Published online Sep 18, 2025. doi: 10.13105/wjma.v13.i3.111716
Revised: July 16, 2025
Accepted: August 29, 2025
Published online: September 18, 2025
Processing time: 64 Days and 23.4 Hours
Aortic root dilation, linked to bicuspid aortic valve (BAV) or tricuspid aortic valve (TAV), risks aneurysm and dissection. Valve-sparing aortic root replacement (VSARR) preserves native valves, avoiding prosthetic valve complications. Long-term VSARR durability, especially in BAV patients, is debated. We hypothesize that VSARR outcomes differ between BAV and TAV patients in short-term and long-term settings.
To investigate short-term and long-term outcomes of VSARR in BAV vs TAV patients.
This Preferred Reporting Items for Systematic Reviews and Meta-Analyses-compliant meta-analysis included observational studies comparing VSARR in adult BAV vs TAV patients. PubMed, ScienceDirect, and EMBASE were searched from inception to June 2025. Outcomes included mortality, reintervention, and procedural times. Pooled relative risk (RR) and mean differences (MD) with 95%CI were calculated. Risk of bias was assessed using Risk of Bias in Non-randomized Studies of Interventions; evidence certainty via GRADE.
Thirteen observational studies involving 1419 BAV and 2349 TAV patients were included. In-hospital mortality (RR = 0.34, 95%CI: 0.10–1.14, P = 0.08) and reoperation (RR = 1.04, 95%CI: 0.64–1.69, P = 0.87) showed no significant differences. All-cause mortality risk was significantly lower in BAV patients (RR = 0.34, 95%CI: 0.13–0.86, P = 0.02). Overall reintervention risk was significantly greater in BAV patients (RR = 2.64, 95%CI: 1.96–3.55, P < 0.00001). Aortic cross-clamp (MD = 3.35 minutes, 95%CI: -5.06 to 11.76, P = 0.43) and cardiopulmonary bypass times (MD = 3.96 minutes, 95%CI: -10.26 to 18.18, P = 0.59) showed no significant differences but substantial heterogeneity. The certainty of evidence was moderate for reintervention, low for mortality risk and in-hospital reoperation, and very low for procedural times.
VSARR demonstrates comparable short-term safety between BAV and TAV patients. However, BAV patients face a significantly higher long-term reintervention risk, highlighting the need for tailored strategies and further research.
Core Tip: Valve-sparing aortic root replacement (VSARR) preserves native valves in patients with bicuspid aortic valve (BAV) or tricuspid aortic valve (TAV), reducing prosthetic valve complications. Our meta-analysis reveals comparable short-term safety between BAV and TAV patients but a significantly higher long-term reintervention risk in BAV patients. These findings highlight the need for tailored surgical strategies and vigilant long-term monitoring to optimize outcomes in BAV patients undergoing VSARR.
- Citation: Adugna LF, Asfeha NF, Musa ME, Haile EA, Hailu SZ, Anjulo MT, Tafesse HT, Khan ZH, Hussain A. Comparison of outcomes following valve-sparing aortic root replacement in patients with bicuspid and tricuspid aortic valves: A meta-analysis. World J Meta-Anal 2025; 13(3): 111716
- URL: https://www.wjgnet.com/2308-3840/full/v13/i3/111716.htm
- DOI: https://dx.doi.org/10.13105/wjma.v13.i3.111716
Aortic root dilation, a critical condition often linked to bicuspid aortic valve (BAV) or tricuspid aortic valve (TAV), increases the risk of life-threatening complications such as aortic aneurysm and dissection[1,2]. Surgical intervention is frequently required to prevent catastrophic outcomes, with valve-sparing aortic root replacement (VSARR) gaining prominence as a technique that preserves the native aortic valve[3]. Unlike traditional composite valve conduit pro
The choice of VSARR is particularly relevant for patients with BAV, a common congenital anomaly, and TAV, as both groups present unique anatomical and physiological challenges[7]. While VSARR has been widely adopted, its application in BAV patients remains debated due to concerns about long-term valve durability and the need for reintervention[8]. The growing body of clinical research underscores the importance of comparing outcomes between BAV and TAV patients to refine surgical strategies and improve patient prognosis.
Current VSARR techniques, including the David reimplantation and Yacoub remodeling procedures, aim to stabilize the aortic root while preserving valve function[6]. These approaches have demonstrated comparable short-term outcomes, with low in-hospital mortality and reoperation risk for both BAV and TAV patients[9]. However, challenges persist, particularly in BAV patients, who often require additional leaflet repairs due to cusp prolapse or commissural fusion, which can potentially increase the risk of recurrent aortic regurgitation (AR)[10]. Long-term data suggest that BAV patients may face higher reintervention rates, driven by factors such as aortic stenosis, endocarditis, or progressive valve dysfunction[8]. Additionally, variations in surgical expertise, patient selection, and the use of adjunctive techniques, such as aortic annuloplasty, complicate outcome comparisons, highlighting the need for standardized approaches and robust evidence.
Despite advances in VSARR, the comparative long-term outcomes for BAV vs TAV patients remain incompletely understood. A meta-analysis by Zuo et al[9] provided critical insights, indicating similar perioperative outcomes but a higher long-term reintervention rate in BAV patients. Emerging studies have provided new data on patient characteristics, surgical techniques, and long-term outcomes for VSARR in patients with BAV and TAV. However, these findings have not been systematically synthesized to address unresolved questions about valve durability, reintervention risks, and factors influencing differential outcomes. An updated and comprehensive analysis is needed to clarify these uncertainties and inform clinical practice. This meta-analysis aimed to evaluate the short-term and long-term outcomes of VSARR in BAV compared to TAV patients, integrating recent studies to build a robust evidence base. We seek to identify factors contributing to outcome differences and enhance surgical decision-making for patients with aortic root dilation.
The review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[11] and is registered with PROSPERO (No. CRD420251088958).
We systematically searched PubMed, Cochrane Central Register of Controlled Trials, and EMBASE databases to identify studies comparing outcomes of VSARR in patients with BAV and TAV. The search included all studies published up to June 2025, without language or publication date restrictions. Search terms (“Aortic Valve”[MeSH Terms] OR “valve-sparing aortic root replacement”[Title/Abstract] OR “VSARR”[Title/Abstract] OR “aortic root replacement”[Title/Abstract] OR “David procedure”[Title/Abstract]) AND (“Bicuspid”[Title/Abstract] OR “Bicuspid Aortic Valve”[Title/Abstract] OR “BAV”[Title/Abstract]) AND (“Tricuspid Aortic Valve”[Title/Abstract] OR “TAV”[Title/Abstract]). Additional studies were identified by manually reviewing reference lists of included articles and relevant reviews. Detailed Search String for each database is given in the Supplementary material.
Studies were included if they were observational studies or randomized controlled trials comparing outcomes of VSARR in adult patients (aged ≥ 18 years) with BAV vs TAV, reporting at least one of the following outcomes: (1) In-hospital mortality; (2) Reoperation rate; (3) Long-term mortality; and (4) Freedom from reintervention. Eligible studies involved VSARR techniques, such as reimplantation or remodeling, with or without adjunctive procedures like leaflet repair, and provided quantitative data suitable for meta-analysis, with no restrictions on language or publication date.
Studies were excluded if they were single-arm studies focusing solely on BAV or TAV, non-VSARR interventions, case reports, case series with fewer than 10 patients, editorials, reviews, or studies lacking comparative data or clear outcome reporting for both BAV and TAV groups. Pediatric studies, non-human studies, and duplicate or non-peer-reviewed publications were also excluded.
Studies were selected through a systematic screening process based on predefined inclusion and exclusion criteria. Two independent reviewers (Adugna LF and Asfeha NF) assessed the titles and abstracts of all retrieved studies to identify those meeting the eligibility criteria. Full-text articles of potentially relevant studies were then reviewed to confirm their inclusion. Any discrepancies between the reviewers were resolved through discussion and consensus among the research team. This rigorous selection process ensured the inclusion of only high-quality, relevant studies in the meta-analysis.
Two reviewers (Hailu SZ and Khan ZH) independently extracted data using a standardized form, including study details (author, country, study period, sample size, follow-up duration), patient characteristics [age, male gender, preoperative AR grades, ejection fraction (EF), Marfan syndrome, aortic dissection, prolapse correction], and outcomes. Primary outcomes were in-hospital mortality, in-hospital reoperation, overall mortality, and reintervention rates (overall; short-term: < 1 year; mid-term: 1–5 years; long-term: > 5 years). Secondary outcomes included aortic cross-clamp time (ACX) and cardiopulmonary bypass (CPB) time. Data were collected for the BAV and TAV groups. Discrepancies were resolved by consensus.
The risk of bias assessment for the included non-randomized studies was conducted using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool, which evaluates potential biases across seven domains: (1) Bias due to confounding; (2) Bias in the selection of participants; (3) Bias in classification of interventions; (4) Bias due to deviations from intended interventions; (5) Bias due to missing data; (6) Bias in measurement of outcomes; and (7) Bias in the selection of the reported result. Each study was independently assessed by two reviewers, with discrepancies resolved through discussion or consultation with a third reviewer. The risk of bias for each domain and overall was categorized as low, moderate, serious, critical, or no information[12].
Statistical analyses were conducted using Review Manager version 5.4. Dichotomous outcomes, such as mortality and reoperation rates, were analyzed using relative risk (RR) with 95%CI. Continuous outcomes, including ACX and CPB time, were evaluated through mean differences (MD). Forest plots were created for each outcome to facilitate visual data interpretation and funnel plots were generated to assess potential publication bias. A P value less than 0.05 was considered indicative of statistical significance. Sensitivity analyses, including leave-one-out methods, were conducted to assess the impact of individual studies on heterogeneity and model selection. Subsequently, the quality of evidence for each outcome was appraised using the GRADE approach, which considers factors such as risk of bias, inconsistency, imprecision, indirectness, and effect size to determine the overall certainty of the evidence.
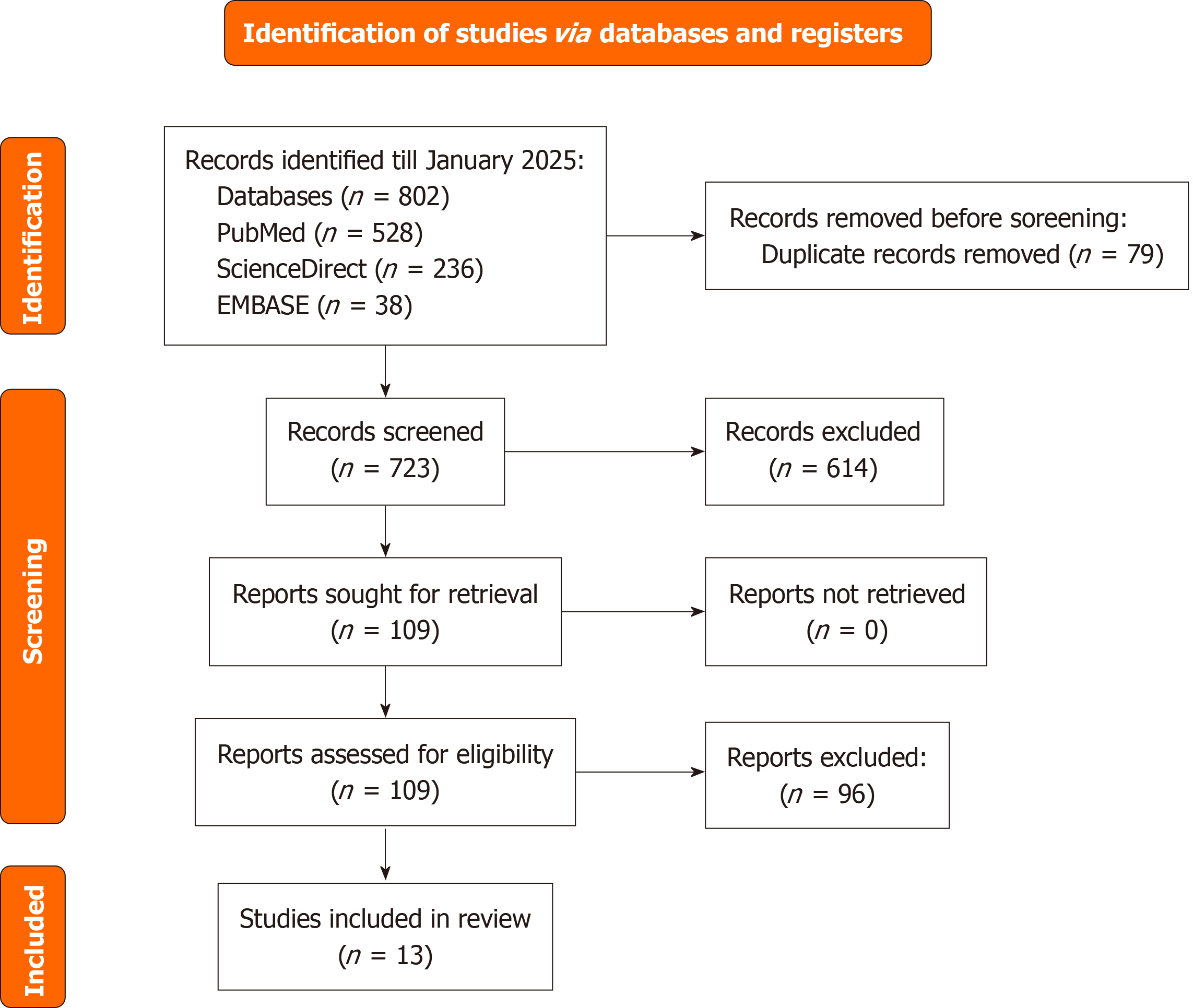
The systematic search of PubMed, ScienceDirect, and EMBASE, conducted up to July 2025, yielded 802 records. After removing 79 duplicates, 723 records underwent title and abstract screening, of which 614 were excluded due to irrelevance, non-human studies, or inappropriate study designs. The full-text review was performed for 109 articles, with 96 excluded for lack of comparative BAV vs TAV data, insufficient outcome reporting, single-arm studies, and duplicate or overlapping data. Ultimately, 13 studies were included in the meta-analysis (Figure 1)[13-25].
The meta-analysis included 13 studies conducted across seven countries (Germany, Spain, Lithuania, the United States, Canada, Italy, and Belgium) from 1988 to 2023, with follow-up durations ranging from 4.1 years to 15 years. A total of 1419 patients with BAV and 2349 with TAV underwent VSARR, with sample sizes ranging from 24 to 414 for BAV and ranging from 63 to 589 for TAV groups. Mean or median age, reported in 10 studies, ranged from 40 years to 54 years for BAV patients and ranged from 36 years to 62 years for TAV patients, with BAV patients generally younger. Male gender, reported in 10 studies, predominated in both groups, with 77.2%–100% of BAV patients and 67.6%–93.1% of TAV patients being male. Three studies (Schäfers et al[14], 2015, Miyahara et al[15], 2020, Malvindi et al[16], 2012) did not report age or gender data. Detailed study characteristics are presented in Table 1[13-25].
| Ref. | Country | Study period | Follow-up (years) | Sample size (n) | Age (years) | Male gender | |||
| BAV | TAV | BAV | TAV | BAV | TAV | ||||
| Aicher et al[25], 2007 | Germany | 1995–2006 | 10 | 81 | 193 | 52 ± 12 | 62 ± 15 | 69 (85.2) | 132 (68.4) |
| Martín et al[19], 2017 | Spain | 2004–2015 | 5 | 57 | 103 | 46.0 ± 11.8 | 57.5 ± 17.8 | 57 (100) | 89 (88.1) |
| Karciauskas et al[21], 2019 | Lithuania | 2004–2016 | 10 | 29 | 63 | 42.4 ± 12 | 55.3 ± 14.9 | 56 (88.9) | 27 (93.1) |
| Schäfers et al[14], 2015 | Spain | 1995–2013 | 15 | 290 | 431 | 54 ± 15 | – | – | – |
| Kvitting et al[17], 2013 | United States | 1993–2009 | 10 | 63 | 170 | 43 ± 12 | 36 ± 13 | 50 (79.4) | 115 (67.6) |
| Bavaria et al[18], 2015 | United States | 2004–2013 | 5 | 40 | 89 | 46 ± 12 | 45 ± 15 | 35 (87.5) | 63 (70.8) |
| Shrestha et al[20], 2018 | Germany | 1993–2015 | 8.7 | 24 | 173 | 40 (30–47) | 49 (35–62) | 21 (87.5) | 123 (71.1) |
| Ouzounian et al[22], 2019 | Canada | 1988–2012 | 8.2 | 45 | 135 | 40 ± 13 | 41 ± 14 | 121 (89.6) | 39 (86.7) |
| Malvindi et al[16], 2012 | Italy | 2002–2011 | 5 | 24 | 108 | – | – | – | – |
| Miyahara et al[15], 2020 | Germany | 1995–2018 | 15 | 414 | 589 | – | – | – | – |
| Mokashi et al[13], 2022 | United States | 2002–2017 | 8 | 71 | 71 | 48 ± 12 | 49 ± 12 | 57 (80.3) | 52 (73.2) |
| François et al[24], 2025 | Belgium | 2000-2023 | 7.9 | 65 | 171 | 41.9 ± 14.4 | 48.7 ± 18.2 | 51 (77.2) | 129 (74.6) |
| Levine et al[23], 2023 | United States | 2005-2020 | 4.1 | 156 | 156 | 45.00 ± 12.72 | 46.00 ± 12.72 | 131 (84) | 133 (85.3) |
The meta-analysis included 13 studies with a total of 1419 BAV and 2349 TAV patients undergoing VSARR, with sample sizes ranging from 24 to 414 for BAV and ranging from 63 to 589 for TAV groups. Preoperative AR data showed a mix of severity grades, with grade I AR reported in 19–224 patients and grade III AR in 20–436 patients across both groups, though reporting was inconsistent. EF, reported in eight studies, ranged from 53.6% ± 7.5% to 88% in BAV patients and ranged from 48.7% ± 10.1% to EF ≥ 60% in TAV patients, indicating generally preserved ventricular function. Marfan syndrome was more prevalent in TAV patients (1–91 cases) than BAV patients (0–60 cases) across seven studies reporting this variable. Aortic dissection was less common, with 0–29 cases in BAV and 0–59 cases in TAV groups (five studies). Prolapse correction was frequently performed, particularly in BAV patients (10–70 cases) compared to TAV patients (3–103 cases) in eight studies. Variability in reporting limited pooled estimates for some characteristics, with detailed data presented in Table 2[13-25].
| Ref. | Sample | Aortic regurgitation grade I | EF (%) | Marfan syndrome | Dissection | Prolapse correction | ||||||||
| BAV | TAV | Grade I | Grade II | Grade III | Grade IV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | |
| Aicher et al[25], 2007 | 81 | 193 | 71 | 64 | 116 | 23 | – | – | 0 | 5 | 6 | 40 | 70 | 103 |
| Martín et al[19], 2017 | 57 | 103 | 224 | 37 | 3 | 3 | 85.40 | ≥ 55 | 107 | 7 | – | – | 47 | 56 |
| Karciauskas et al[21], 2019 | 29 | 63 | 5 | 47 | 38 | 2 | 53.6 ± 7.5 | 48.7 ± 10.1 | – | – | 1 | 6 | 28 | 53 |
| Schäfers et al[14], 2015 | 290 | 431 | 56 | 183 | 436 | 21 | – | – | – | – | 29 | 59 | – | – |
| Kvitting et al[17], 2013 | 63 | 170 | 89 | 64 | 52 | 28 | 62 | 61 | 3 | 91 | 0 | 0 | 42 | 63 |
| Bavaria et al[18], 2015 | 40 | 89 | 19 | 38 | 20 | 28 | 58 ± 9 | 56 ± 10 | 0 | 47 | – | – | 40 | 13 |
| Shrestha et al[20], 2018 | 24 | 173 | 21 | 29 | 50 | 97 | – | – | 60 | – | – | – | 11 | 14 |
| Ouzounian et al[22], 2019 | 45 | 135 | – | – | – | – | 75 | ≥ 60 | 4 | 71 | – | – | 42 | 59 |
| Malvindi et al[16], 2012 | 24 | 108 | – | – | – | – | 88 | > 45 | 5 | 1 | – | – | 10 | 3 |
| Miyahara et al[15], 2020 | 414 | 589 | – | – | – | – | – | – | – | – | – | – | – | – |
| Mokashi et al[13], 2022 | 71 | 71 | 58 | 33 | 29 | 22 | 57 ± 5.4 | 57 ± 5.6 | – | – | 0 | 1 | – | – |
| François et al[24], 2025 | 65 | 171 | – | – | – | – | – | – | 5 (7.6) | 64 (36.9) | 4 (6.1) | 26 (15) | – | – |
| Levine et al[23], 2023 | 156 | 156 | – | – | – | – | 56.33 ± 4.49 | 56.67 ± 3.74 | – | – | – | – | – | – |
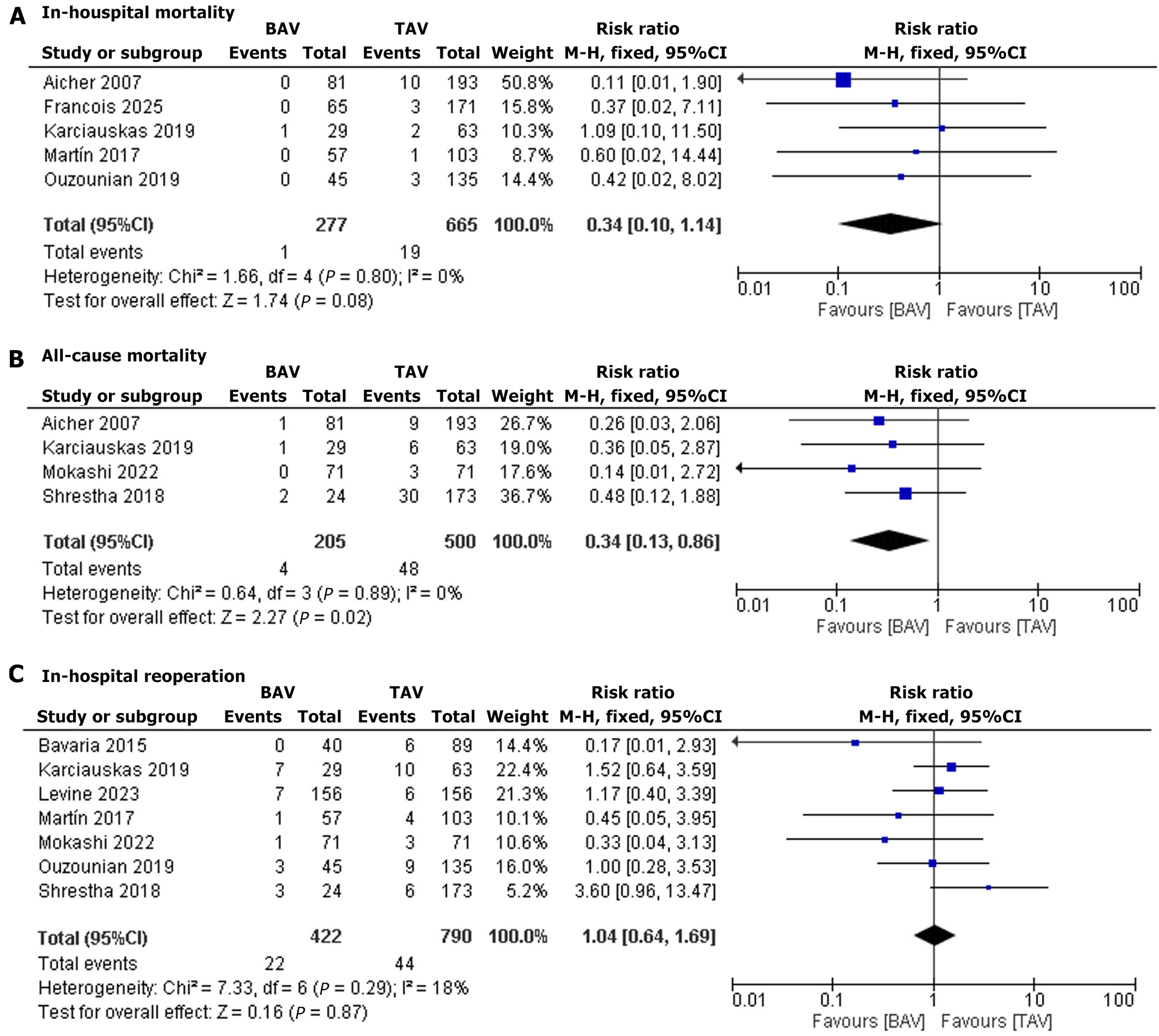
In-hospital mortality: Five studies reported in-hospital mortality. The pooled risk ratio was 0.34 (95%CI: 0.10–1.14, P = 0.08), indicating no significant difference in mortality risk between BAV and TAV patients. Heterogeneity was low (I2 = 0%) (Figure 2A)[19,21,22,24,25].
All-cause mortality: Four studies reported overall mortality. The pooled RR was 0.34 (95%CI: 0.13–0.86, P = 0.02), showing a significantly lower mortality risk in BAV patients compared to TAV patients. Heterogeneity was low (I2 = 0%) (Figure 2B)[13,20,21,25].
In-hospital reoperation: Seven studies reported in-hospital reoperation. The pooled RR was 1.04 (95%CI: 0.64–1.69, P = 0.87), suggesting similar reoperation risks for BAV and TAV patients. Heterogeneity was low (I2 = 18%) (Figure 2C)[13,18-23].
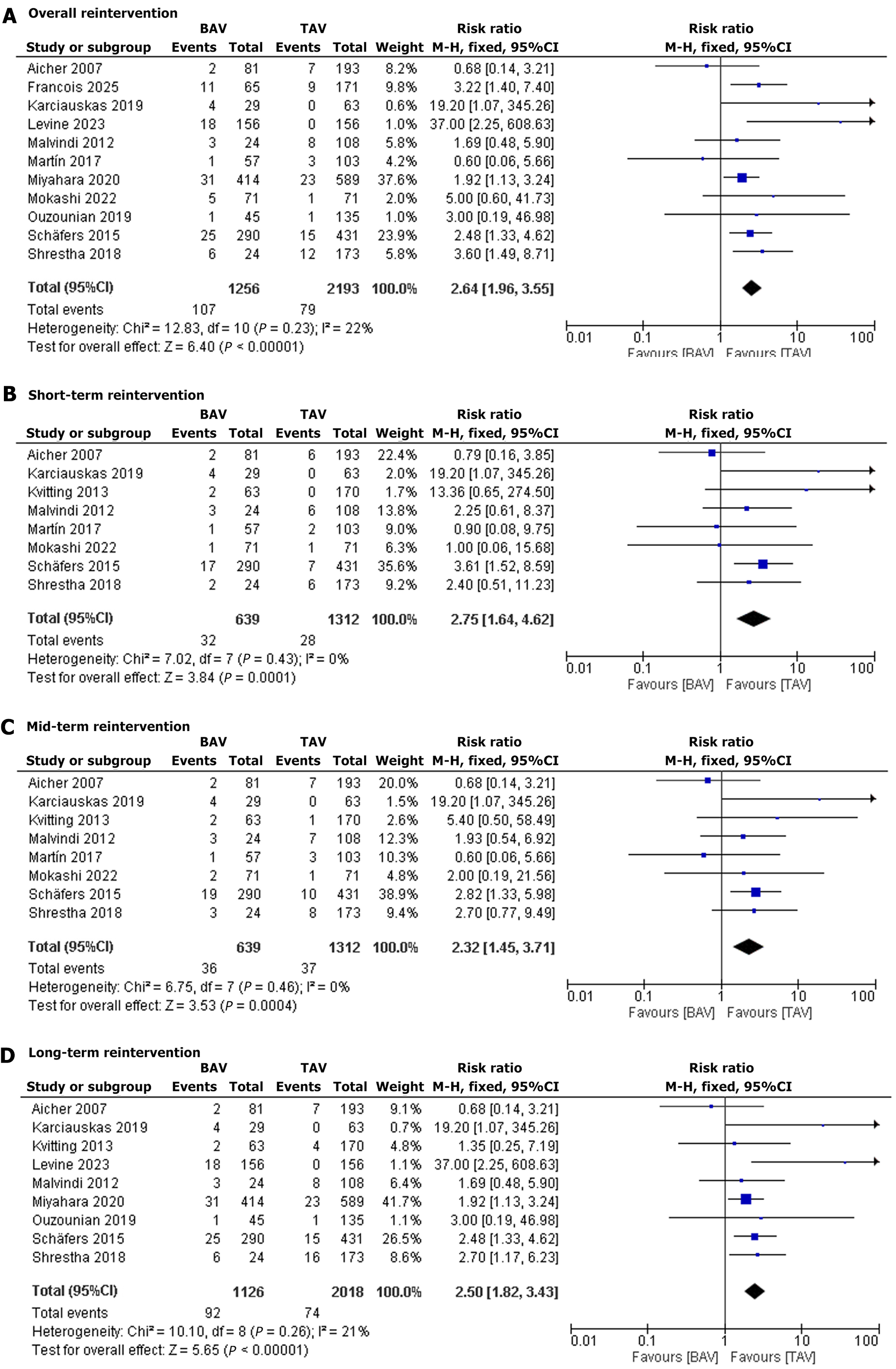
Overall reintervention: Eleven studies reported reintervention events. The pooled RR was 2.64 (95%CI: 1.96–3.55, P < 0.00001), indicating a significantly greater reintervention risk in BAV patients compared to TAV patients. Heterogeneity was low (I2 = 22%) (Figure 3A)[13-16,19-25].
Short-term reintervention: Eight studies reported short-term reintervention. The pooled RR was 2.75 (95%CI: 1.64–4.62, P = 0.0001), showing a significantly increased mortality risk in BAV patients compared to TAV patients. Heterogeneity was low (I2 = 0%) (Figure 3B)[13,14,16,17,19-21,25].
Mid-term reintervention: Eight studies reported mid-term reintervention. The pooled RR was 2.32 (95%CI: 1.45–3.71, P = 0.0004), suggesting a significantly elevated mortality risk in BAV patients compared to TAV patients. Heterogeneity was low (I2 = 0%) (Figure 3C)[13,14,16,17,19-21,25].
Long-term reintervention: Nine studies reported long-term reintervention. The pooled RR was 2.50 (95%CI: 1.82–3.43, P < 0.00001), indicating a significantly higher mortality risk in BAV patients compared to TAV patients. Heterogeneity was low (I2 = 21%) (Figure 3D)[14-17,20-23,25].
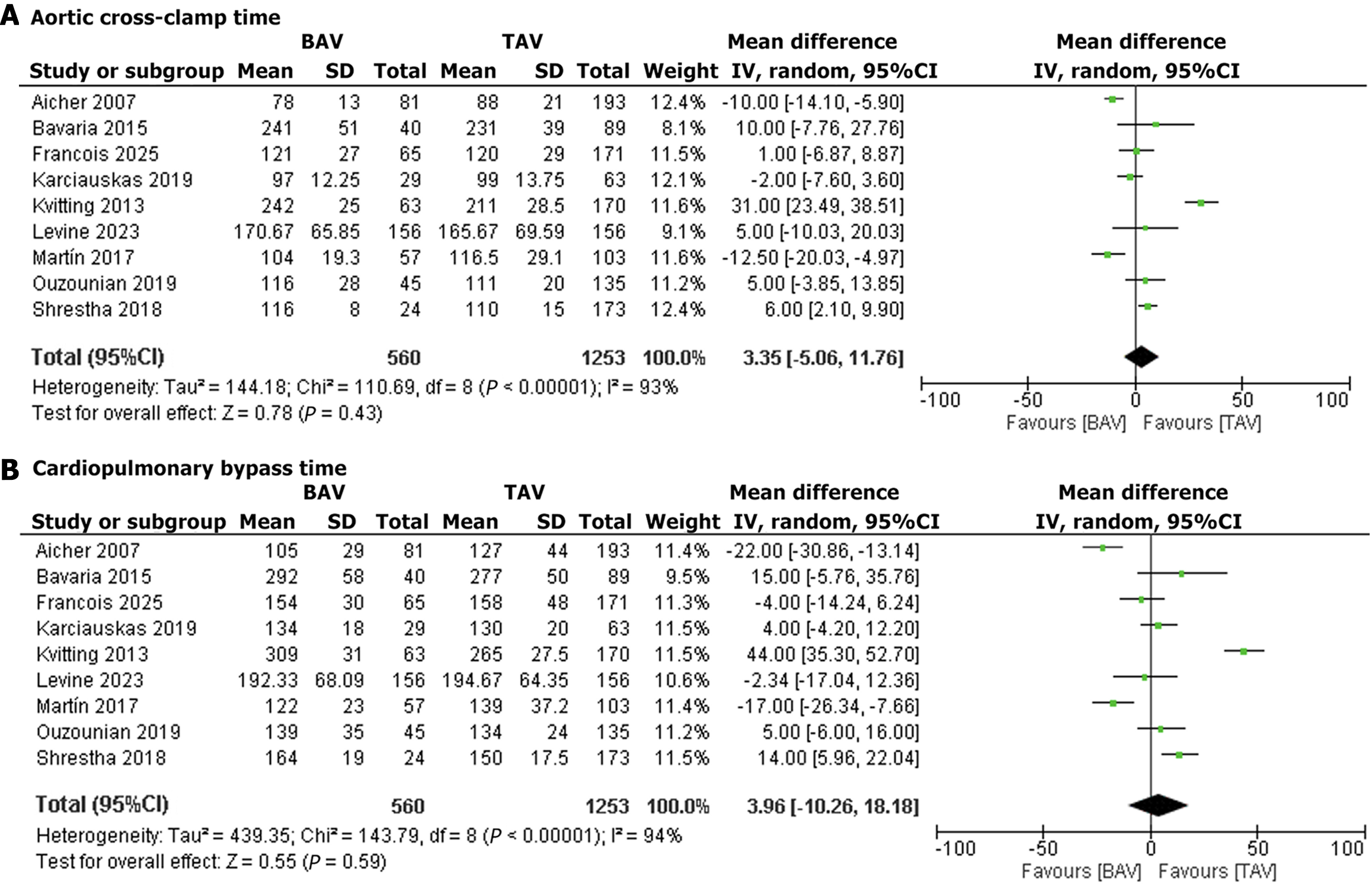
ACX time: Nine studies reported ACX time. The pooled MD was 3.35 minutes (95%CI: -5.06 to 11.76, P = 0.43), suggesting no significant difference in clamp time between BAV and TAV patients. Heterogeneity was substantial (I2 = 93%) that dropped significantly upon exclusion of the Kvitting et al[17], 2013 and Aicher et al[25], 2007 (I2 = 72%) (Figure 4A)[17-25].
CPB time: Nine studies reported CBP time. The pooled MD was 3.96 minutes (95%CI: -10.26 to 18.18, P = 0.59), indicating comparable bypass times for BAV and TAV patients. Heterogeneity was substantial (I2 = 94%), and dropped significantly upon exclusion of the Kvitting et al[17], 2013 and Aicher et al[25], 2007 (I2 = 79%) studies (Figure 4B)[17-25]. Effect sizes for all clinical outcomes in BAV vs TAV patients are summarized in Supplementary Table 1.
The ROBINS-I quality assessment revealed that the 13 included studies generally exhibited low-to-moderate risk of bias, supporting the reliability of the meta-analysis findings on VSARR outcomes in BAV and TAV patients. Five studies were rated as low risk overall, indicating robust study design and minimal bias, while six had moderate risk, primarily due to potential confounding or incomplete outcome reporting. Two studies, both older with smaller cohorts, were rated as serious risk, largely driven by missing data, which may limit their contribution to long-term outcome estimates. Detailed assessments are visualized in Supplementary Figures 1 and 2[13-25].
A total of nine outcomes were assessed across multiple observational studies, with the number of studies ranging from 4 to 11 for each outcome. While the risk of bias was not serious for most outcomes, one outcome (all-cause mortality) was downgraded due to a serious risk of bias in a contributing study.
Outcomes such as overall, short-term, mid-term, and long-term reintervention demonstrated moderate certainty of evidence, consistently indicating a significantly increased risk. Conversely, in-hospital mortality, all-cause mortality, and in-hospital reoperation showed low certainty of the evidence, primarily due to serious imprecision (wide confidence intervals crossing the null effect) or identified risk of bias. Finally, procedural times, specifically ACX time and CPB time, were associated with very low certainty of evidence, largely driven by substantial heterogeneity and serious imprecision, suggesting no significant difference between groups (Supplementary Table 2).
Publication bias was assessed using funnel plots for all outcomes. No evidence of publication bias was observed for in-hospital mortality, all-cause mortality, in-hospital reoperation, overall reintervention, short-term reintervention, mid-term reintervention, and long-term reintervention. ACX time and CPB time showed publication bias, indicated by asymmetrical funnel plots with gaps in the lower left quadrant, suggesting potential underrepresentation of smaller studies with non-significant or negative results (Supplementary Figure 3).
This meta-analysis provides comprehensive evidence regarding the comparative outcomes of VSARR between patients with BAV and TAV, revealing important insights that should inform clinical decision-making and patient counseling. The findings demonstrate comparable in-hospital mortality risk between BAV and TAV patients undergoing VSARR, indicating similar short-term safety profiles[9]. However, a significantly lower long-term mortality risk was observed in BAV patients, likely reflecting their younger age profile at the time of surgery[9]. The most clinically significant finding is the substantially higher reintervention risk in BAV patients, which persisted across short-term, mid-term, and long-term intervals. This increased reintervention rate can be attributed to several anatomical and pathophysiological factors unique to bicuspid valves, including asymmetric leaflet configuration, altered commissural anatomy, and frequent requirement for concomitant cusp repair[26,27]. The mechanism of failure also differs between valve types, with reintervention in BAV patients more likely due to progressive calcification and aortic stenosis, while TAV patients typically require reintervention for aortic insufficiency[26]. BAV patients often require additional procedures such as cusp plication (34% of cases) and commissure repair (8% of cases), which may contribute to the increased long-term failure rate[27].
The analysis found comparable procedural complexity between BAV and TAV patients, as evidenced by similar ACX and CPB times, although substantial heterogeneity was observed for these outcomes. This finding contrasts with historical concerns about increased technical difficulty in BAV repair, suggesting that modern surgical techniques and standardized repair protocols have helped standardize the approach to bicuspid valve preservation[28,29]. The comparable operative times indicate that experienced centers can achieve similar technical efficiency regardless of valve morphology, though the significant heterogeneity suggests variation in surgical techniques and institutional practices across centers. These findings have direct implications for patient selection and preoperative counseling, as the significantly higher reintervention risk in BAV patients must be carefully discussed with patients and families, particularly for younger BAV patients who have longer life expectancies and may face multiple reinterventions over their lifetimes[9,26,27].
The decision-making process should incorporate several factors beyond valve morphology, including patient age, life expectancy, and individual risk tolerance for reintervention[30,31]. For very young BAV patients (< 40 years), the higher reintervention risk may still be acceptable due to the decades of anticoagulation avoidance provided by valve preservation[30,32]. The results emphasize the critical importance of surgical expertise and center volume in achieving optimal outcomes with VSARR procedures, as studies have consistently demonstrated that the best results are achieved at high-volume centers with dedicated aortic valve specialists[33,34]. The United States National Marfan Foundation has established benchmarks requiring operative mortality < 1% and 10-year freedom from valve reoperation > 90% for centers performing these procedures, and this meta-analysis supports these recommendations, particularly for BAV patients, where the technical challenges are greater[35]. Centers should carefully evaluate their outcomes and consider referring complex BAV cases to specialized aortic centers if their reintervention rates exceed acceptable thresholds[30,33].
The higher reintervention risk in BAV patients necessitates enhanced long-term surveillance protocols, with current guidelines recommending annual echocardiographic evaluation; however, the timing and intensity of follow-up may need to be individualized based on valve morphology[26,34]. BAV patients may benefit from more frequent imaging, particularly beyond the 5-year mark when their reintervention risk appears to accelerate[32]. Recent advances in cardiac imaging, including four-dimensional (4D) flow magnetic resonance imaging (MRI) and advanced echocardiographic techniques, may help identify early signs of valve deterioration before clinical symptoms develop. The findings of this analysis are largely consistent with previous systematic reviews and meta-analyses examining VSARR outcomes, including a recent comprehensive meta-analysis by Zuo et al[9], similarly reporting no significant differences in early mortality risk but identifying trends toward higher reintervention rates in BAV patients. However, the current analysis provides more robust evidence with larger patient numbers and longer follow-up periods, and the observed reintervention rates align closely with large single-center series[24,25].
The patterns observed in this VSARR meta-analysis differ from those reported in surgical aortic valve replacement literature, where studies of prosthetic valve replacement generally show comparable long-term outcomes between BAV and TAV patients when modern prostheses are used[36,37]. This suggests that the anatomical complexity of bicuspid valves poses greater challenges for repair techniques than for replacement procedures, highlighting the importance of individualized treatment algorithms that consider not only valve morphology but also the specific pathology present[36]. While valve replacement may neutralize the anatomical disadvantages of bicuspid valves, repair techniques must work within the constraints of the existing anatomy[38]. The substantial heterogeneity observed in procedural time outcomes (e.g., ACX time: I2 = 93%; CPB time: I2 = 94%) in this meta-analysis likely stems from multiple sources, including variations in surgical technique, patient anatomy, institutional experience, and the learning curve associated with BAV-specific repairs. Differences in surgical approaches, such as the choice of annuloplasty techniques or valve-sparing procedures, may contribute significantly to variability in procedural times, as BAV repairs often require tailored strategies to address complex anatomical variations like bicuspid valve morphology or asymmetric commissural orientation. Patient-specific factors, such as aortic root dimensions or leaflet calcification, may further complicate repairs and prolong operative times. Additionally, institutional experience and surgeon familiarity with BAV-specific techniques likely play a critical role, as centers with higher case volumes may achieve more consistent outcomes due to refined protocols and expertise. The learning curve for BAV repairs, which demand precise intraoperative assessments like effective height measurement or commissural orientation analysis, may also contribute to heterogeneity, particularly in less experienced centers. To address these challenges, standardized surgical approaches and outcome reporting are essential. The AVIATOR Registry, supported by the Heart Valve Society, represents a pivotal step toward achieving this goal by collecting homogeneous data across multiple centers[39,40]. Future research should focus on identifying optimal surgical techniques for different anatomical variants, establishing evidence-based selection criteria, and promoting the universal adoption of objective assessment tools, such as effective height measurement and systematic commissural orientation analysis, to enhance repair quality, durability, and consistency across institutions.
The findings support the use of VSARR as a safe option for both BAV and TAV patients in experienced centers, offering the benefit of avoiding prosthetic valve complications, such as anticoagulation-related risks and thromboembolism[3,4]. However, the higher reintervention risk in BAV patients necessitates meticulous patient selection and detailed preoperative counseling, particularly for younger patients who may prioritize anticoagulation avoidance but face a greater likelihood of future surgeries[30,32]. Surgeons should clearly communicate the trade-offs between VSARR and valve replacement, emphasizing the need for long-term monitoring, especially in BAV patients. Referral to high-volume centers with specialized aortic valve expertise is recommended for complex BAV cases to optimize outcomes. Enhanced surveillance protocols, including more frequent echocardiographic evaluations for BAV patients beyond five years, are critical to detect early valve deterioration and guide timely interventions.
This meta-analysis has some limitations. First, the included studies were only observational, with no randomized controlled trials available, introducing potential confounding and selection bias that may affect the validity of the findings, despite the low-to-moderate risk of bias in most included studies. Second, substantial heterogeneity in procedural time outcomes reflects variability in surgical techniques, institutional practices, and reporting, which limits the precision of these findings. Third, incomplete reporting of patient characteristics, such as preoperative AR severity and comorbidities, as well as unmeasured anatomical variables like leaflet symmetry, commissural orientation, and aortic root dimensions, which can significantly influence clinical outcomes, precluded comprehensive pooled analyses of potential confounders. Fourth, the predominance of data from high-volume centers may reduce generalizability to less specialized settings. Finally, evidence of publication bias in procedural time outcomes suggests possible underrepresentation of smaller studies with non-significant results, which may affect the robustness of these findings.
Future research should prioritize standardizing VSARR techniques to minimize heterogeneity and enhance outcomes, particularly for BAV patients. Prospective, multicenter studies with uniform outcome reporting, such as those supported by the AVIATOR Registry, are essential to elucidate long-term valve durability and refine patient selection criteria[39,40]. Exploring the utility of advanced imaging modalities, such as 4D flow MRI, could improve early detection of valve deterioration and inform personalized surveillance strategies[26]. Comparative studies of VSARR vs valve replacement in BAV patients across diverse populations and surgical settings are needed to develop evidence-based treatment algorithms. Additionally, investigating innovative repair techniques, such as advanced annuloplasty or tissue-engineered grafts, may improve the durability of BAV repairs[28,29].
This meta-analysis of 13 studies, encompassing 1419 BAV and 2349 TAV patients, establishes VSARR as a safe and viable option for both valve morphologies, with comparable short-term mortality and procedural complexity. However, the significantly higher long-term reintervention risk in BAV patients highlights the need for tailored surgical strategies, rigorous long-term surveillance, and referral to specialized centers. These findings emphasize the importance of individualized decision-making and underscore the necessity for standardized techniques and further research to optimize outcomes in BAV patients undergoing VSARR.
| 1. | Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bossé Y, Limongelli G, Bossone E, Benson DW, Lancellotti P, Isselbacher EM, Enriquez-Sarano M, Sundt TM 3rd, Pibarot P, Evangelista A, Milewicz DM, Body SC; BAVCon Investigators. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129:2691-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, John Manolis A, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, von Allmen RS, Vrints CJ; Authors/Task Force members. Corrigendum to: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2015;36:2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | David TE. Aortic valve sparing operations: a review. Korean J Thorac Cardiovasc Surg. 2012;45:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax. 1968;23:338-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 940] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Castrovinci S, Tian DH, Murana G, Cefarelli M, Berretta P, Alfonsi J, Yan TD, Di Bartolomeo R, Di Eusanio M. Aortic Root Replacement With Biological Valved Conduits. Ann Thorac Surg. 2015;100:337-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617-21; discussion 622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 778] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 553] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Attinger-Toller A, Bhindi R, Perlman GY, Murdoch D, Weir-McCall J, Blanke P, Barbanti M, Sathananthan J, Ruile P, Gandolfo C, Saia F, Nietlispach F, Wood D, Leipsic J, Webb JG. Mid-term outcome in patients with bicuspid aortic valve stenosis following transcatheter aortic valve replacement with a current generation device: A multicenter study. Catheter Cardiovasc Interv. 2020;95:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Zuo Y, Tan R, Qin C. Outcomes of valve-sparing aortic root replacement in patients with bicuspid aortic valve and tricuspid aortic valve: a systematic review and meta-analysis. J Cardiothorac Surg. 2023;18:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Matalanis G, Shi WY, Hayward PA. Correction of leaflet prolapse extends the spectrum of patients suitable for valve-sparing aortic root replacement. Eur J Cardiothorac Surg. 2010;37:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50909] [Article Influence: 10181.8] [Reference Citation Analysis (2)] |
| 12. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12470] [Article Influence: 1247.0] [Reference Citation Analysis (2)] |
| 13. | Mokashi SA, Rosinski BF, Desai MY, Griffin BP, Hammer DF, Kalahasti V, Johnston DR, Rajeswaran J, Roselli EE, Blackstone EH, Svensson LG. Aortic root replacement with bicuspid valve reimplantation: Are outcomes and valve durability comparable to those of tricuspid valve reimplantation? J Thorac Cardiovasc Surg. 2022;163:51-63.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Schäfers HJ, Raddatz A, Schmied W, Takahashi H, Miura Y, Kunihara T, Aicher D. Reexamining remodeling. J Thorac Cardiovasc Surg. 2015;149:S30-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Miyahara S, Karliova I, Giebels C, Schneider U, Matsushima S, Schäfers HJ. Aortic root remodeling in bicuspid and tricuspid aortic valves-long-term results. Indian J Thorac Cardiovasc Surg. 2020;36:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Malvindi PG, Raffa GM, Basciu A, Citterio E, Cappai A, Ornaghi D, Tarelli G, Settepani F. Bicuspidy does not affect reoperation risk following aortic valve reimplantation. Interact Cardiovasc Thorac Surg. 2012;14:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kvitting JP, Kari FA, Fischbein MP, Liang DH, Beraud AS, Stephens EH, Mitchell RS, Miller DC. David valve-sparing aortic root replacement: equivalent mid-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg. 2013;145:117-126, 127.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Bavaria JE, Desai N, Szeto WY, Komlo C, Rhode T, Wallen T, Vallabhajosyula P. Valve-sparing root reimplantation and leaflet repair in a bicuspid aortic valve: comparison with the 3-cusp David procedure. J Thorac Cardiovasc Surg. 2015;149:S22-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Martín CE, García Montero C, Serrano SF, González A, Mingo S, Moñivas V, Centeno J, Forteza A. The influence of Marfans and bicuspid valves on outcomes following aortic valve reimplantation. J Card Surg. 2017;32:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Shrestha ML, Beckmann E, Abd Alhadi F, Krueger H, Meyer-Bockenkamp F, Bertele S, Koigeldiyev N, Kaufeld T, Fleissner F, Korte W, Schmitto J, Cebotari S, Harringer W, Haverich A, Martens A. Elective David I Procedure Has Excellent Long-Term Results: 20-Year Single-Center Experience. Ann Thorac Surg. 2018;105:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Karciauskas D, Mizariene V, Jakuska P, Ereminiene E, Orda P, Ordiene R, Vaskelyte JJ, Nedzelskiene I, Kinduris S, Benetis R. Early and long-term results of aortic valve sparing aortic root reimplantation surgery for bicuspid and tricuspid aortic valves. Perfusion. 2019;34:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Ouzounian M, Feindel CM, Manlhiot C, David C, David TE. Valve-sparing root replacement in patients with bicuspid versus tricuspid aortic valves. J Thorac Cardiovasc Surg. 2019;158:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Levine D, Patel P, Zhao Y, Childress P, Chung M, Leshnower BG, Kurlansky P, Smith CR, Chen EP, Takayama H. Bicuspid aortic valve durability with valve-sparing aortic root replacement: comparison to tricuspid valve. Eur J Cardiothorac Surg. 2023;63:ezad030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 24. | François K, Claus I, Verlinden J, De Backer J, Martens T, Czapla J, Philipsen T, Bové T. Valve-Sparing Root Replacement in Bicuspid and Tricuspid Aortic Valves: Long-Term Outcomes Into the Second Decade. Ann Thorac Surg. 2025;S0003-4975(25)00447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Aicher D, Urbich C, Zeiher A, Dimmeler S, Schäfers HJ. Endothelial nitric oxide synthase in bicuspid aortic valve disease. Ann Thorac Surg. 2007;83:1290-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Beckmann E, Martens A, Krüger H, Korte W, Kaufeld T, Stettinger A, Haverich A, Shrestha ML. Aortic valve-sparing root replacement in patients with bicuspid aortic valve: long-term outcome with the David I procedure over 20 years. Eur J Cardiothorac Surg. 2020;58:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Marom G, Weltert LP, Raanani E, Chirirchilli I, Giebels C, Irace FG, De Paulis R, Schäfers HJ. Systematic adjustment of root dimensions to cusp size in aortic valve repair: a computer simulation. Interdiscip Cardiovasc Thorac Surg. 2024;38:ivae024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Lansac E, de Kerchove L. Aortic valve repair techniques: state of the art. Eur J Cardiothorac Surg. 2018;53:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Patlolla SH, Saran N, Dearani JA, Stulak JM, Schaff HV, Greason KL, Daly RC, King KS, Pochettino AB. Outcomes and risk factors of late failure of valve-sparing aortic root replacement. J Thorac Cardiovasc Surg. 2022;164:493-501.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Arnaoutakis GJ, Sultan I, Siki M, Bavaria JE. Bicuspid aortic valve repair: systematic review on long-term outcomes. Ann Cardiothorac Surg. 2019;8:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Schneider U, Hofmann C, Schöpe J, Niewald AK, Giebels C, Karliova I, Schäfers HJ. Long-term Results of Differentiated Anatomic Reconstruction of Bicuspid Aortic Valves. JAMA Cardiol. 2020;5:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Tran A, Shih E, Harrington KB, Schaffer JM, Banwait JK, Wang Z, DiMaio JM, Mack MJ, Ryan WH, Brinkman WT; Baylor Scott & White Aortic Root Analysis Working Group. Midterm durability of valve-sparing root replacement in bicuspid and tricuspid aortic valves. Proc (Bayl Univ Med Cent). 2024;37:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Liebrich M, Kruszynski MK, Roser D, Meisner C, Doll KN, Hemmer WB, Weimar T. The David procedure in different valve pathologies: a single-center experience in 236 patients. Ann Thorac Surg. 2013;95:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Schneider U, Feldner SK, Hofmann C, Schöpe J, Wagenpfeil S, Giebels C, Schäfers HJ. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg. 2017;153:S65-S71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 35. | Halim SA, Edwards FH, Dai D, Li Z, Mack MJ, Holmes DR, Tuzcu EM, Thourani VH, Harrison JK, Brennan JM. Outcomes of Transcatheter Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Disease: A Report From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2020;141:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 36. | Yoon SH, Bleiziffer S, De Backer O, Delgado V, Arai T, Ziegelmueller J, Barbanti M, Sharma R, Perlman GY, Khalique OK, Holy EW, Saraf S, Deuschl F, Fujita B, Ruile P, Neumann FJ, Pache G, Takahashi M, Kaneko H, Schmidt T, Ohno Y, Schofer N, Kong WKF, Tay E, Sugiyama D, Kawamori H, Maeno Y, Abramowitz Y, Chakravarty T, Nakamura M, Kuwata S, Yong G, Kao HL, Lee M, Kim HS, Modine T, Wong SC, Bedgoni F, Testa L, Teiger E, Butter C, Ensminger SM, Schaefer U, Dvir D, Blanke P, Leipsic J, Nietlispach F, Abdel-Wahab M, Chevalier B, Tamburino C, Hildick-Smith D, Whisenant BK, Park SJ, Colombo A, Latib A, Kodali SK, Bax JJ, Søndergaard L, Webb JG, Lefèvre T, Leon MB, Makkar R. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol. 2017;69:2579-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 37. | Schneider U, Karliova I, Giebels C, Ehrlich T, Schäfers H. Concepts and techniques of bicuspid aortic valve repair. J Vis Surg. 2020;6:3-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Marom G, Haj-Ali R, Rosenfeld M, Schäfers HJ, Raanani E. Aortic root numeric model: annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg. 2013;145:406-411.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Chauvette V, Kluin J, de Kerchove L, El Khoury G, Schäfers HJ, Lansac E, El-Hamamsy I. Outcomes of valve-sparing surgery in heritable aortic disorders: results from the AVIATOR registry. Eur J Cardiothorac Surg. 2022;62:ezac366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | de Heer F, Lansac E, El-Hamamsy I, Pibarot P, De Kerchove L, El Khoury G, Schäfers HJ, Takkenberg JJM, Kluin J. The AVIATOR registry: the importance of evaluating long-term patient outcomes. Ann Cardiothorac Surg. 2019;8:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/