Published online Aug 28, 2021. doi: 10.13105/wjma.v9.i4.377
Peer-review started: June 16, 2021
First decision: July 6, 2021
Revised: July 17, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: August 28, 2021
Processing time: 79 Days and 1.6 Hours
The quality of life of women with endometriosis is substantially adversely affected by the pelvic pain caused by this disease. However, the choice of medication for endometriosis remains controversial, and no drug has been clearly proven to be superior to others.
To assess the efficacy and safety of dienogest, a synthetic progestin, in the treatment of women with painful symptoms of endometriosis.
PubMed, EMBASE, the Cochrane Library, and the Web of Science databases were searched from their inceptions to January 21, 2020 for randomized controlled trials (RCTs) that compared dienogest with other popular prescription drugs for the treatment of endometriosis. Two reviewers extracted the data. Mean difference (MD) values and risk ratios (RRs) with 95% confidence intervals (CIs) were calculated.
Ultimately, seven RCTs with a total of 1493 participants met the requirements for this review. Dienogest was found to more effective than placebo in alleviating endometriosis-related pain (MD = -32.93, 95%CI: -44.63 to -21.23), but led to a more significant decline in plasma estradiol concentrations than placebo (MD = -44.7, 95%CI: -62.24 to -24.69). Dienogest was superior to gonadotropin-releasing hormone analogues (GnRH-a) in relieving pain (MD = -2.41, 95%CI: -3.58 to -1.24). Moreover, compared with dienogest, GnRH-a were significantly more likely to lead to the loss of bone mineral density (MD = 2.77, 95%CI: 0.16 to 5.37) and were significantly associated with a higher incidence of headaches (RR = 0.68, 95%CI: 0.52 to 0.91) and hot flushes (RR = 0.43, 95%CI: 0.18 to 1.02).
This meta-analysis demonstrated that dienogest may be a better pain-relief treatment for endometriosis patients, due to its high efficacy and tolerability.
Core Tip: We comprehensively and systematically analyzed the safety and effectiveness of dienogest for the treatment of endometriosis-related pain. Only high-quality randomized controlled trials that compared dienogest with other drugs, such as gonadotrophin-releasing hormone analogues and placebo, were included in the meta-analysis. The results provide guidelines for the standardization of the clinical use of medications for endometriosis and would improve the choice of medications for patients.
- Citation: Lin SC, Wang XY, Fu XL, Yang WH, Wu H, Bai Y, Shi ZN, Du JP, Wang BJ. Systematic review and Meta-analysis of efficacy and safety of dienogest in treatment of endometriosis. World J Meta-Anal 2021; 9(4): 377-388
- URL: https://www.wjgnet.com/2308-3840/full/v9/i4/377.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i4.377
Endometriosis is defined as the presence of endometrial glands and stroma outside of the uterine cavity, and affects about 200 million women worldwide[1-3]. Although the pathogenesis of endometriosis remains unclear, it is generally accepted that its basic feature is the existence of ectopic endometrium. The chronic inflammatory reaction induced by ectopic endometrial lesions is the central process leading to endometriosis-related pain[4,5]. The resulting painful symptoms, such as dysmenorrhea, dyspa
The management of endometriosis has continuously improved, and a patient’s choice of treatment methods, such as medication, surgery, or assisted reproductive technology, usually depends on her age and fertility requirements[4]. Medication is the first-line therapeutic option for women who do not wish to conceive in the immediate future, considering that surgery has a high rate of recurrence and risk of complications and can reduce the ovarian reserve[1]. There are also many widely available pharmacological treatments for endometriosis, such as painkillers, non-steroidal anti-inflammatories (NSAIDs), combined oral contraceptives (COCs), progestins, and gonadotrophin-releasing hormone analogues (GnRH-a)[9,10]. Although each type of drug is widely used worldwide, they also have clear disadvan
Dienogest, a 19-nortestosterone and progesterone derivative, is a fourth-generation synthetic oral progestin that was designed to treat endometriosis. It binds highly selectively to progesterone receptors, thereby exerting potent progestogenic effects with little androgenic, mineralocorticoid, or glucocorticoid activity[11]. Crucially, dienogest suppresses the growth of endometrioid tissue and also exerts anti-inflammatory, antiproliferative, and antiangiogenic effects[12-14]. It has also shown good effectiveness and a favorable safety profile in long-term clinical application and follow-up studies. However, it remains uncertain whether dienogest is superior to other drugs.
The aim of this systematic review and Meta-analysis was to evaluate the efficacy and safety of dienogest for the treatment of endometriosis in women of reproductive age. We integrated and Meta-analyzed data from high-quality randomized controlled trials (RCTs) to assess differences between the treatment effects of dienogest and those of other drugs. This revealed strong evidence that dienogest should be the drug of first choice for treating endometriosis.
To identify all relevant literature, a systematic search of the Cochrane Library, EMBASE, PubMed, and the Web of Science was conducted on January 21, 2020, using combinations of the following search terms: (endometriosis OR adenomyosis OR EMT OR EM OR uterine adenomyosis OR endometrioma) AND dienogest AND (placebo OR blank control OR drug). The search process and determination of eligibility were conducted independently by two investigators (Wang XF and Fu XL). Disagreements about eligibility or search criteria were mitigated through discussion with a third reviewer (Lin SC).
We only included trials in which women had signs and symptoms of endometriosis or adenomyosis. Trials that met all of the following criteria were included: (1) Prospective RCTs; (2) English language studies; (3) Patients in the experimental group were treated with dienogest, whereas those in the control group received other medications, such as GnRH-a, placebo, COCs, or NSAIDs; and (4) The primary outcome was an improve
Meta-analyses, reviews, case reports, conference abstracts, cohort studies, retrospective studies, and trials without available data were excluded. Studies involving adolescent or menopausal women were also excluded.
Two authors (Wang XF and Fu XL) independently extracted the data from the studies that met all of the inclusion criteria and none of the exclusion criteria. The data extracted were the first author’s name, the publication year, the study design, the interventions, the number of participants, and the inclusion criteria.
The primary efficacy outcome was a change in the visual analogue scale (VAS) scores of endometriosis-related pain before and after the trial, and the secondary efficacy outcome was a change in the number of analgesics administered. The outcomes used to evaluate safety were changes in bone mineral density (BMD) and estrogen concentration, the incidence of hot flushes and headaches, and the occurrence of other adverse events.
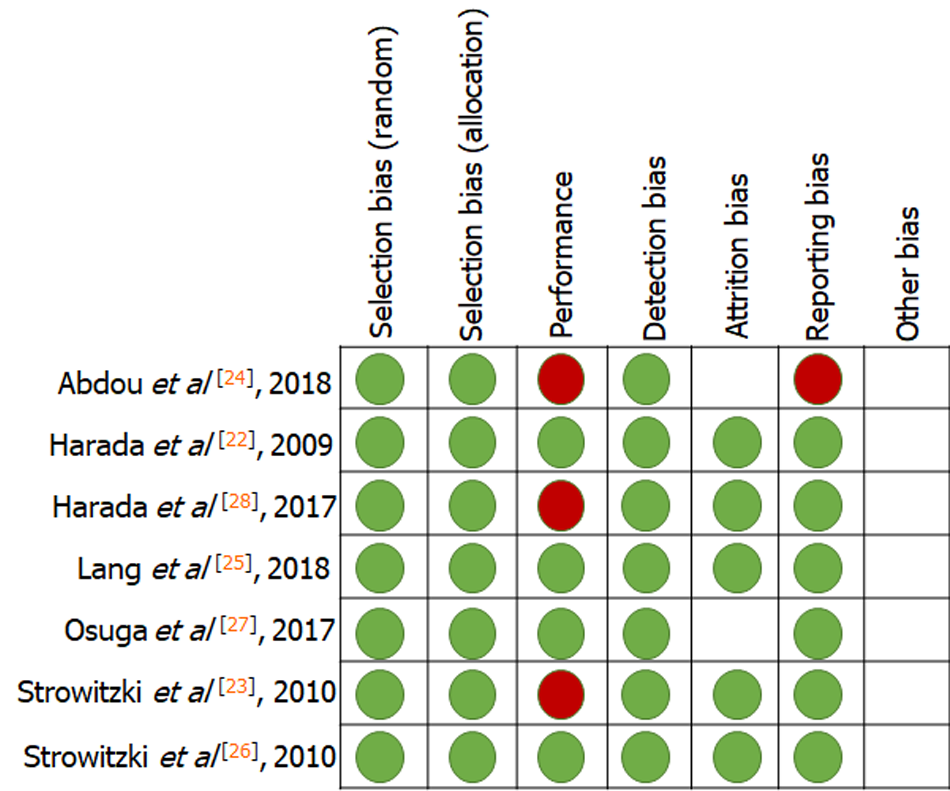
To assess the quality of each included study, the Cochrane risk-of-bias assessment tool was used. Each type of risk was graded as low, high, or unclear for the included RCTs. Seven criteria related to the risk of bias were assessed in each study: (1) Random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. Two authors (Wang XF and Fu X) indepen
Review Manager (RevMan) 5.3 software was used to conduct the Meta-analysis. Continuous data and dichotomous data are reported as the mean ± standard deviation (SDs) and the number of outcome events/total number, respectively. We also calcu
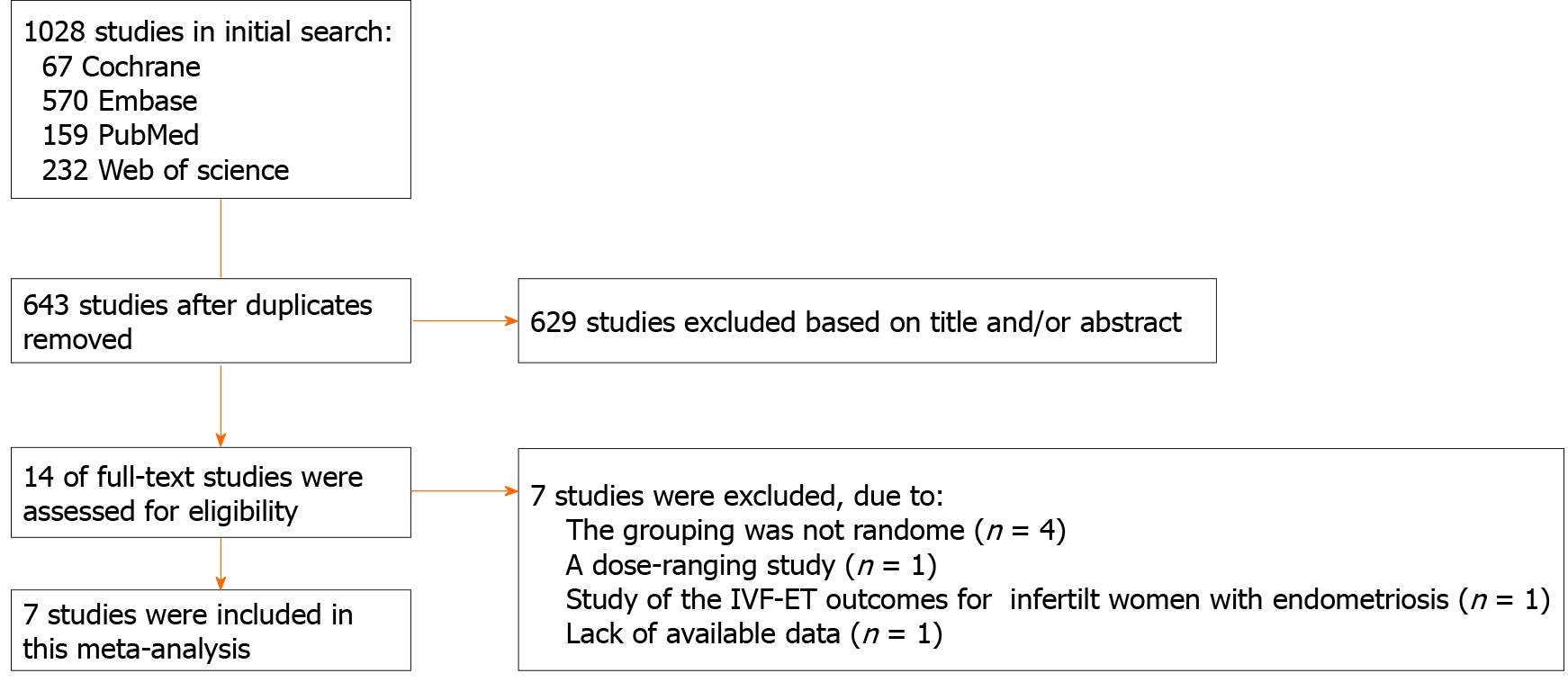
The search strategy identified 1028 trials. Initially, 385 duplicates were excluded, and a further 629 trials were excluded based on the title and/or abstract. The remaining 14 trials were further assessed by reading the full text. Four trials were excluded because randomization was not adopted in the grouping process[15-18]. One dose-ranging study was excluded because it did not compare dienogest with other treatments[19]. One trial’s detailed data were not provided, and it was also excluded as the author did not respond to a request for data[20]. One trial investigating the benefits of dienogest treatment before in vitro fertilization and embryo transfer was excluded, as it did not measure the specific outcomes analyzed in this meta-analysis[21]. Finally, seven RCTs with a high level of evidence were eligible for the analysis[22-28]. The procedure for study selection is presented in Figure 1.
Table 1 presents the characteristics of the studies included in this meta-analysis. The seven prospective RCTs included a total of 1493 women from ten countries: Germany, Italy, Ukraine, Austria, Spain, Poland, Portugal, Japan, China, and Egypt. Two milligrams of dienogest per day was the dosage in all studies. Efficacy was assessed in terms of changes in VAS scores for endometriosis-related pain and changes in the intake of supportive analgesic medication (SAM). Safety was assessed in terms of changes in BMD and serum estradiol (E2) concentrations, and the incidence of headaches and hot flushes. The results of quality assessment are shown in Figure 2. All eligible trials indicated a low risk of bias.
| Ref. | Study design | Country | Blind | Duration | Intervention | Sample size | Efficacy assessments | Safety assessments |
| Harada et al[22], 2009 | RCT | Japan | Double-blind | 24 wk | 2 mg/d DNG vs 900 μg/d buserelin | 137/134 | VAS | BMD change Hot flashes Headache |
| Strowitzki et al[23], 2010 | RCT | Germany Austria Spain Poland Italy Portugal | Open-label | 24 wk | 2 mg/d DNG vs 3.75 mg/4wk leuprolide | 124/128 | VAS | Hot flashes Headache |
| Abdou et al[24], 2018 | RCT | Egypt | Open-label | 12 wk | 2 mg/d DNG vs 3.75 mg/4 wk leuprolide | 130/131 | VAS | Hot flashes Headache |
| Lang et al[25], 2018 | RCT | China | Double-blind | 24 wk | 2 mg/d DNG vs placebo | 130/132 | VAS SAM intake | BMD change E2 |
| Strowitzki et al[26], 2010 | RCT | Germany Italy Ukraine | Double-blind | 12 wk | 2 mg/d DNG vs placebo | 102/96 | SAM intake | BMD change E2 Headache |
| Osuga et al[27], 2017 | RCT | Japan | Double-blind | 16 wk | 2 mg/d DNG vs placebo | 34/33 | VAS SAM intake | E2 Hot flashes |
| Harada et al[28], 2017 | RCT | Japan | DNG: Un-blind Placebo: Blind | 24 wk | 2 mg/d DNG vs placebo | 53/129 | VAS |
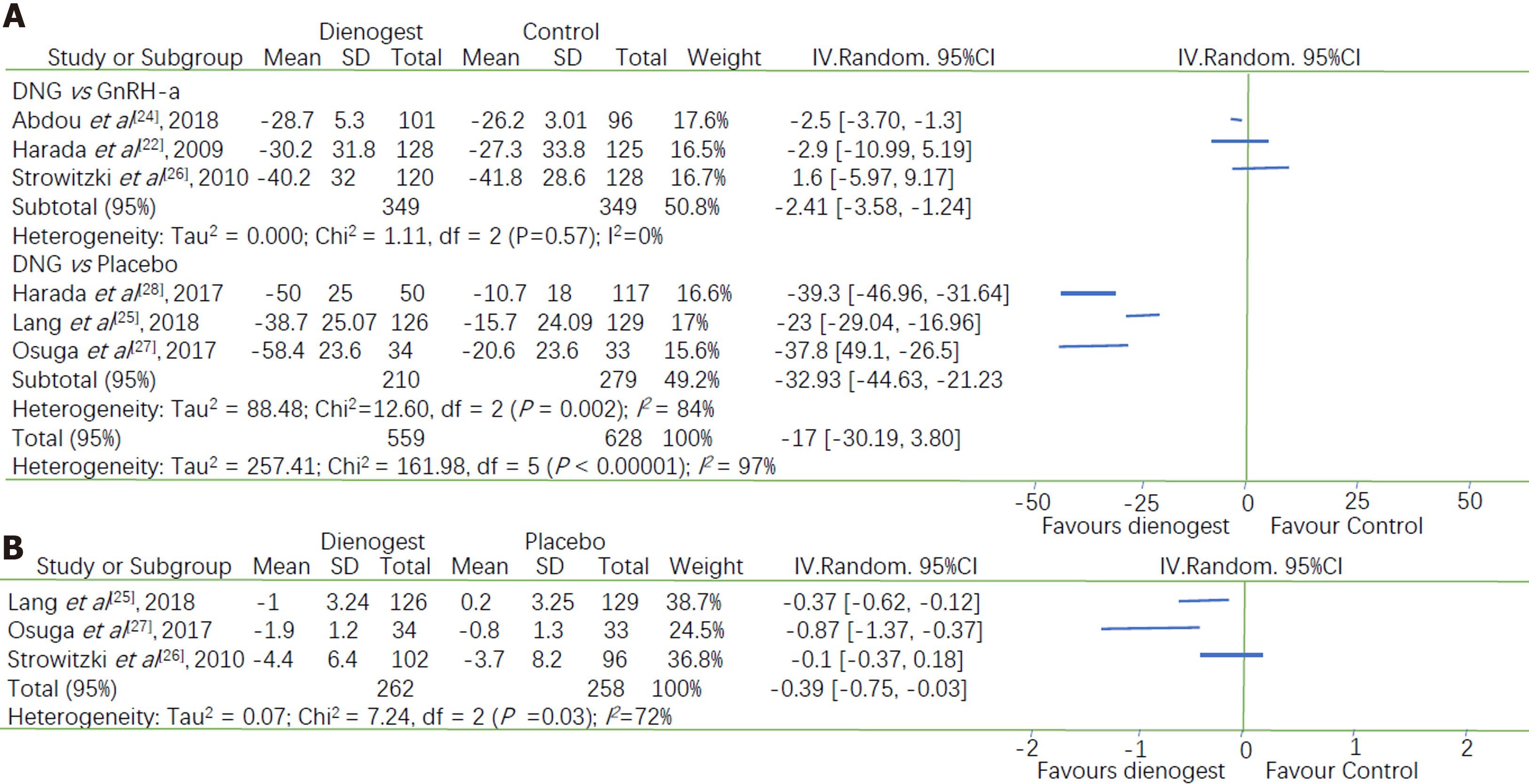
All seven studies assessed the efficacy of dienogest in comparison with that of other drugs (placebo or GnRH-a) in terms of changes in VAS scores at the end of the treatment period. However, the data of one study were unclearly presented[26]. Our meta-analysis revealed a statistically significant advantage of dienogest, compared with other drugs, in terms of the relief of endometriosis- or adenomyosis-associated pain (MD = -17, 95%CI: -30.19 to -3.80). Considering the rather high heterogeneity between the studies [an I2 of 97% (P < 0.00001)], we conducted a subgroup analysis of the different categories of alternative drugs.
Dienogest was found to be significantly superior to GnRH-a for pain relief (MD = -2.41, 95%CI: -3.58 to -1.24) with a very low heterogeneity (I2 = 0.0%, P = 0.57). Dienogest was also significantly superior to placebo with respect to pain relief [MD = -32.93, 95%CI: -44.63 to -21.23], but with a high heterogeneity (I2 = 84%, P = 0.002) (Figure 3A). Subgroup analysis reduced the heterogeneity within each group but did not eliminate it. Therefore, we performed a sensitivity analysis by excluding each study, and then analyzing the effect of this exclusion. This revealed that the RCT performed by Lang et al[25] was the probable source of heterogeneity, as its elimination decreased I2 from 84% to 0.0%. The combined results of the other two articles also indicated that dienogest was superior to lacebo for pain relief (MD = -38.83, 95%CI: -45.17 to -32.49). Visual inspection of the funnel plot of the changes in VAS scores reveals its asymmetry, which suggests that there was some degree of publication bias and that more studies are needed to validate the results (SupplementaryFigure 1).
Three studies that compared dienogest with placebo reported the change in the level of SAM intake, and the combined results indicated a significantly larger reduction of SAM intake in the dienogest group than the placebo group (MD = -0.39, 95%CI: -0.75 to -0.03), with an I2 of 72% (P = 0.03) (Figure 3B). Subgroup analyses of two studies (Osuga et al[27] and Lang et al[25]) demonstrated a high heterogeneity, i.e., a minimum I2 of 52%. The sensitivity analysis subsequent to removal of either of those studies indicated that the statistical difference between dienogest and placebo was not significant (P = 0.08 and 0.24 for Osuga et al[27] and Lang et al[25], respectively). Thus, additional studies are required to validate this result.
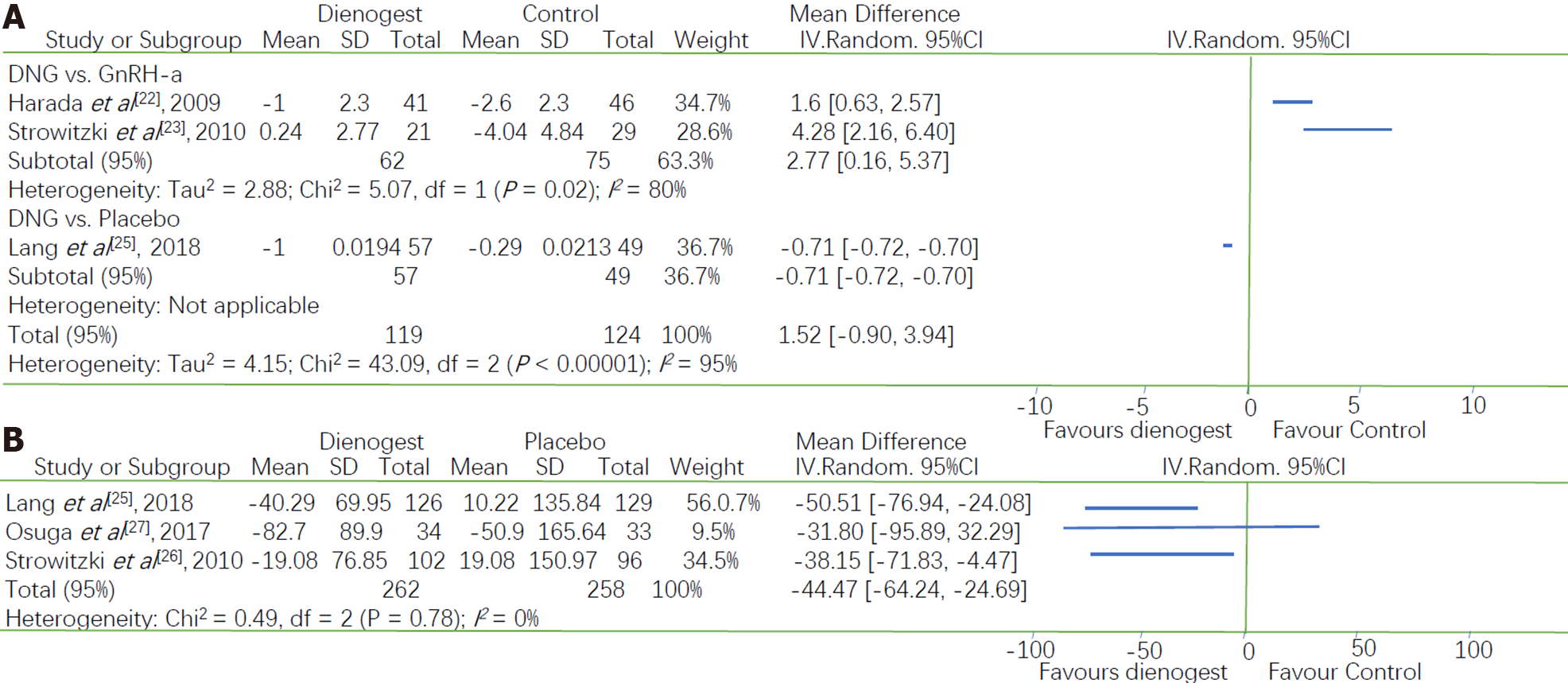
Three studies investigated the effect of dienogest on BMD, and we found that there was a statistically significant difference between dienogest and the other drugs with respect to changes in BMD. Two studies compared dienogest with GnRH-a, whereas one study compared dienogest with placebo. Therefore, a subgroup analysis was conducted based on the type of drug used as the control. A greater reduction in BMD was observed in the GnRH-a group than in the dienogest group (MD = 2.77, 95%CI: 0.16 to 5.37), but there was a significant heterogeneity between these two trials (P = 0.02, I2 = 80%). Furthermore, a smaller reduction in BMD was observed in the placebo group than in the dienogest group (MD = -0.71, 95%CI: -0.72 to -0.70) (Figure 4A).
Serum E2 concentrations were measured in three studies in which dienogest was compared with placebo. The E2 concentrations were lower in the dienogest groups than in the placebo groups. A Meta-analysis of the three studies showed that the decrease in the E2 concentration was -44.47 pg/mL [95%CI: -62.24 to -24.69]. The pooled measures revealed an I2 value of 0.0% (P = 0.78), indicating a homogeneity between studies (Figure 4B).
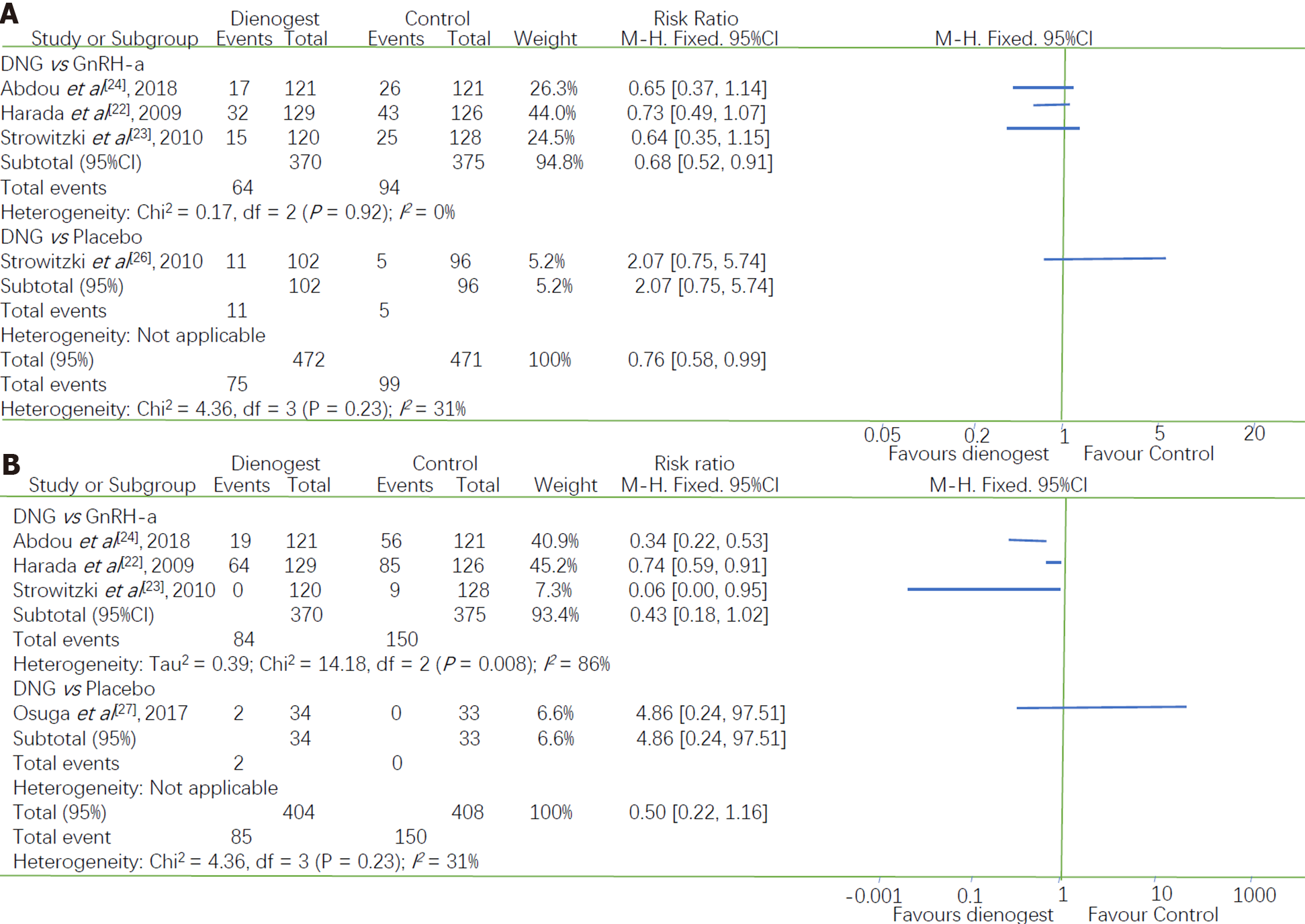
Headaches were one of the most common drug-related adverse effects reported during treatment. Four studies reported the incidence of headache after treatment: Three studies comparing dienogest with GnRH-a and one comparing dienogest with placebo. Their I2 of 31% (P = 0.23) indicated that there was a high heterogeneity between these studies. Therefore, the studies were divided into two subgroups according to the type of drug used in the control group. Women in the dienogest group were less likely to experience headaches than those in the GnRH-a group (RR = 0.68, 95%CI: 0.52 to 0.91), with I2 of 0.0% indicating that there was no heterogeneity between the studies in this group. The only RCT that compared dienogest with a placebo did not show a significant difference in the incidence of headaches (RR = 2.07, 95%CI: 0.75 to 5.74, P = 0.16) (Figure 5A).
Three studies comparing dienogest with GnRH-a and one study comparing dienogest with placebo reported the incidence of hot flushes. Due to the high heterogeneity between these studies (I2 = 81%), we performed a subgroup analysis based on the type of drug used as the control. Only one study compared dienogest with placebo, reporting that the difference in the incidence of hot flushes was not statistically significant (RR = 4.86, 95%CI: 0.24 to 97.51).
The subgroup analysis showed that the incidence of hot flushes was greater in women treated with GnRH-a than in women treated with dienogest (RR = 0.43, 95%CI: 0.18 to 1.02; I2 = 86%, P = 0.0008) (Figure 5B). After excluding the study by Harada et al[22] from the sensitivity analysis, the I2 decreased from 86% to 39%. However, the combined results of the two remaining studies indicated that the difference between dienogest and GnRH-a with respect to the incidence of hot flushes was not statistically significant (P = 0.06). Given these conflicting results, more data are required to clarify the differences between the incidence of this adverse effect resulting from dienogest or GnRH-a treatment.
The chronic nature of endometriosis means that lifelong management must be the focus of clinical decision making, for which patients urgently need safer and more effective drugs. To comprehensively evaluate the efficacy and safety of dienogest in the treatment of endometriosis, we performed a Meta-analysis of seven RCTs that included a total of 1493 patients. The results showed that the pain-relieving ability of dienogest was superior to that of placebo and GnRH-a. Dienogest also exhibited significant safety advantages over GnRH-a, due to its low incidence of adverse effects. Taken together, these findings indicate that dienogest should be the first-line treatment for endometriosis.
The superior pain-relieving ability of dienogest may be attributable to the greater decrease in serum E2 concentrations observed in dienogest-treated patients than in those who received other treatments. This is consistent with the fact that a randomized, dose-controlled study indicated that a daily 2-mg dose of dienogest moderately suppresses E2 production and reliably inhibits ovulation[29], and that a moderate decrease in estrogen concentration effectively suppresses the growth of endometrial tissue and does not result in hypoestrogenic adverse effects, in accordance with the threshold theory proposed by Barbieri[30]. The patients’ dependence on supportive analgesics also decreased during treatment with dienogest, providing indirect evidence for the drug’s pain-relieving effect. In addition, in the safety analysis, no difference was observed in the incidence of headaches and hot flushes between the two groups. An extended study investigating the effects of dienogest treatment over 53 wk found that it induced a sustained reduction in endometriosis-associated pelvic pain, with low rates of treatment-related adverse events[31].
The superiority of dienogest over GnRH-a for relieving endometriosis-related pain was also supported by a -2.41 mm difference in VAS pain scores. In concordance with our findings, another meta-analysis concluded that dienogest was superior to GnRH-a in this regard, with a -2.17 mm difference in VAS pain scores[32]. Gerlinger et al[33] suggested a non-inferiority margin of 10 mm for endometriosis related pian measured using a VAS. Therefore, although the -2.41 mm difference in the VAS pain score is statistically significant, it is less than the suggested minimal clinically significant difference of 10 mm, and thus does not prove that dienogest is superior to GnRH-a in relieving endometriosis-related pain. However, the two drugs appear equivalent in terms of their pain-relieving ability, and more RCTs are required to confirm whether they clinically differ in this ability.
The safety of drugs used for the long-term management of endometriosis must not be overlooked. Dienogest showed significant safety benefits over GnRH-a, as the latter was more likely to lead to decreased BMD and an increased incidence of headaches and hot flushes. GnRH-a are an effective therapy for endometriosis, but they are also associated with hypoestrogenism and decreased BMD if taken for more than 6 mo. Patients are prescribed an add-back therapy to prevent this adverse event[34]. In contrast, dienogest can be taken for a long term with fewer adverse effects. A pooled analysis from four European RCTs confirmed the favorable safety and tolerability of dienogest in both short- and long-term use[35]. Thus, dienogest appears a better option for patients who require chronic treatment.
Research on the mechanism by which dienogest relieves the pelvic pain of endometriosis remains exploratory. Some researchers have found that dienogest can inhibit the production of tumor necrosis factor alpha and interleukin 1 beta by agonizing progesterone receptors, and ultimately inhibit the production of nerve growth factor, which has been shown to be an important factor in the pelvic pain of endometriosis[36-39]. It has also been suggested that the high efficacy of dienogest in relieving endometriosis pain is related to the expression of nuclear factor kappa-B (NF-κB) and Bcl-2, because dienogest can increase the activity of NF-κB and thus increase the apoptosis of endometrial mesenchymal cells[13,40]. Accordingly, a fuller understanding of the mechanism of action dienogest is required, as this may optimize its clinical applicability.
The main strength of this meta-analysis is that we only included RCTs with high levels of evidence, which included a total of 1493 patients, most of whom were clearly diagnosed by histology or laparoscopy. Second, our analysis of the safety and effectiveness of dienogest for the treatment of endometriosis was comprehensive and systematic. The results of this meta-analysis demonstrate how the clinical use of medications for endometriosis could be standardized and enhance patients’ ability to select the best treatment.
However, there are some limitations of our study. First, the included studies differed in the category of disease, the ethnicity of participants, the types of GnRH-a used for treatment, the administration route, and the duration of treatment, which may account for the heterogeneity between studies that we observed. For example, in analyzing the changes in BMD between the two studies, Gerlinger et al[32] attributed the heterogeneity observed between these studies to ethnicity. Moreover, estrogen plays a crucial role in the growth and maintenance of the skeleton, and Asian women have higher blood concentrations of estrogens than Caucasian women[41]. In addition, we conducted subgroup and sensitivity analyses to determine the sources of heterogeneity. Second, although the most frequent drug-related effect of dienogest in all included studies was irregular uterine bleeding (spotting and breakthrough bleeding), our safety assessments did not analyze this side effect. Although patients used daily diaries to record bleeding patterns in four of the included trials, these data cannot be used to accurately estimate the severity of bleeding. As such, an approach that can quantitatively evaluate irregular genital bleeding is needed to investigate this adverse effect. Third, our meta-analysis only included a small number of studies, and none compared dienogest with other therapies, such as compound COCs or levonorgestrel intrauterine devices, which have been proven effective in the treatment of endometriosis and adenomyosis. Finally, more studies are required to evaluate the pharmacoeconomics of drugs that are commonly used to treat endometriosis.
This Meta-analysis systematically evaluated the efficacy and safety of dienogest for the treatment of endometriosis. The results showed that dienogest is superior to placebo and GnRH-a in terms of pain relief and is better tolerated than GnRH-a. This demonstrates that dienogest may be the best medication for endometriosis patients seeking pain relief. Further high-quality RCTs are warranted to confirm these findings.
Endometriosis is one of the common gynecological diseases in reproductive women and the concomitant pelvic pain has substantial negative effects on patients’ quality of life. In recent years, significant advances have been made in the treatment of endometriosis, and drugs remain the primary treatment option for women of childbearing age. However, there is no agreement on which drugs are most effective and tolerated for the treatment of endometriosis.
Several well-designed randomized controlled trials (RCTs) have demonstrated that dienogest is effective in relieving endometriosis-related pain and has a tolerable adverse-effect profile. However, these RCTs have not unambiguously demonstrated whether dienogest is superior to other drugs.
This study was conducted to evaluate the effectiveness and safety of dienogest compared with other drugs for the treatment of endometriosis.
This meta-analysis only included RCTs that compared dienogest with other drugs in the treatment of endometriosis. Review Manager (RevMan) 5.3 software was used to calculate mean difference (MD) values and risk ratios (RRs) with 95% confidence intervals (CIs).
This study included seven RCTs with 1493 participants and demonstrated that dienogest was more effective than placebo in alleviating endometriosis-related pain (MD = -32.93, 95%CI: -44.63 to -21.23), and led to a more significant decrease in plasma estradiol concentrations than placebo (MD = -44.7, 95%CI: -62.24 to -24.69). The combined results showed that dienogest was superior to GnRH-a in relieving pain (MD = -2.41, 95%CI: -3.58 to -1.24). Furthermore, adverse events were more frequent in patients in the GnRH-a group, including the loss of BMD (MD = 2.77, 95%CI: 0.16 to 5.37), headaches (RR = 0.68, 95%CI: 0.52 to 0.91), and hot flushes (RR = 0.43, 95%CI: 0.18 to 1.02).
Dienogest is an effective and tolerable therapeutic for the treatment of endometriosis-related pain in women of reproductive age.
The results of this meta-analysis provide insights on how the clinical use of medications for endometriosis could be standardized and should improve the choice of medications for patients.
| 1. | Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 657] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 2. | Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1079] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 3. | Rogers PA, D'Hooghe TM, Fazleabas A, Gargett CE, Giudice LC, Montgomery GW, Rombauts L, Salamonsen LA, Zondervan KT. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod Sci. 2009;16:335-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J. Endometriosis. Endocr Rev. 2019;40:1048-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 569] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 5. | Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 6. | Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1326] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 7. | De Graaff AA, D'Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L; WERF EndoCost Consortium, Simoens S. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28:2677-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | Young K, Fisher J, Kirkman M. Women's experiences of endometriosis: a systematic review and synthesis of qualitative research. J Fam Plann Reprod Health Care. 2015;41:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Laganà AS, Vitale SG, Granese R, Palmara V, Ban Frangež H, Vrtačnik-Bokal E, Chiofalo B, Triolo O. Clinical dynamics of Dienogest for the treatment of endometriosis: from bench to bedside. Expert Opin Drug Metab Toxicol. 2017;13:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Brockman R. Medication and transference in psychoanalytically oriented psychotherapy of the borderline patient. Psychiatr Clin North Am. 1990;13:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 374] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Andres Mde P, Lopes LA, Baracat EC, Podgaec S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet. 2015;292:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Mita S, Shimizu Y, Notsu T, Imada K, Kyo S. Dienogest inhibits Toll-like receptor 4 expression induced by costimulation of lipopolysaccharide and high-mobility group box 1 in endometrial epithelial cells. Fertil Steril. 2011;96:1485-1489.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Fischer OM, Kaufmann-Reiche U, Moeller C, Fuhrmann U. Effects of dienogest on surgically induced endometriosis in rats after repeated oral administration. Gynecol Obstet Invest. 2011;72:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Katayama H, Katayama T, Uematsu K, Hiratsuka M, Kiyomura M, Shimizu Y, Sugita A, Ito M. Effect of dienogest administration on angiogenesis and hemodynamics in a rat endometrial autograft model. Hum Reprod. 2010;25:2851-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Caruso S, Iraci M, Cianci S, Casella E, Fava V, Cianci A. Quality of life and sexual function of women affected by endometriosis-associated pelvic pain when treated with dienogest. J Endocrinol Invest. 2015;38:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Angioni S, Pontis A, Malune ME, Cela V, Luisi S, Litta P, Vignali M, Nappi L. Is dienogest the best medical treatment for ovarian endometriomas? Gynecol Endocrinol. 2020;36:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Fawzy M, Mesbah Y. Comparison of dienogest vs triptorelin acetate in premenopausal women with adenomyosis: a prospective clinical trial. Arch Gynecol Obstet. 2015;292:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Caruso S, Iraci M, Cianci S, Vitale SG, Fava V, Cianci A. Effects of long-term treatment with Dienogest on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain. J Pain Res. 2019;12:2371-2378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Köhler G, Faustmann TA, Gerlinger C, Seitz C, Mueck AO. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4mg of dienogest daily for endometriosis. Int J Gynaecol Obstet. 2010;108:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Takenaka M, Yano R, Hiraku Y, Shibata M, Hatano K, Yamamoto S, Sato K, Yamamoto K, Morishige K. Exploratory study of pre-surgical medications with dienogest or leuprorelin in laparoscopic cystectomy of endometrial cysts. J Obstet Gynaecol Res. 2015;41:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Tamura H, Yoshida H, Kikuchi H, Josaki M, Mihara Y, Shirafuta Y, Shinagawa M, Tamura I, Taketani T, Takasaki A, Sugino N. The clinical outcome of Dienogest treatment followed by in vitro fertilization and embryo transfer in infertile women with endometriosis. J Ovarian Res. 2019;12:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Harada T, Momoeda M, Taketani Y, Aso T, Fukunaga M, Hagino H, Terakawa N. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis--a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Abdou AM, Ammar IMM, Alnemr AAA, Abdelrhman AA. Dienogest Versus Leuprolide Acetate for Recurrent Pelvic Pain Following Laparoscopic Treatment of Endometriosis. J Obstet Gynaecol India. 2018;68:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Lang J, Yu Q, Zhang S, Li H, Gude K, von Ludwig C, Ren X, Dong L. Dienogest for Treatment of Endometriosis in Chinese Women: A Placebo-Controlled, Randomized, Double-Blind Phase 3 Study. J Womens Health (Larchmt). 2018;27:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Strowitzki T, Faustmann T, Gerlinger C, Seitz C. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2017;108:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Harada T, Kosaka S, Elliesen J, Yasuda M, Ito M, Momoeda M. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: a randomized controlled trial. Fertil Steril. 2017;108:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Klipping C, Duijkers I, Remmers A, Faustmann T, Zurth C, Klein S, Schuett B. Ovulation-inhibiting effects of dienogest in a randomized, dose-controlled pharmacodynamic trial of healthy women. J Clin Pharmacol. 2012;52:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 225] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Petraglia F, Hornung D, Seitz C, Faustmann T, Gerlinger C, Luisi S, Lazzeri L, Strowitzki T. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2012;285:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Gerlinger C, Faustmann T, Hassall JJ, Seitz C. Treatment of endometriosis in different ethnic populations: a meta-analysis of two clinical trials. BMC Womens Health. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis-associated pelvic pain measured on a visual analog scale: analyses of two placebo-controlled, randomized trials. Health Qual Life Outcomes. 2010;8:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Olive DL. Optimizing gonadotropin-releasing hormone agonist therapy in women with endometriosis. Treat Endocrinol. 2004;3:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 35. | Strowitzki T, Faustmann T, Gerlinger C, Schumacher U, Ahlers C, Seitz C. Safety and tolerability of dienogest in endometriosis: Pooled analysis from the european clinical study program. International journal of women's health 2015; 7: 393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Mita S, Shimizu Y, Sato A, Notsu T, Imada K, Kyo S. Dienogest inhibits nerve growth factor expression induced by tumor necrosis factor-α or interleukin-1β. Fertil Steril. 2014;101:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Maeda N, Izumiya C, Taniguchi K, Matsushima S, Mita S, Shimizu Y, Fukaya T. Dienogest improves human leucocyte antigen-DR underexpression and reduces tumour necrosis factor-α production in peritoneal fluid cells from women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;177:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertil Steril. 2005;83:1530-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Takeuchi A, Koga K, Miyashita M, Makabe T, Sue F, Harada M, Hirata T, Hirota Y, Fujii T, Osuga Y. Dienogest reduces proliferation, NGF expression and nerve fiber density in human adenomyosis. Eur J Obstet Gynecol Reprod Biol. 2016;207:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Ureyen Ozdemir E, Adali E, Islimye Taskin M, Yavasoglu A, Aktug H, Oltulu F, Inceboz U. Effects of ranibizumab and zoledronic acid on endometriosis in a rat model. Arch Gynecol Obstet. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocr Rev. 1994;15:275-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rakhshan V S-Editor: Liu M L-Editor: Wang TQ P-Editor: Ma YJ