Injection of Bleeding Gastroduodenal Artery Pseudoaneurysm: 1611. Am College Gastroenterol. 2017;112:S876. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Jhajharia A, Wanjari S, Ashdhir P, Sharma D, Pokharna R, Nijhawan S. Endoscopic ultrasound-guided thrombin injection for management of visceral artery pseudoaneurysm: A novel approach. Indian J Gastroenterol. 2018;37:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Society of Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Harada R, Mitri RD S-Editor: Fan JR L-Editor: A P-Editor: Liu JH