Published online Aug 28, 2021. doi: 10.13105/wjma.v9.i4.353
Peer-review started: April 3, 2021
First decision: June 18, 2021
Revised: June 28, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: August 28, 2021
Processing time: 152 Days and 20.4 Hours
Previous meta-analyses related smoking to death or severe infection from coronavirus disease 2019 (COVID-19) in hospitalized patients, but considered only a few studies, did not adjust for demographics and comorbidities, and inadequately defined smoking.
To review and meta-analyse epidemiological evidence on smoking and COVID-19, considering a range of endpoints, populations and smoking definitions and the effect of adjustment.
Studies were identified from publications in English up to 30 September, 2020 involving at least 100 individuals, carried out in Europe, Israel, America or Australasia, not restricted to those with specific other diseases, and providing information relating smoking to various COVID-related endpoints. Meta-analyses were carried out for combinations of population and endpoint, with variation studied by smoking definition, adjustment level and other factors.
From 96 publications, 74 studies were identified, 37 in the United States, 10 in the United Kingdom, with up to four in the other countries. Three involved over a million individuals, and 37 involved less than a thousand. Adjusted results for smoking were available in 42 studies, with adjustment not considered in 20 studies. Results were considered by endpoint. No significant effect of smoking on COVID-19 positivity was seen in the general population, but there was a reduced risk in those tested. Best-adjusted estimates for current (vs never) smoking were 0.87 (95% confidence interval: 0.52-1.47) in the general population and 0.52 (0.43-0.64) in those tested. For those hospitalized due to COVID-19, unadjusted rates were significantly increased in current smokers (1.20, 1.01-1.42) and ever smokers (1.64, 1.41-1.91), but those adjusted for comorbidities showed no increase for current (0.82, 0.52-1.30) or ever smokers (1.00, 0.76-1.32). There was little evidence to suggest that smoking was associated with intensive care admission. For those hospitalized with COVID-19, best-adjusted estimates were 0.88 (0.72-1.08) for current smokers and 1.10 (0.99-1.22) for ever smokers. In those hospitalized with COVID-19, smoking was not significantly related to subsequent mechanical ventilation, with best-adjusted estimates of 1.12 (0.60-2.09) for current smokers and 1.05 (0.88-1.25) for ever smokers. For those hospitalized with severe COVID-19, best-adjusted estimates were 0.74 (0.49-1.12) for current smokers and 1.15 (0.87-1.51) for ever smokers; few estimates were adjusted for comorbidities. While smoking was associated with increased mortality in unadjusted analyses, the association disappeared after adjustment for comorbidities. For example, in those hospitalized with COVID-19, the unadjusted estimate for ever smokers of 1.59 (1.37-1.83) reduced to 1.07 (0.82-1.38) when adjusted for comorbidities. Studies on those with severe COVID-19 showed that smoking tended to be associated with worsening of the disease. However, no estimate was adjusted, even for demographics. Estimates did not clearly vary by location or study size, and there was too little evidence to usefully study variations by age, amount smoked or years quit.
The increased COVID-19 death rate in smokers seen in unadjusted analyses disappears following adjustment for demographics and comorbidities. Among those tested, smoking is associated with lower COVID-19 infection rates.
Core Tip: Detailed analyses of 74 studies related smoking to being tested for coronavirus disease 2019 (COVID-19), having COVID-19, or suffering death or severe disease due to COVID-19. Various smoking indices were studied, as were the effects of adjusting for other factors. Although many studies provided limited unreliable results, consistent evidence showed that of those tested, smokers were less likely to have COVID-19. Among those positive for or hospitalized with COVID-19, there was a clear association between smoking and COVID-19 death and severity in unadjusted analyses, which disappeared following adjustment for comorbidities and demographics. Any adverse effects in smokers appear to derive from their poorer prior health status.
- Citation: Lee PN, Hamling JS, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in Europe, Israel, America and Australasia on smoking and COVID-19. World J Meta-Anal 2021; 9(4): 353-376
- URL: https://www.wjgnet.com/2308-3840/full/v9/i4/353.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i4.353
In a previous project commenting on publications on smoking and coronavirus disease 2019 (COVID-19), we considered over 100 papers published up to the end of September 2020. Among these were various meta-analyses falling into two groups.
Eight publications[1-8] considered smoking prevalence in hospitalized patients, generally agreeing it was substantially less than expected from national statistics. This evidence does not necessarily show smoking protects against acquiring COVID-19. Smoking may be markedly under-reported in studies based on medical records. Also, among those with COVID-19, smokers might be less likely than non-smokers to be hospitalized. These meta-analyses ignored relevant information from studies of those tested for COVID-19, or of the general population, where smoking habits were collected pre-pandemic, as well as more recent studies of hospitalized patients.
The other meta-analyses[3,6,9-28] concerned hospitalized patients, relating smoking to severity, progression or death from COVID-19, mainly from studies in China. While these generally reported positive associations which were often statistically significant, many meta-analysis estimates were unadjusted even for age, with comorbidities present pre-pandemic rarely considered. These meta-analyses also varied on the index of smoking used, which was not always clearly defined.
Here we describe meta-analyses aimed at avoiding the limitations of the early meta-analyses by considering more studies, not limiting attention to hospitalized patients, and paying particular attention to the definition of smoking and the effects of adjustment, as well as the reliability of the smoking data.
To limit the scope of the study and provide timely results various restrictions to the studies were made, as described in the methods section. Notably studies in China and other parts of Asia, except for Israel, were excluded. A recent review[29] classified few studies from Asia as “good” or “fair”, most having much missing data and/or not reliably distinguishing current, former, ever and never smoking status. We were also aware of a large study in Israel[30] without these weaknesses.
Due to the more comprehensive data included, and better quality of the studies considered, the meta-analyses we describe should provide much better insight into the relationships of smoking to various COVID-related outcomes than do the meta-analyses referred to above.
Full details of the methods used are given in Supplementary material 1 and are sum
Pre-defined criteria stipulated for practical reasons that studies should be described in English and were detected in searches up to September 2020. They should also be conducted in Europe, Israel, America, or Australasia, as discussed above. America here includes all the countries in South and Central America, as well as the United States and Canada. Studies restricted to individuals with specific other diseases were excluded as being less generalizable. Studies of less than 100 individuals were excluded as they provided inadequate power to detect reliable results, and as there were adequate numbers of larger studies. Studies should provide information relating smoking to the probability of one or more of the following relevant endpoints: being tested for COVID-19, having confirmed COVID-19, having self-reported COVID-19, being hospitalized with COVID-19, requiring mechanical ventilation for COVID-19, requiring intensive care for COVID-19, having severe/progressive COVID-19, or dying from COVID-19 or from any cause. The studies may concern various at-risk populations, including the general population, those tested for COVID-19, those positive for COVID-19, those hospitalized with COVID-19, or those with severe COVID-19.
As part of our earlier project, we carried out a first PubMed search on April 7, 2020, and then carried out further daily searches up to September 30, 2020.
Publications identified in our study as being of initial interest were then examined to identify studies satisfying our inclusion/exclusion criteria, and relevant meta-analyses. The meta-analyses were then examined for additional relevant studies meeting our inclusion criteria. Further studies were then sought from meta-analyses identified in further searches, from a further more detailed look at our original searches, and from examining reference lists of publications identified as relevant.
To avoid double-counting results from the same study reported in multiple publications, the relevant publications were examined to identify publications from the same study.
Data from each publication were entered onto a study database and a linked effect estimate database. The study database recorded information on: publications considered; study title; study location; sexes, ages and races considered; study dates; study type; nature of population studied; sample size; definition of severe COVID-19 (if relevant); method of COVID-19 diagnosis; whether adjusted effect estimates were available; the confounding variables studied; and whether adjusted results on smoking were reported and, if not, why not (e.g., smoking not significant in the adjusted model). It also recorded information on the smoking index for which results were available (e.g., current vs never smoking), the source of the smoking data (e.g., medical records), the extent of missing data, the percentage of smokers in the population studied, and whether dose-response data were available, as well as details of the endpoints and at-risk populations studied.
The effect estimate database included details of each individual effect estimate entered. Effect estimates were entered for every available combination of endpoints within a population, smoking index and level of adjustment (separated into unadjusted (U), adjusted for demographics only (D), adjusted also for comorbidities but not post-infection variables (C), and adjusted also for variables including post-infection responses to COVID-19 (P). Where available, effect estimates were entered by sex, age group and smoking dose (amount, duration, time since quitting). Other factors recorded included the publication the effect estimate was derived from; the population and endpoint considered; the smoking comparison; the type of effect estimate [odds ratio (OR), relative risk (RR) or hazard ratio (HR)]; the adjustment factors considered; the number of cases and at-risk subdivided by the smoking variable; the effect estimate and its lower and upper 95% confidence interval; and whether the estimate was given in the paper or was derived from the data presented. Derivation could be from the 2 × 2 table of numbers for unadjusted data, or using the method described by Hamling et al[31] to derive adjusted estimates for other smoking indices from estimates given in the publication (e.g., ever vs never estimates from those for current vs never and former vs never).
All data were entered by Hamling JS or Coombs KJ and checked by Lee PN, with any disagreements discussed and resolved.
For each studied combination of endpoint within a population (e.g., died while hospitalized with COVID-19), meta-analyses were carried out relating the endpoint to each of six indices of smoking; ever vs never, current vs non-current, current vs never, former vs never, a combined index most closely approximating to current vs never, and a combined index most closely approximating to ever vs never. The combined index for current vs never smoking includes, from each study reporting the endpoint/population combination, results in the following preference order (most to least preferred) – current vs never, current vs non-current, smoker (undefined) vs non-smoker, tobacco use vs none, ever vs never, and former vs never. The combined index for ever vs never smoking uses the preference - ever vs never, former vs never, smoker (undefined) vs non-smoker, tobacco use vs none, current vs never, and current vs non-current.
For each endpoint within a combination of population and smoking index, the results to be meta-analysed were selected using a first preference on level of adjustment and then a second preference on type of effect estimate. For adjustment, the order of preference (first to last) was adjustment for factors including comorbidities, adjustment for demographics only, unadjusted, and adjustment for factors measuring responses to COVID-19 infection (the lowest preference, as this may be a form of over-adjustment). For type of effect estimate, the preference order was HR, then RR, then OR.
Where the numbers of estimates permitted, the meta-analyses compare estimates by level of adjustment, type of estimate, sex, location and study size.
Fixed-effect and random-effects meta-analyses were conducted using the method of Fleiss and Gross[32] with heterogeneity quantified by H, the ratio of the heterogeneity chisquared to its degrees of freedom. H is directly related to the I2 statistic[33] by the formula I2 = 100(H−1)/H. For all meta-analyses, Egger’s test of publication bias[34] was included.
All analyses were carried out using RoeLee release 63, build 52, available from RoeLee Statistics Ltd (http://www.roelee.co.uk).
Figure 1 summarizes the results of the literature searches, with fuller details, including reasons for rejecting papers (Supplementary material 2). Overall, 98 publications met the selection criteria.
Twelve references represented multiple publications from the same study. Allowing for this, data were entered for 76 separate studies. Subsequently, during data entry, it became apparent that two studies[35,36] provided no data for any of the endpoints considered, and only compared smoking prevalence with published data in the population at large. These studies were not further evaluated.
Supplementary material 3 summarizes the details for the 74 studies, including references. Studies were identified by the six character codes shown there.
Thirty-seven studies were from the United States, 10 from the United Kingdom (including seven restricted to England), four each from France, Israel, Italy, Mexico and Spain, two from Switzerland and one each from Australia, Brazil and Denmark. Also one study was conducted in the United Kingdom and Italy, and one in multiple European countries.
In 31 studies, the population considered was patients hospitalized with COVID-19, with a further four patients admitted to the intensive care unit (ICU). Also 19 studies considered those with a positive COVID-19 test, 11 included those who were tested for COVID-19 and seven included the general population. One study included hospital patients and non-COVID-19 controls, while another included those tested for COVID-19 as well as control groups not tested.
All studies included both sexes, and none selected individuals on race or ethnicity. One study was restricted to adults aged 47-87 years, with a further 41 restricted to adults, generally with a minimum age of 18 years, but sometimes having lower limits ranging from 15 years to 23 years. The remaining 32 studies did not refer to any age restriction. As shown in Supplementary material 3, the number of individuals with smoking data varied widely between the studies. The largest was OPENSA in England involving over 16 million individuals, with two other United Kingdom studies over a million, and three others over 100000. In contrast, 37 studies involved less than 1000 individuals.
It is also shown in Supplementary material 3 that some studies reported analyses for subsets of their populations, and for various endpoints. These endpoints included being tested for COVID-19, having confirmed or having self-reported COVID-19, being hospitalized with COVID-19, requiring intensive care or mechanical ventilation, having severe or progressive COVID-19, and mortality, either from COVID-19 or from all causes combined.
Thirty-six studies allowed calculation of separate effect estimates for current, former and ever smokers. Eight reported results for current smoking only, 19 for smoking history only, and eight for smoking undefined. One (TWIGG) reported results for tobacco use undefined, one (CHAND) had two source papers - one providing results for current smokers only, one for ever smokers only - and another (GUPTA) gave numbers of current or former smokers combined and adjusted results for current vs non-current smokers. The smoking data were mainly extracted from medical records, although in some cases the data came from a questionnaire or other sources, with no details given in six studies. Percentages with missing data on smoking were available in 35 studies, with 13 being over 20%.
Adjusted results for smoking were presented for 42 studies. In 12, only predictors other than smoking appeared in the adjusted model, either because smoking was not significant in univariate analyses, so was not considered in multivariate analyses, or because smoking dropped out of the multivariate modelling. In 20 studies, no adjusted results were presented.
In 54 studies, COVID-19 diagnosis was by reverse transcriptase-polymerase chain reaction or simply by polymerase chain reaction, but there were various alternatives, as indicated in Supplementary material 3.
Effect estimates were available for all but one study (KNIGHT), which stated only that “Ever cigarette smoking was predictive of death (P < 0.05)” without providing quantitative detail.
Overall there were 738 effect estimates: 548 ORs, 122 RRs and 68 HRs. The studies providing most estimates were GU (100), BIOBNK (86) and VETERA (52), with 18 other studies providing 10 or more estimates. Fourteen studies provided only one estimate. There were 153 estimates for current vs never smoking, 202 for current vs non-smoking, 142 for former vs never smoking, 223 for ever vs never smoking, 16 for smoker vs non-smoker not otherwise stated, and two for tobacco vs no-tobacco not otherwise stated. One study (HIPPIS) provided 15 effect estimates by amount smoked, and one (TOOLKI) provided four estimates by years quit. No other study provided dose-response data.
Of the 738 estimates, 432 (58.5%) were unadjusted, 110 (14.9%) adjusted for demographics only, 169 (22.9%) adjusted for variables including co-morbidities but not responses to COVID-19, and 27 (3.7%) adjusted for a list including responses to COVID-19.
One study (YANOVE) provided effect estimates subdivided jointly by sex and age group. Four others (BIOBNK, GDEM, HOPKIN, MIYARA) provided estimates subdivided by sex only, and three others (GDEM, SINAI, VETERA) provided estimates subdivided by age group only.
The at-risk population was the general population for 155 (21.0%) effect estimates, those tested for COVID-19 for 106 (14.4%), those positive for COVID-19 for 222 (30.1%), those hospitalized with COVID-19 for 188 (25.5%), and those in intensive care for 22 (3.0%). Other populations were considered for 45 (6.1%) effect estimates, with GU providing 36 of these, all based on populations representing a combination of a group considered above (e.g., tested for COVID-19) and unmatched controls. Other such populations included a mixture of those positive for COVID-19 and those untested (two estimates from BIOBNK), those hospitalized but including non-COVID cases (five from MEINI), those tested for COVID-19 but not hospitalized (one from ADORNI) and those hospitalized with COVID-19 but not in ICU (one from PELLAU).
The endpoints considered in the 738 effect estimates were tested for COVID-19 in 41 (5.6%), positive for COVID-19 in 189 (25.6%), hospitalized for COVID-19 in 128 (17.3%), in intensive care in 92 (12.5%), mechanical ventilation in 58 (7.9%), severe COVID-19 (defined variously) in 58 (7.9%), and died in 172 (23.3%).
The effect estimates concerned many different combinations of endpoint within population, the commonest being hospitalized among those positive for COVID-19 (98 effect estimates), positive within those tested (90), died among those hospitalized (82) and positive among the general population (79).
The results below are considered by endpoint and within endpoint by population. Meta-analysis results are shown in the main tables for endpoint/population combinations that have data from at least five studies, with the individual study data summarized in Supplementary material 4 for combinations with data from fewer than five studies. Fuller details, including the individual effect estimates meta-analysed, the extent of heterogeneity between the estimates, and results of tests for publication bias, are provided in Supplementary material 5.
Four studies provided effect estimates (Supplementary Table 1). GU compared tested individuals with unmatched controls, and the remaining studies (BIOBNK, ADORNI and HOPKIN) compared those tested and not tested. BIOBNK provided estimates from multiple publications, the results from two being shown in SupplementaryTable 1, with the others providing little extra information.
| Selection of estimates1 | Statistic2 | Ever vs never | Current vs non-current | Current vs never | Former vs never | Closest to current | Closest to never | |
| Population = general3 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.04 (0.79-1.37) | 0.76 (0.56-1.02) | 0.87 (0.52-1.47) | 1.09 (0.94-1.28) | 0.78 (0.57-1.06) | 0.89 (0.73-1.09) |
| n | 5 | 7 | 5 | 5 | 7 | 7 | ||
| C | EE (95%CI) | 1.10 (0.70-1.72) | 0.93 (0.43-2.01) | 0.96 (0.42-2.17) | 1.10 (0.86-1.40) | 0.96 (0.42-2.17) | 1.10 (0.70-1.72) | |
| n | 3 | 3 | 3 | 3 | 3 | 3 | ||
| U | EE (95%CI) | 0.71 (0.63-0.80) | 0.56 (0.43-0.75) | 0.45 (0.36-0.55) | 0.92 (0.80-1.05) | 0.56 (0.42-0.75) | 0.65 (0.55-0.77) | |
| n | 1 | 3 | 1 | 1 | 3 | 3 | ||
| All estimates | U | EE (95%CI) | 1.04 (0.83-1.29) | 0.79 (0.51-1.22) | 0.90 (0.46-1.78) | 1.13 (0.97-1.31) | 0.80 (0.53-1.22) | 0.89 (0.74-1.08) |
| n | 5 | 7 | 5 | 5 | 7 | 7 | ||
| Population = tested for COVID-194 | ||||||||
| Best-adjusted | All | EE (95%CI) | 0.71 (0.60-0.84) | 0.61 (0.52-0.71) | 0.52 (0.43-0.64) | 0.97 (0.86-1.09) | 0.55 (0.47-0.65) | 0.70 (0.63-0.77) |
| n | 10 | 13 | 8 | 8 | 17 | 17 | ||
| C | EE (95%CI) | 0.77 (0.56-1.05) | 0.60 (0.45-0.79) | 0.53 (0.34-0.83) | 0.90 (0.76-1.08) | 0.58 (0.43-0.80) | 0.77 (0.64-0.92) | |
| n | 4 | 5 | 4 | 4 | 5 | 5 | ||
| U | EE (95%CI) | 0.67 (0.52-0.86) | 0.62 (0.51-0.75) | 0.49 (0.42-0.58) | 1.04 (0.85-1.28) | 0.55 (0.45-0.67) | 0.67 (0.58-0.78) | |
| n | 6 | 8 | 4 | 4 | 11 | 11 | ||
| All estimates | U | EE (95%CI) | 0.72 (0.62-0.85) | 0.61 (0.50-0.73) | 0.53 (0.46-0.61) | 0.97 (0.87-1.09) | 0.55 (0.46-0.66) | 0.71 (0.64-0.79) |
| n | 10 | 12 | 8 | 8 | 17 | 17 | ||
The results presented are somewhat conflicting, with the BIOBNK study reporting estimates above 1.00, generally significant, and tending to decrease with increasing adjustment, while ADORNI and HOPKIN, both only provided unadjusted results, with significant estimates below 1.00. GU shows a reduced probability of testing for current smoking and an increased probability of testing for former and ever smoking, with the estimates reducing with increasing adjustment.
Meta-analysis of this conflicting data was not attempted.
Table 1 presents the meta-analysis results from six studies where the at-risk population was the general population, and 15 where it was those tested for COVID-19. The “best-adjusted” results are those where, for each study, an effect estimate was selected in the order of preference C, D, U and P for level of adjustment. Results are shown for all the best-adjusted results and for those where C and U were the best-adjusted results available, which form the great majority of the best-adjusted results. The “all estimates U” results give estimates for the totality of unadjusted results, including those not included in the best-adjusted results being superseded by results for the same study with level of adjustment C or D. The results are consistent with no effect of smoking on positivity in the general population, but a reduced risk of positivity, particularly among current smokers, in those tested for COVID-19. No clear effects of adjustment were seen in either analysis.
Four other studies presented results for COVID-19 positivity based on other populations (Supplementary Table 2). In BIOBNK, COVID-19 positives were compa
| Selection of estimates1 | Statistic2 | Ever vs never | Current vs non-current | Current vs never | Former vs never | Closest to current | Closest to ever | |
| Population = positive for COVID-193 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.34 (1.13-1.60) | 1.00 (0.86-1.16) | 1.05 (0.84-1.32) | 1.31 (0.93-1.85) | 1.31 (1.12-1.54) | 1.38 (1.17-1.63) |
| n | 19 | 13 | 11 | 11 | 22 | 22 | ||
| C | EE (95%CI) | 1.00 (0.76-1.32) | 0.90 (0.76-1.07) | 0.82 (0.52-1.30) | 0.87 (0.67-1.12) | 0.96 (0.79-1.18) | 0.93 (0.80-1.08) | |
| n | 6 | 6 | 4 | 4 | 8 | 8 | ||
| U | EE (95%CI) | 1.53 (1.14-2.06) | 1.03 (0.75-1.41) | 1.13 (0.81-1.59) | 1.77 (1.04-3.04) | 1.44 (1.03-2.01) | 1.71 (1.24-2.36) | |
| n | 10 | 6 | 6 | 6 | 11 | 11 | ||
| All estimates | U | EE (95%CI) | 1.64 (1.41-1.91) | 0.97 (0.71-1.32) | 1.20 (1.01-1.42) | 1.75 (1.35-2.27) | 1.47 (1.21-1.77) | 1.72 (1.46-2.02) |
| n | 18 | 13 | 11 | 11 | 21 | 21 | ||
Table 2 shows the meta-analysis results based on 19 studies of those positive for COVID-19. While the unadjusted estimates show increased hospitalization rates in former and ever smokers, those adjusted for comorbidities show no indication of an increase for any index of smoking.
Supplementary Table 3 shows additional results from three studies. BIOBNK provided results based on the general population, GDEM and GU provided results on those tested for COVID-19, and GU provided results based on those hospitalized and unmatched controls. The results are rather conflicting, with GU showing markedly lower hospitalization rates in current smokers, and the other studies increased rates. In BIOBNK, adjustment tended to reduce the associations, although they remained statistically significant. GDEM reported only results adjusted for demographics and GU reported only unadjusted results.
| Selection of estimates1 | Statistic2 | Ever vs never | Current vs non-current | Current vs never | Former vs never | Closest to current | Closest to ever | |
| Population = positive for COVID-193 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.57 (1.14-2.17) | 0.87 (0.67-1.12) | 0.94 (0.60-1.48) | 1.55 (1.00-2.42) | 1.13 (0.82-1.54) | 1.40 (1.09-1.80) |
| n | 7 | 6 | 5 | 5 | 8 | 8 | ||
| C | EE (95%CI) | 1.68 (1.15-2.46) | 0.85 (0.77-0.94) | 0.61 (0.11-3.36) | 1.58 (0.97-2.57) | 1.14 (0.54-2.41) | 1.30 (0.74-2.31) | |
| n | 2 | 2 | 1 | 1 | 3 | 3 | ||
| U | EE (95%CI) | 1.42 (0.94-2.14) | 0.94 (0.57-1.57) | 0.98 (0.60-1.60) | 1.55 (0.91-2.65) | 0.98 (0.60-1.60) | 1.42(0.94-2.14) | |
| n | 4 | 4 | 4 | 4 | 4 | 4 | ||
| All estimates | U | EE (95%CI) | 1.52 (1.04-2.22) | 1.03 (0.56-1.88) | 0.92 (0.60-1.43) | 1.68 (1.05-2.68) | 1.11 (0.62-2.00) | 1.61 (1.13-2.29) |
| n | 5 | 6 | 5 | 5 | 6 | 6 | ||
| Population = hospitalized with COVID-194 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.10 (0.99-1.22) | 0.90 (0.72-1.12) | 0.88 (0.72-1.08) | 1.21 (0.99-1.47) | 1.02 (0.87-1.21) | 1.11 (0.98-1.25) |
| n | 9 | 9 | 6 | 6 | 14 | 14 | ||
| C | EE (95%CI) | 1.01 (0.88-1.16) | 0.80 (0.61-1.04) | 0.81 (0.61-1.07) | 1.07 (0.92-1.24) | 0.81 (0.61-1.07) | 1.01 (0.88-1.16) | |
| n | 1 | 1 | 1 | 1 | 1 | 1 | ||
| U | EE (95%CI) | 1.22 (1.04-1.43) | 1.09 (0.93-1.27) | 0.96 (0.70-1.31) | 1.30 (1.00-1.70) | 1.16 (1.05-1.28) | 1.19 (1.08-1.30) | |
| n | 8 | 7 | 5 | 5 | 12 | 12 | ||
| All estimates | U | EE (95%CI) | 1.20 (1.09-1.32) | 1.00 (0.87-1.13) | 0.89 (0.74-1.06) | 1.28 (1.10-1.48) | 1.05 (0.95-1.17) | 1.14 (1.04-1.24) |
| n | 9 | 9 | 6 | 6 | 14 | 14 | ||
Table 3 shows the meta-analysis results based on estimates from eight studies of those positive for COVID-19 and 14 of those hospitalized with COVID-19. SupplementaryTable 4 shows the results from one general population study, and one study comparing intensive care patients with unmatched controls. Most estimates considered in Table 3 are unadjusted, not even for age, and show little evidence of an association between smoking and ICU admission. Exceptionally, the data in Supplementary Table 4 show reduced admission rates in current smokers, and increased admission rates in former smokers, tending to diminish and become marginally significant after adjustment in the HIPPIS study.
| Selection of estimates1 | Statistic2 | Ever vs never | Current vs non-current | Current vs never | Former vs never | Closest to current | Closest to ever | |
| Population = hospitalized for COVID-193 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.05 (0.88-1.25) | 1.11 (0.72-1.71) | 1.12 (0.60-2.09) | 1.16 (0.87-1.55) | 1.08 (0.86-1.36) | 1.11 (0.92-1.35) |
| n | 12 | 8 | 5 | 5 | 15 | 15 | ||
| C | EE (95%CI) | 1.04 (0.79-1.36) | 1.94 (0.40-9.49) | 2.77 (0.28-27.2) | 2.43 (0.32-18.5) | 0.99 (0.77-1.29) | 1.04 (0.79-1.36) | |
| n | 7 | 2 | 2 | 2 | 7 | 7 | ||
| U | EE (95%CI) | 1.06 (0.94-1.21) | 1.12 (0.60-2.09) | 0.76 (0.59-0.98) | 1.10 (0.96-1.27) | 1.16 (0.80-1.67) | 1.24 (0.99-1.54) | |
| n | 5 | 5 | 3 | 3 | 7 | 7 | ||
| All estimates | U | EE (95%CI) | 1.07 (0.92-1.26) | 1.10 (0.80-1.51) | 1.09 (0.60-1.95) | 1.12 (0.98-1.28) | 1.12 (0.98-1.38) | 1.17 (1.00-1.37) |
| n | 10 | 8 | 5 | 5 | 13 | 13 | ||
Fourteen studies provided results where the population involved patients hospitalized with COVID-19. While the best-adjusted effect estimates in Table 4 were greater than 1.0 for each smoking index, none were statistically significant at P < 0.05.
Supplementary Table 5 summarizes the results from three studies where the population was those tested for COVID-19. VETERA, which provided the most detailed results, did not demonstrate any clear association, with estimates for ever and for former smoking significantly increased when unadjusted, but close to 1.0 and non-significant when adjusted for comorbidities. In ESSVRD, a significant unadjusted increase again was non-significant after adjustment for comorbidities. Exceptionally, MUNOZP reported a very high OR for ever smoking after adjustment for comorbidities.
| Selection of estimates2 | Statistic3 | Ever vs never | Current vs non-current | Current vs never | Former vs never | Closest to current | Closest to ever | ||
| Population = positive4 | |||||||||
| Best-adjusted | All | EE (95%CI) | 1.39 (1.07-1.80) | 1.04 (0.91-1.20) | 1.27 (0.82-1.95) | 1.20 (0.62-2.32) | 1.10 (0.97-1.25) | 1.10 (0.97-1.25) | |
| n | 7 | 5 | 4 | 4 | 8 | 8 | |||
| C | EE (95%CI) | - | 1.02 (0.88-1.18) | - | - | 1.02 (0.88-1.18) | 1.02 (0.88-1.18) | ||
| n | 0 | 1 | 0 | 0 | 1 | 1 | |||
| U | EE (95%CI) | 1.30 (0.94-1.79) | 1.26 (0.82-1.93) | 1.27 (0.82-1.95) | 1.20 (0.62-2.32) | 1.35 (0.95-1.92) | 1.30 (0.94-1.79) | ||
| n | 5 | 4 | 4 | 4 | 5 | 5 | |||
| All estimates | U | EE (95%CI) | 1.63 (1.27-2.10) | 1.17 (1.09-1.25) | 1.27 (0.82-1.95) | 1.20 (0.62-2.32) | 1.48 (1.13-1.94) | 1.45 (1.12-1.86) | |
| n | 7 | 5 | 4 | 4 | 8 | 8 | |||
| Population = hospitalized5 | |||||||||
| Best-adjusted | All | EE (95%CI) | 1.15 (0.87-1.51) | 1.05 (0.93-1.18) | 0.74 (0.49-1.12) | 1.07 (0.87-1.32) | 1.08 (0.94-1.25) | 1.09 (0.98-1.22) | |
| n | 7 | 5 | 2 | 2 | 10 | 10 | |||
| C | EE (95%CI) | 1.35 (0.66-2.75) | 0.76 (0.50-1.15) | 0.77 (0.51-1.17) | 1.06 (0.86-1.31) | 1.23 (0.46-3.27) | 1.35 (0.66-2.75) | ||
| n | 2 | 1 | 1 | 1 | 2 | 2 | |||
| U | EE (95%CI) | 1.38 (1.04-1.83) | 1.12 (1.04-1.21) | 0.29 (0.03-2.64) | 1.25 (0.56-2.76) | 1.17 (0.99-1.37) | 1.14 (1.06-1.23) | ||
| n | 4 | 3 | 1 | 1 | 6 | 6 | |||
| All estimates | U | EE (95%CI) | 1.40 (1.20-1.63) | 1.14 (1.04-1.24) | 0.89 (0.54-1.46) | 1.48 (1.23-1.80) | 1.17 (1.08-1.27) | 1.21 (1.12-1.29) | |
| n | 6 | 5 | 2 | 2 | 9 | 9 | |||
Table 5 shows the meta-analysis results from five studies on those positive for COVID-19 and 10 studies on those hospitalized with COVID-19. As shown in Table 5, definitions of severity varied by study. Few effect estimates were adjusted for comorbidities. The smoking indices were generally associated with a small increase in severity, but this was only significant at P<0.05 in one of the 12 best-adjusted meta-analysis estimates.
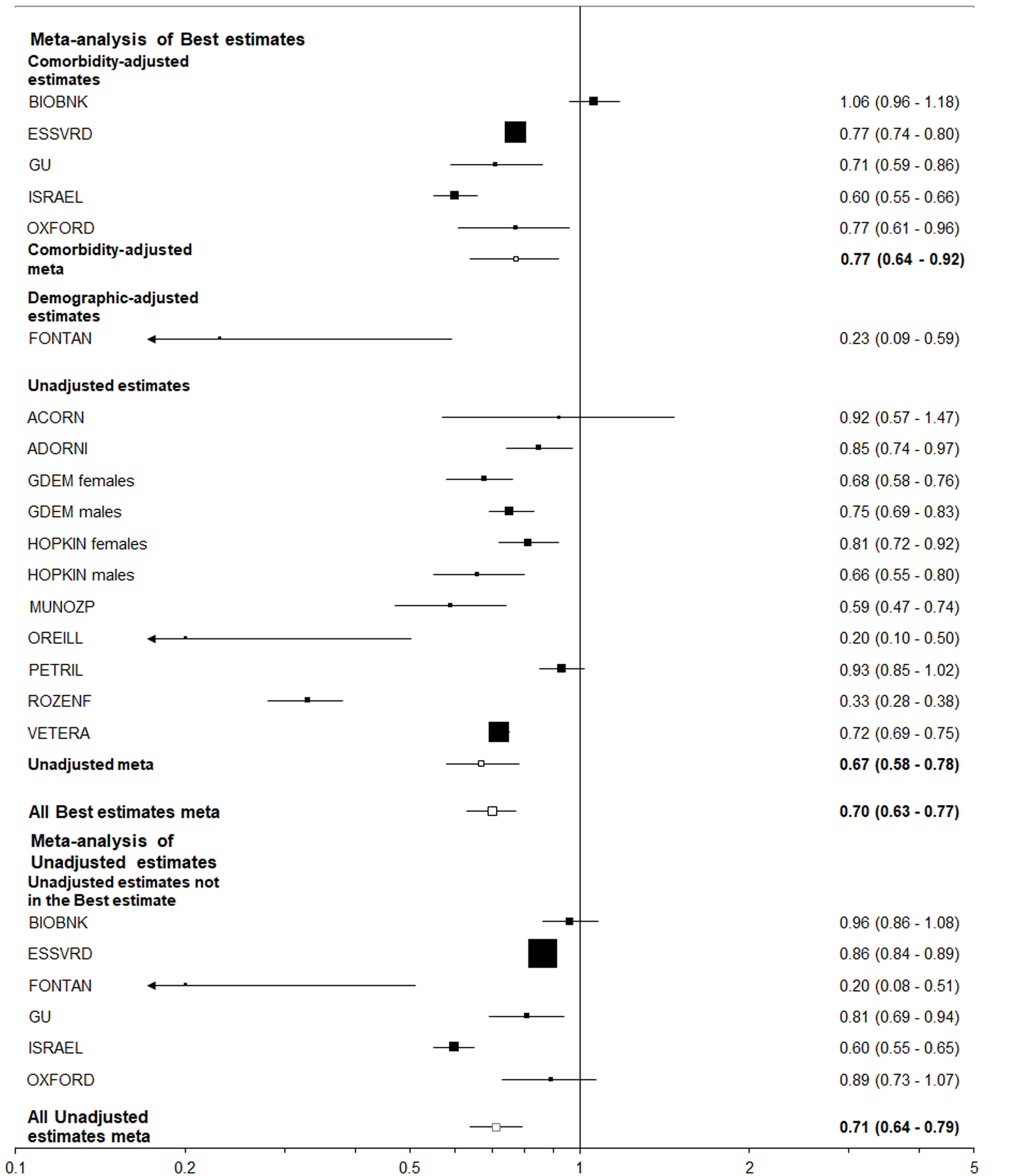
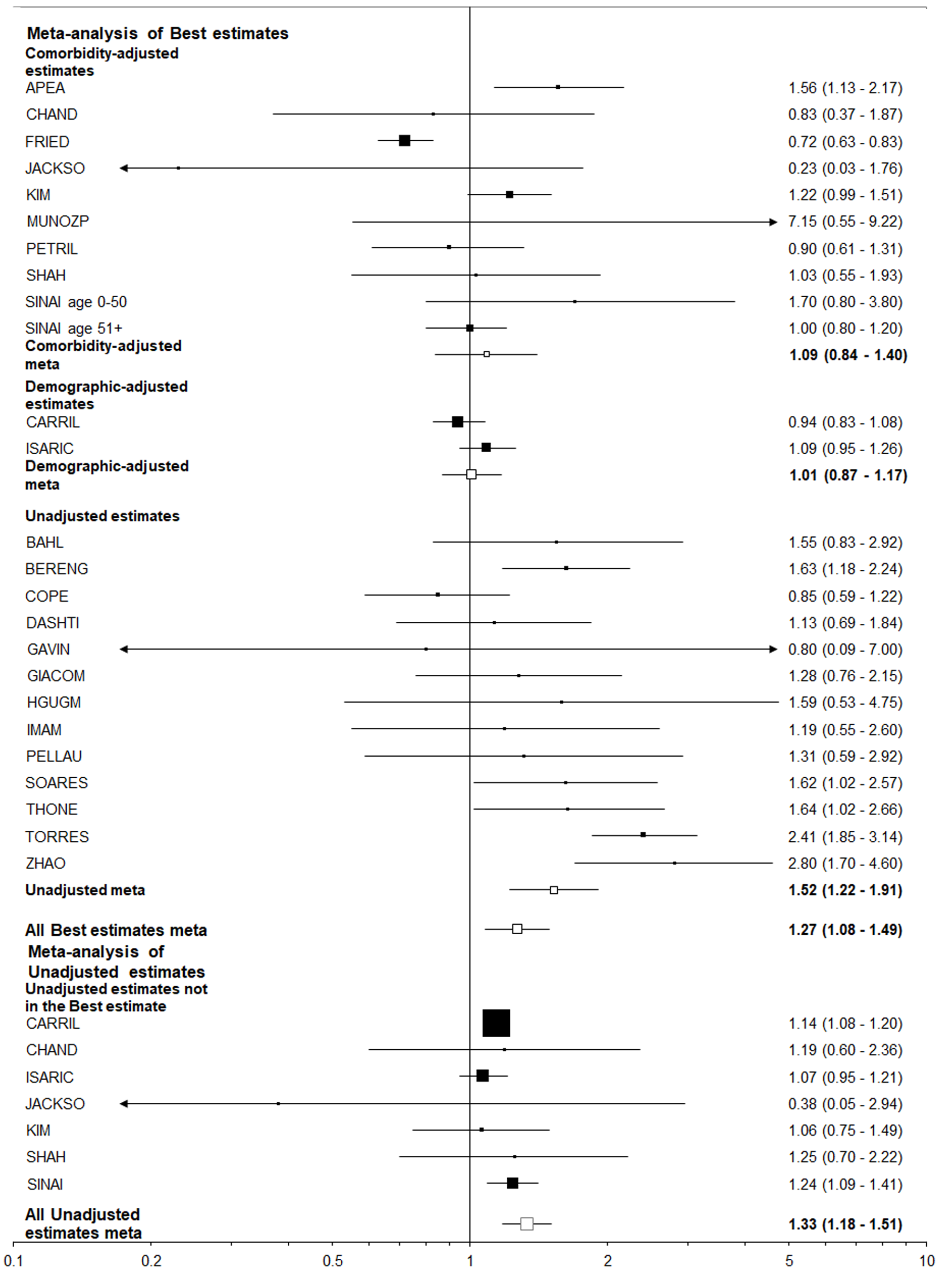
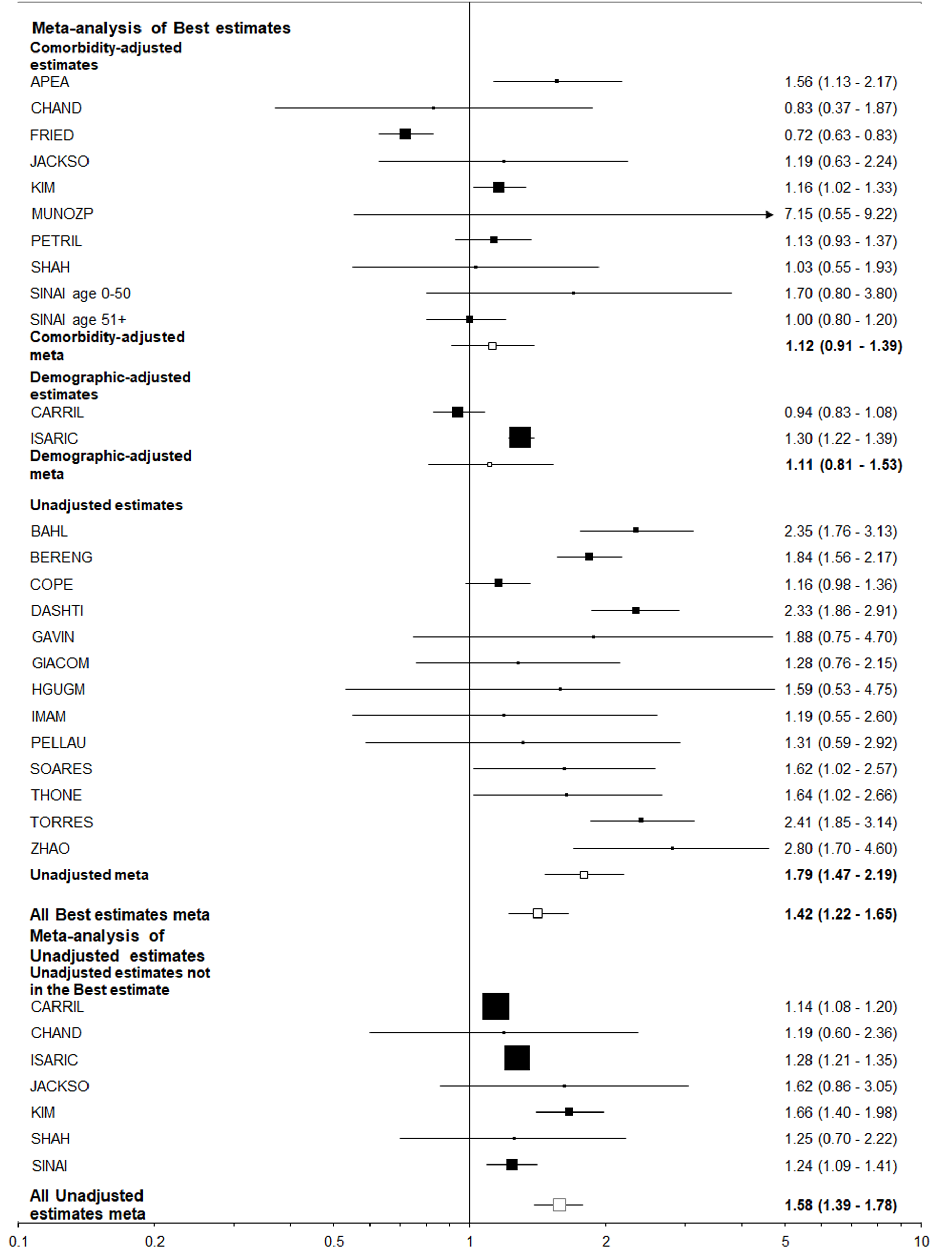
Table 6 summarizes the results using data from 25 studies of those hospitalized with COVID-19, and eight studies of those positive for COVID-19 regardless of hospitalization. The estimates adjusted for comorbidities were virtually never statistically significant and usually close to 1.00, but the unadjusted estimates were nearly always elevated and often statistically significant. This was very clearly illustrated by the results for “closest to ever smoking” where about a two-fold increase was seen for the unadjusted results, with little or no increase seen for the comorbidity adjusted results, regardless of the population studied. It is also clear that higher unadjusted estimates were seen for former or ever smoking than for current smoking, perhaps because former smokers tend to be older than current or never smokers.
| Selection of estimates1 | Statistic2 | Ever vs never | Current vs non-current | Current vs never | Former vs never | Closest to current | Closest to ever | |
| Population = hospitalized with COVID-193 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.42 (1.19-1.70) | 0.99 (0.89-1.10) | 1.12 (0.98-1.29) | 1.61 (1.34-1.92) | 1.27 (1.08-1.49) | 1.42 (1.22-1.65) |
| n | 18 | 10 | 10 | 10 | 25 | 25 | ||
| C | EE (95%CI) | 1.07 (0.82-1.38) | 0.64 (0.12-3.35) | 1.02 (0.70-1.49) | 1.15 (1.02-1.29) | 1.09 (0.84-1.40) | 1.12 (0.91-1.39) | |
| n | 8 | 2 | 3 | 3 | 10 | 10 | ||
| U | EE (95%CI) | 1.78 (1.40-2.26) | 0.95 (0.77-1.16) | 1.18 (0.91-1.52) | 1.92 (1.48-2.50) | 1.52 (1.22-1.91) | 1.79 (1.47-2.19) | |
| n | 9 | 6 | 6 | 6 | 13 | 13 | ||
| All estimates | U | EE (95%CI) | 1.59 (1.37-1.83) | 1.00 (0.88-1.13) | 1.12 (0.97-1.29) | 1.80 (1.48-2.17) | 1.33 (1.18-1.51) | 1.58 (1.39-1.78) |
| n | 15 | 10 | 9 | 9 | 20 | 20 | ||
| Population = with COVID-194 | ||||||||
| Best-adjusted | All | EE (95%CI) | 1.41 (0.93-2.14) | 0.96 (0.92-1.01) | 0.98 (0.72-1.32) | 1.61 (0.79-3.29) | 1.08 (0.82-1.42) | 1.34 (0.96-1.86) |
| n | 7 | 6 | 5 | 5 | 8 | 8 | ||
| C | EE (95%CI) | 0.99 (0.87-1.13) | 0.96 (0.92-1.01) | 0.85 (0.67-1.07) | 1.04 (0.91-1.19) | 0.96 (0.92-1.01) | 0.97 (0.93-1.02) | |
| n | 3 | 4 | 3 | 3 | 4 | 4 | ||
| U | EE (95%CI) | 2.07 (1.12-3.82) | 0.93 (0.60-1.46) | 1.49 (0.94-2.36) | 2.70 (1.11-6.58) | 1.59 (1.40-1.80) | 2.07 (1.12-3.82) | |
| n | 3 | 2 | 2 | 2 | 3 | 3 | ||
| All estimates | U | EE (95%CI) | 2.04 (1.39-3.02) | 1.06 (0.39-2.90) | 1.07 (0.72-1.58) | 2.58 (1.31-5.07) | 1.52 (0.89-2.60) | 2.22 (1.51-3.27) |
| n | 5 | 5 | 4 | 4 | 6 | 6 | ||
Table 6 does not include results where the population studied was those admitted to the ICU, as these form a subset of those reported in Table 7; see the next section.
Results were also available from three studies based on other populations (Supplementary Table 6). OPENSA provided estimates based on the general population, and these seem consistent with the pattern shown in Table 6. For former smoking, for example, an unadjusted estimate of 2.53 [95% confidence interval (CI): 2.43-2.63] reduced to 1.19 (1.14-1.24) after adjustment for comorbidities. Two other studies only reported unadjusted results. PELLAU found no increase in smokers (undefined) when comparing hospitalized patients with and without COVID-19, while GU reported an increased risk of death in former and ever smokers and a decreased risk in current smokers, whether the tested population was considered or whether decedents were compared to unmatched controls.
Not considered above was the KNIGHT study, which provided no effect estimates, merely stating that ever cigarette smoking predicted death from COVID-19.
As shown in Table 7, nine studies reported results on the endpoint worsened or died, based on those with severe disease. Most estimates relate to death among those in the ICU or those requiring mechanical ventilation. There was a tendency for smoking to be positively associated with the endpoint. However, each estimate was unadjusted even for demographic variables.
Two studies reported results for endpoints worse than hospitalization among those tested for COVID-19 (see Supplementary Table 7). Based on estimates adjusted for demographics only, GDEM reported a significant increased risk of pneumonia in current smokers, but no increase in intensive care admissions or need for mechanical ventilation. Similar to the results shown in Supplementary Table 6 for death, and based on unadjusted estimates, GU reported an increased risk of ICU admission in former and ever smokers and a decreased risk in current smokers.
Table 8 compares best-adjusted effect estimates by level of adjustment, effect estimate type, location and study size separately for the indices of smoking closest to current smoking and closest to ever smoking, and for the six combinations of endpoint and population where data were available for at least 10 studies.
| Factor/level | Endpoint | CP | H | IC | MV | S | M |
| Population | T | CP | H | H | H | H | |
| Estimates | 17 | 22 | 14 | 15 | 10 | 25 | |
| Estimates for closest to current smoking | |||||||
| Adjustment | U | 0.55 (0.45-0.67) | 1.44 (1.03-2.01) | 1.16 (1.05-1.28) | 1.16 (0.80-1.67) | 1.17 (0.99-1.37) | 1.52 (1.22-1.91) |
| D | 0.23 (0.09-0.59) | 1.52 (1.18-1.95) | 0.73 (0.56-0.96) | 0.85 (0.67-1.07) | 1.02 (0.89-1.16) | 1.01 (0.87-1.17) | |
| C | 0.58 (0.43-0.80) | 0.96 (0.79-1.18) | 0.81 (0.61-1.07) | 0.99 (0.77-1.29) | 1.23 (0.46-3.27) | 1.09 (0.84-1.40) | |
| PC | - | - | - | - | 0.71 (0.41-1.23) | - | |
| P | NS | < 0.05 | < 0.001 | NS | NS | < 0.05 | |
| Estimate type | OR | 0.54 (0.46-0.63) | 1.26 (0.97-1.64) | 1.07 (0.87-1.32) | 1.13 (0.88-1.44) | 1.09 (0.93-1.27) | 1.35 (1.07-1.70) |
| RR | 0.64 (0.31-1.34) | 1.25 (1.20-1.31) | 0.90 (0.71-1.15) | 0.73 (0.56-0.95) | - | 1.11 (0.87-1.40) | |
| HR | - | 1.10 (0.97-1.24) | - | - | 1.02 (0.64-1.63) | 1.15 (0.89-1.50) | |
| P | NS | NS | NS | < 0.05 | NS | NS | |
| Location | United States | 0.45 (0.37-0.54) | 1.27 (1.11-1.46) | 1.00 (0.81-1.22) | 1.02 (0.83-1.26) | 1.06 (0.72-1.56) | 1.21 (0.95-1.53) |
| Other | 0.64 (0.55-0.73) | 1.11 (0.48-2.57) | 1.08 (0.79-1.49) | 1.05 (0.65-1.71) | 1.10 (1.02-1.17) | 1.34 (1.07-1.69) | |
| P | < 0.01 | NS | NS | NS | NS | NS | |
| Study size | < 500 | - | 0.99 (0.58-1.69) | 1.25 (0.91-1.71) | 1.35 (1.02-1.80) | 1.02 (0.65-1.59) | 1.29 (0.97-1.72) |
| 500- | 0.40 (0.27-0.61) | 1.42 (1.17-1.72) | 1.07 (0.69-1.65) | 0.92 (0.34-2.46) | 1.62 (1.14-2.29) | 1.57 (1.20-2.05) | |
| 5000- | 0.51 (0.39-0.66) | 1.43 (0.97-2.10) | 0.64 (0.29-1.42) | 0.83 (0.70-0.98) | 0.81 (0.56-1.19) | 1.03 (0.83-1.28) | |
| 50,000+ | 0.67 (0.51-0.87) | 1.00 (0.85-1.18) | 0.97 (0.72-1.31) | 0.98 (0.61-1.59) | 1.09 (0.99-1.19) | 0.94 (0.82-1.07) | |
| P | NS | < 0.05 | NS | < 0.05 | NS | < 0.01 | |
| Estimates for closest to ever smoking | |||||||
| Adjustment | U | 0.67 (0.58-0.78) | 1.71 (1.24-2.36) | 1.19 (1.08-1.30) | 1.24 (0.99-1.54) | 1.14 (1.06-1.23) | 1.79 (1.47-2.19) |
| D | 0.23 (0.09-0.59) | 1.41 (1.04-1.92) | 0.73 (0.56-0.96) | 0.85 (0.67-1.07) | 1.02 (0.89-1.16) | 1.11 (0.81-1.53) | |
| C | 0.77 (0.64-0.92) | 0.93 (0.80-1.08) | 1.01 (0.88-1.16) | 1.04 (0.79-1.36) | 1.35 (0.66-2.75) | 1.12 (0.91-1.39) | |
| PC | - | - | - | - | 0.71 (0.41-1.23) | - | |
| P | < 0.05 | < 0.001 | < 0.01 | NS | NS | < 0.01 | |
| Estimate type | OR | 0.67 (0.59-0.76) | 1.39 (1.08-1.79) | 1.16 (0.99-1.37) | 1.14 (0.91-1.42) | 1.10 (0.98-1.24) | 1.53 (1.19-1.96) |
| RR | 0.87 (0.59-1.27) | 1.25 (1.20-1.31) | 1.01 (0.89-1.15) | 1.02 (0.88-1.18) | - | 1.16 (1.05-1.29) | |
| HR | - | 1.03 (0.95-1.11) | - | - | 1.02 (0.64-1.63) | 1.28 (1.14-1.44) | |
| P | NS | < 0.001 | NS | NS | NS | NS | |
| Location | United States | 0.65 (0.50-0.84) | 1.38 (1.14-1.67) | 1.10 (0.99-1.23) | 1.08 (0.90-1.30) | 1.15 (0.87-1.51) | 1.43 (1.11-1.84) |
| Other | 0.74 (0.66-0.82) | 1.31 (0.64-2.71) | 1.08 (0.79-1.49) | 1.05 (0.65-1.71) | 1.10 (1.02-1.17) | 1.43 (1.19-1.73) | |
| P | NS | NS | NS | NS | NS | NS | |
| Study size | < 500 | - | 1.21 (0.84-1.75) | 1.25 (0.93-1.69) | 1.29 (0.95-1.76) | 1.06 (0.74-1.52) | 1.36 (1.05-1.75) |
| 500- | 0.55 (0.37-0.80) | 1.32 (0.99-1.76) | 1.04 (0.92-1.18) | 1.24 (0.77-2.00) | 1.62 (1.14-2.29) | 1.70 (1.34-2.16) | |
| 5000- | 0.64 (0.50-0.81) | 1.71 (1.18-2.49) | 1.48 (1.10-2.00) | 0.83 (0.70-0.98) | 1.00 (0.83-1.22) | 1.27 (0.96-1.68) | |
| 50000+ | 0.81 (0.73-0.89) | 0.97 (0.88-1.07) | 0.97 (0.73-1.28) | 1.10 (0.79-1.52) | 1.09 (0.99-1.19) | 0.94 (0.82-1.07) | |
| P | < 0.05 | < 0.01 | NS | < 0.05 | NS | < 0.001 | |
For level of adjustment, the results echo those summarized above, with adjustment for comorbidities eliminating unadjusted associations of both current and ever smoking with hospitalization within those COVID-19 positive, and with ICU admission and death within those hospitalized for COVID-19.
Significant variation by type of effect estimate was only seen in two of the 12 analyses, and where it was seen may reflect the fact that the unadjusted estimates were typically ORs.
There is no convincing evidence that effect estimates vary by location.
Large studies, involving 50000 or more individuals, showed no significant increases with smoking in any of the analyses shown. Again, higher effect estimates seen in smaller studies may reflect a greater tendency for such studies to report unadjusted results.
Although meta-analyses were attempted by sex, none of them included sex-specific results from more than two studies, and the results are not shown in Table 8. There were even fewer studies reporting results by age, or by sex and age jointly, so meta-analyses by these factors were not attempted.
Only one study, HIPPIS, reported results by amount smoked in current smokers, and only one, TOOLKI, by quit duration in former smokers. Neither study showed a significant dose-response relationship for any of the endpoint/population combinations considered (results not shown).
Table 9 summarizes the results from the meta-analyses of the 10 endpoint/population combinations shown in Tables 1 to 6. Results for the best estimates are shown, where estimates adjusted for comorbidities (and not responses to COVID-19) were preferred, with those adjusted for demographics preferred to unadjusted estimates, and those adjusted for factors including responses to the infection being least preferred. Results are also presented for comorbidity-adjusted estimates and for unadjusted estimates. The results show no consistent evidence of publication bias.
| Endpoint | Population | Smoking index = closest to current smoking | Smoking index = closest to ever smoking | ||||
| Best estimate2 | Comorbidity adjusted3 | Unadjusted4 | Best estimate2 | Comorbidity adjusted3 | Unadjusted4 | ||
| Positive | General | 0.78 (0.57-1.06)5 | 0.96 (0.42-2.17) | 0.56 (0.42-0.75)5 | 0.89 (0.73-1.09) | 1.10 (0.70-1.72) | 0.65 (0.55-0.77) |
| Positive | Tested | 0.55 (0.47-0.65) | 0.58 (0.43-0.80) | 0.55 (0.45-0.67) | 0.70 (0.63-0.77) | 0.77 (0.64-0.92) | 0.67 (0.58-0.78) |
| Hospitalized | Positive | 1.31 (1.12-1.54) | 0.96 (0.79-1.18) | 1.44 (1.03-2.01) | 1.38 (1.17-1.63) | 0.93 (0.80-1.08) | 1.71 (1.24-2.36) |
| ICU admission | Positive | 1.13 (0.82-1.54) | 1.14 (0.54-2.41) | 0.98 (0.60-1.60) | 1.40 (1.09-1.80)5 | 1.30 (0.74-2.31) | 1.42 (0.94-2.14) |
| ICU admission | Hospitalized | 1.02 (0.87-1.21) | 0.81 (0.61-1.07) | 1.16 (1.05-1.28) | 1.11 (0.98-1.25) | 1.01 (0.88-1.16) | 1.19 (1.08-1.30) |
| Mechanically ventilated | Hospitalized | 1.08 (0.86-1.36) | 0.99 (0.77-1.29)5 | 1.16 (0.80-1.67) | 1.11 (0.92-1.35) | 1.04 (0.79-1.36)5 | 1.24 (0.99-1.54) |
| Severe | Positive | 1.10 (0.97-1.25)5 | 1.02 (0.88-1.18) | 1.35 (0.95-1.92) | 1.10 (0.97-1.25)5 | 1.02 (0.88-1.18) | 1.30 (0.94-1.79) |
| Severe | Hospitalized | 1.08 (0.94-1.25) | 1.23 (0.46-3.27) | 1.17 (0.99-1.37) | 1.09 (0.98-1.22) | 1.35 (0.66-2.75) | 1.14 (1.06-1.23) |
| Died | Positive | 1.08 (0.82-1.42) | 0.96 (0.92-1.01) | 1.59 (1.40-1.80) | 1.34 (0.96-1.86) | 0.97 (0.93-1.02) | 2.07 (1.12-3.82) |
| Died | Hospitalized | 1.27 (1.08-1.49)5 | 1.09 (0.84-1.40) | 1.52 (1.22-1.91) | 1.42 (1.22-1.65) | 1.12 (0.91-1.39) | 1.79 (1.47-2.19) |
The clearest result shows that, of those tested for COVID-19, smoking was associated with a reduced risk of positivity (Figures 2 and 3), with less clear evidence of a negative association between smoking and positivity seen among the general population.
In contrast, all the best estimates for the other eight endpoint/population combinations, each of which relate to adverse events in those positive for, or hospitalized with COVID-19, were greater than 1 (ranging from 1.02 to 1.42), with five of the 16 estimates statistically significant (at P < 0.05). However, there was a clear difference between estimates unadjusted for other risk factors, where nine of the 16 estimates were significant, and those adjusted for comorbidities where none were significant and six were below 1.0. This difference is strikingly seen for the most commonly considered endpoint/population combination - died among those hospitalized - where (Figures 4 and 5) unadjusted estimates of 1.52 (95%CI: 1.22-1.91) for the smoking index closest to current smoking and 1.79 (1.47-2.19) for that closest to ever smoking can be contrasted with comorbidity adjusted estimates of, respectively, 1.09 (0.84-1.40) and 1.12 (0.91-1.39).
A major limitation of the available data is that, of the 73 studies which provided effect estimates, adjusted results were available for only 42. In most epidemiological contexts, effect estimates adjusted for age and sex are a basic starting point for analysis, but this was not so here. Given the different age distribution of current, former and never smokers and the strong age relationship to severe COVID-19 and death, unadjusted estimates would seem likely to be biased, as would the analyses which adjusted for variables representing a response to the virus. The most useful analyses were based on estimates adjusted for demographics only, or those adjusted also for comorbidities. These answer different questions. Analyses adjusted for demographics and comorbidities attempted to answer the question “Is a smoker more at risk of COVID-19 related outcome (such as hospitalization, admission to intensive care, undergoing mechanical ventilation or death) than an otherwise equivalent never smoker of the same age, sex and other relevant demographics and health status pre-pandemic?” This is a valid question and is somewhat equivalent to that investigated in a cohort study where smoking, demographics and health status were recorded at baseline, and smoking was related to an outcome occurring during follow-up. In analyses adjusting for demographics only, any increased risk of COVID-19 related outcomes in smokers may be due to their poorer status of health pre-pandemic. While clear answers to both questions would be nice to have, it must be noted that there are very few studies providing effect estimates adjusted only for demographics. Thus, considering deaths in the hospitalized population, only three out of 25 studies with relevant data provided estimates adjusted for demographics only, and none provided comparable unadjusted, demographic adjusted and comorbidity adjusted effect estimates.
Another problem is that some studies provided ORs, some RRs and some HRs. Where results for the relevant 2 × 2 table on exposure × outcome were available, and the ORs and RRs were both estimable, we generally used the OR, using the RR only where the source paper had reported adjusted RRs or HRs. Although we could have used an alternative strategy, it is doubtful whether this would have materially affected our results, given the general consistency of the results by type of effect estimate.
Another possible concern is with the mortality data. Much relates to deaths occurring in patients hospitalized with COVID-19, and many publications implicitly assume all those deaths were due to the virus, when some might have been due to other causes. However, given this proportion is small, it seems probable that this would only result in a minor bias.
More concern relates to the quality of the smoking data. There are two issues here. One is the way smoking was recorded and defined, with eight studies reporting results for smoking undefined, and only 36 distinguishing current and former smoking. Also, only one study reported results by amount smoked, and only one reported results by duration of quit. Furthermore, papers generally did not provide details on the smoking questions asked, or when they were asked.
The other issue is that many studies derived their smoking data from medical records, known to be incomplete and inaccurate[37-39]. While many studies gave no information concerning missing data on smoking, 35 did so, and in 13 the proportion with missing data exceeded 20%, giving concern about the validity of their effect estimates. It would not surprise us to find that, in some studies that did not mention missing data, the “non-smokers” included some individuals never actually asked about their smoking.
Recent publications, particularly by Farsalinos et al[2-4,6-8], have observed that the prevalence of smoking seen in the studies of hospitalized patients was substantially less than reported in national statistics by a factor of four or so, and have suggested that smokers might be protected against getting COVID-19. While the mean percentage of current and former smokers in the studies of hospitalized patients that we considered (current 7.76%, SE 1.00%; ever 33.24%, SE 2.01%) was clearly less than in the studies of the general population (current 15.14%, SE 1.34%; ever 44.0%, SE 1.75%), the difference was only by a factor of 2 or 1.3 rather than about 4. While we also showed a reduced risk in smokers of COVID-19 positivity in those tested (Table 1), the reduction was again much less than the factor reported by Farsalinos et al[6]. Although it is possible that some of the difference between our results and those of Farsalinos et al[6] has arisen as we excluded Asian studies, while their results mainly came from Asia, the fact that two out of the three studies on the general population that we considered found no reduced risk of hospitalization in smokers (SupplementaryTable 3) suggests to us that the low prevalence of smoking seen in hospitalized patients may largely result from incompleteness of data in hospital records, although it would also be consistent with smokers with COVID-19 tending to be less likely to appear for testing or report to hospital.
As noted in the introduction, there were, by the end of September 2020 (the final date of the searches used to produce these results), quite a number of published meta-analyses which relate smoking to adverse events such as deaths or severity of COVID-19[3,6,9-28]. These meta-analyses had various limitations, including little attention to possible data inadequacy, limiting attention to studies of hospitalized patients and considering few, if any, studies conducted outside Asia. They also include paying scant attention to the need for adjusting effect estimates for other risk factors. Many of the studies and meta-analyses we considered demonstrated that dying from COVID-19, for example, was strongly related to various factors associated with smoking, including age, obesity, and a history of respiratory, cardiovascular and other diseases, and yet they attempt to draw conclusions for smoking from unadjusted analyses.
By now, we are aware of further meta-analyses that have been conducted, including those that concentrate on smoking[11,19,29,40,41] and those that consider smoking as one of a list of factors considered[16,20,21,42-55]. While some of the reviews considered far more studies than the earlier reviews, including those in non-Asian populations, the weaknesses seen generally persist. Thus, for example, a recent review of 109 studies[41] limited attention to hospitalized patients, considered only unadjusted effect estimates, hardly mentioned lack of adjustment as a possible weakness, and paid very limited attention to the possibility that smoking data from hospital records may be inadequate.
That meta-analysis[41] described the 109 studies included as being all of moderate or high quality and some of the other reviews also attempted to evaluate study quality. We did not attempt classification of study quality, but given so many studies used medical records as the source of smoking data and failed to present results adjusted even for basic demographics, we doubt very much that we would have considered more than a few studies to be of high or even moderate quality. This view aligns with that of a recent meta-analysis[29] which rejected 201 of 233 studies as being of poor quality, with only one of the studies considered in their meta-analyses considered to be of good quality and the rest classified as fair.
Our meta-analyses have various strengths, including giving careful attention to adjustment, considering many combinations of outcome and population at-risk, and including meta-analyses comparing estimates by factors such as location, study size and type of estimate considered. Weaknesses relate mainly to the poor underlying data quality, much from medical records, and many studies failing to provide adjusted estimates. Almost complete lack of data for males and females separately, and by age group is also a limitation, as is the very limited data on amount smoked or quit duration. Other possible limitations relate to the fact that, with the exception of studies from Israel, we did not consider results from other Asian countries; thus, our conclusions may not necessarily apply to all locations. We also did not consider studies involving less than 100 cases as their results would be less reliable, and studies of patients with specific diseases as their results would not be generalizable.
However, we feel that our meta-analysis provides a good insight into the relationship between smoking and a variety of endpoints relevant to COVID-19.
Based on data from 74 studies conducted in Europe, Israel, America and Australasia, many providing only limited results, there is evidence that, among those tested for COVID-19, smokers are less likely to be positive for the virus. There is also less clear evidence of reduced positivity in smokers in the general population. Among those who are positive for, or hospitalized with, COVID-19 there is a positive association between smoking and both death and severity of COVID-19. This association is most clearly seen for effect estimates unadjusted for other risk factors, and is not evident for estimates adjusted for comorbidities and demographic variables. This suggests that any apparent adverse effect of smoking is due to the poorer prior health status of smokers and that smokers and non-smokers with equivalent demographics and prior health status have a very similar risk of adverse events linked to COVID-19.
Previous meta-analyses relating smoking to coronavirus disease 2019 (COVID-19) are limited by considering few studies, restricting attention to hospitalized patients, giving limited or no attention to the definition of smoking or the reliability of smoking as recorded, and failing to properly consider the effect of adjustment for demographics and comorbidities.
We wished to gain a detailed insight into the effect of smoking on a variety of endpoints in different populations.
To carry out a systematic review, based on epidemiological studies in Europe, Israel, America and Australasia on the relationship of smoking to being tested for COVID-19, being positive for COVID-19, being hospitalized with COVID-19, having severe disease or dying.
Literature searches based on publications in English up to September 30, 2020 identified studies of at least 100 individuals, carried out in Europe, Israel, America and Australasia, and unrestricted to those with specific other diseases, and providing information relating smoking to various COVID-related endpoints. Fixed-effect and random-effects meta-analyses were conducted for combinations of index of smoking, endpoint, population and level of adjustment with heterogeneity studied by level of adjustment, study location, and other factors.
Data were available from 74 studies of highly variable size: 37 in the United States, 10 in the United Kingdom, and up to four elsewhere, with populations most commonly studied being those hospitalized with COVID-19, positive for COVID-19, tested for COVID-19 and the general population. Only 36 studies distinguished current and former smokers, and adjusted results for smoking were only given in 42 studies. Positivity for COVID-19 was reduced among smokers in those tested, but not in the general population. Apparent increases in risk in smokers of hospitalization for COVID-19 among those positive, and of death among those positive and among those hospitalized disappeared following adjustment for pre-existing comorbidities, and there was little evidence of any relationship of smoking with admission to intensive care, being mechanically ventilated or having severe COVID-19, even in the unadjusted results.
There is some evidence that smoking is associated with a reduced risk of being COVID-19 positive. Any apparent adverse effects of smoking on hospitalization rates among those positive, and on death rates seem due to the poorer prior health status of smokers.
Evidence from later studies could consolidate these conclusions, and help to explain why, among those tested for COVID-19, current smokers are less likely to be positive.
We thank Yvonne Cooper for typing various drafts of the paper and obtaining relevant references, and John Fry for comments at various stages.
| 1. | Balabanski LL. An international review of tobacco use and the COVID-19 pandemic: Examining hospitalization, asymptomatic cases, and severity. 2020 Preprint. Available from: medRxiv. [DOI] [Full Text] |
| 2. | Farsalinos K, Barbouni A and Niaura R. Smoking, vaping and hospitalization for COVID-19. 2020 Preprint. Available from: Qeios: Z69O8A.10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Farsalinos K, Poulas K, Polosa R, Barbouni A, Caponnetto P and Niaura R. Prevalence of current smoking and association with adverse outcome in hospitalized COVID-19 Patients: A systematic review and meta-analysis. 2020 Preprint. Available from: Preprint: 2020050113. [DOI] [Full Text] |
| 4. | Farsalinos K, Niaura R, Le Houezec J, Barbouni A, Tsatsakis A, Kouretas D, Vantarakis A, Poulas K. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep. 2020;7:658-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Farsalinos K, Barbouni A, Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med. 2020;15:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 6. | Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11:2040622320935765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Gonzalez-Rubio J, Navarro-Lopez C, Lopez-Najera E, Lopez-Najera A, Jiménez-Díaz L, Navarro-López JD and Nájera A. What is happening with smokers and COVID-19? A Systematic review and a meta-analysis. 2020 Preprint. Available from: Preprints: 2020040540. [DOI] [Full Text] |
| 8. | Tajlil A, Ghaffari S, Pourafkari L, Mashayekhi S, Roshanravan N. Nicotine and smoking in the COVID-19 era. J Cardiovasc Thorac Res. 2020;12:136-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, Quaderi S, Mandal S, Hurst JR. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS One. 2020;15:e0233147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 536] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 10. | Del Sole F, Farcomeni A, Loffredo L, Carnevale R, Menichelli D, Vicario T, Pignatelli P, Pastori D. Features of severe COVID-19: A systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Gülsen A, Yigitbas BA, Uslu B, Drömann D, Kilinc O. The Effect of Smoking on COVID-19 Symptom Severity: Systematic Review and Meta-Analysis. Pulm Med. 2020;2020:7590207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Guo FR. Smoking links to the severity of COVID-19: An update of a meta-analysis. J Med Virol. 2020;92:2304-2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Karanasos A, Aznaouridis K, Latsios G, Synetos A, Plitaria S, Tousoulis D, Toutouzas K. Impact of Smoking Status on Disease Severity and Mortality of Hospitalized Patients With COVID-19 Infection: A Systematic Review and Meta-analysis. Nicotine Tob Res. 2020;22:1657-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Li J, He X, Yuan Yuan, Zhang W, Li X, Zhang Y, Li S, Guan C, Gao Z, Dong G. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2021;49:82-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 16. | Noor FM, Islam MM. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Community Health. 2020;45:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 17. | Patanavanich R, Glantz SA. Smoking is Associated with COVID-19 Progression: A Meta-Analysis. 2020 Preprint. Available from: medRxiv: 20063669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob Res. 2020;22:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 489] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 19. | Patanavanich R, Glantz SA. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: A systematic review and meta-analysis. 2020 Preprint. Available from: medRxiv: 20199802. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Patel U, Malik P, Usman MS, Mehta D, Sharma A, Malik FA, Khan N, Siddiqi TJ, Ahmed J, Patel A, Sacks H. Age-Adjusted Risk Factors Associated with Mortality and Mechanical Ventilation Utilization Amongst COVID-19 Hospitalizations-a Systematic Review and Meta-Analysis. SN Compr Clin Med. 2020;1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Pranata R, Soeroto AY, Huang I, Lim MA, Santoso P, Permana H, Lukito AA. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020;24:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J Med Virol. 2021;93:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 23. | Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: A systematic review and meta-analysis. Respir Med. 2020;171:106096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Simons D, Brown J, Shahab L and Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: A living rapid evidence review. 2020 Preprint. Available from: Qeios: UJR2AW.2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 25. | Simons D, Brown J, Shahab L and Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: A living rapid evidence review 2020 Preprint. Available from: Qeios: UJR2AW.3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Vardavas CI, Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis. 2020;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 751] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 27. | Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, Deng Y, Lin S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. 2020;92:1915-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 465] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 28. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1480] [Article Influence: 246.7] [Reference Citation Analysis (0)] |
| 29. | Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2021;116:1319-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 30. | Israel A, Feldhamer I, Lahad A, Levin-Zamir D and Lavie G. Smoking and the risk of COVID-19 in a large observational population study. 2020 Preprint. Available from: medRxiv. [DOI] [Full Text] |
| 31. | Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 553] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 32. | Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 262] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48457] [Article Influence: 2106.8] [Reference Citation Analysis (4)] |
| 34. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 42451] [Article Influence: 1463.8] [Reference Citation Analysis (4)] |
| 35. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6572] [Article Influence: 1095.3] [Reference Citation Analysis (0)] |
| 36. | Rossato M, Russo L, Mazzocut S, Di Vincenzo A, Fioretto P, Vettor R. Current smoking is not associated with COVID-19. Eur Respir J. 2020;55:2001290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Calhoun PS, Wilson SM, Hertzberg JS, Kirby AC, McDonald SD, Dennis PA, Bastian LA, Dedert EA; VA Mid-Atlantic MIRECC Workgroup, Beckham JC. Validation of Veterans Affairs Electronic Medical Record Smoking Data Among Iraq- and Afghanistan-Era Veterans. J Gen Intern Med. 2017;32:1228-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez-Barradas MC, Sico JJ, Tindle HA, Crothers K. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 39. | Chen LH, Quinn V, Xu L, Gould MK, Jacobsen SJ, Koebnick C, Reynolds K, Hechter RC, Chao CR. The accuracy and trends of smoking history documentation in electronic medical records in a large managed care organization. Subst Use Misuse. 2013;48:731-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Umnuaypornlert A, Kanchanasurakit S, Lucero-Prisno DEI, Saokaew S. Smoking and risk of negative outcomes among COVID-19 patients: A systematic review and meta-analysis. Tob Induc Dis. 2021;19:09. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 41. | Zhang H, Ma S, Han T, Qu G, Cheng C, Uy JP, Shaikh MB, Zhou Q, Song EJ, Sun C. Association of smoking history with severe and critical outcomes in COVID-19 patients: A systemic review and meta-analysis. Eur J Integr Med. 2021;43:101313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLoS One. 2021;16:e0246318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 43. | Chidambaram V, Tun NL, Haque WZ, Majella MG, Sivakumar RK, Kumar A, Hsu AT, Ishak IA, Nur AA, Ayeh SK, Salia EL, Zil-E-Ali A, Saeed MA, Sarena APB, Seth B, Ahmadzada M, Haque EF, Neupane P, Wang KH, Pu TM, Ali SMH, Arshad MA, Wang L, Baksh S, Karakousis PC, Galiatsatos P. Factors associated with disease severity and mortality among patients with COVID-19: A systematic review and meta-analysis. PLoS One. 2020;15:e0241541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 44. | Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15:e0243191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 45. | Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V, Tassara A, Cid C, Catalano HN, Agarwal A, Foroutan F, Rada G. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020;15:e0241955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 416] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 46. | Katzenschlager S, Zimmer AJ, Gottschalk C, Grafeneder J, Seitel A, Maier-Hein L, Benedetti A, Larmann J, Weigand MA, McGrath S, Denkinger CM. Can we predict the severe course of COVID-19 - a systematic review and meta-analysis of indicators of clinical outcome? 2020 Preprint. Available from: medRxiv: 20228858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Mehraeen E, Karimi A, Barzegary A, Vahedi F, Afsahi AM, Dadras O, Moradmand-Badie B, Seyed Alinaghi SA, Jahanfar S. Predictors of mortality in patients with COVID-19-a systematic review. Eur J Integr Med. 2020;40:101226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 48. | Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, Sarriá Cabrera MA, Maffei de Andrade S, Sequí-Dominguez I, Martínez-Vizcaíno V. Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15:e0241742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 49. | Rahman A, Sathi NJ. Risk factors of the severity of COVID-19: A meta-analysis. Int J Clin Pract 2021; 75: e13916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Salah HM, Sharma T, Mehta J. Smoking Doubles the Mortality Risk in COVID-19: A Meta-Analysis of Recent Reports and Potential Mechanisms. Cureus. 2020;12:e10837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Setiati S, Harimurti K, Safitri ED, Ranakusuma RW, Saldi SRF, Azwar MK, Marsigit J, Pitoyo Y, Widyaningsih W. Risk factors and laboratory test results associated with severe illness and mortality in COVID-19 patients: A systematic review. Acta Med Indones. 2020;52:227-245. [PubMed] |
| 52. | Silverio A, Di Maio M, Citro R, Esposito L, Iuliano G, Bellino M, Baldi C, De Luca G, Ciccarelli M, Vecchione C, Galasso G. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc Disord. 2021;21:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 53. | Zhang T, Huang WS, Guan W, Hong Z, Gao J, Gao G, Wu G, Qin YY. Risk factors and predictors associated with the severity of COVID-19 in China: a systematic review, meta-analysis, and meta-regression. J Thorac Dis. 2020;12:7429-7441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Xiang G, Xie L, Chen Z, Hao S, Fu C, Wu Q, Liu X, Li S. Clinical risk factors for mortality of hospitalized patients with COVID-19: systematic review and meta-analysis. Ann Palliat Med. 2021;10:2723-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Xie J, Wang Q, Xu Y, Zhang T, Chen L, Zuo X, Liu J, Huang L, Zhan P, Lv T, Song Y. Clinical characteristics, laboratory abnormalities and CT findings of COVID-19 patients and risk factors of severe disease: a systematic review and meta-analysis. Ann Palliat Med. 2021;10:1928-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Royal Statistical Society, United Kingdom
Specialty type: Infectious diseases
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Samadder S S-Editor: Liu M L-Editor: Webster JR P-Editor: Ma YJ