Published online Sep 18, 2025. doi: 10.13105/wjma.v13.i3.108018
Revised: June 16, 2025
Accepted: August 12, 2025
Published online: September 18, 2025
Processing time: 160 Days and 4.5 Hours
Hepatic encephalopathy (HE) remains a significant neuropsychiatric complication of liver disease, characterized by a spectrum of cognitive, behavioral, and motor impairments. Recent advances in the understanding of its pathophysiology have identified inflammation and gut-liver-brain axis interactions along with conventional theories on ammonia toxicity. Rifaximin and lactulose continue to be the first-line therapies. However, novel approaches like ammonia-lowering agents such as glycerol phenylbutyrate, ornithine phenylacetate, zinc and L-ornithine L-aspartate, gut microbiota modulation by probiotics, fecal microbiota transplan
Core Tip: The review article explores the current approach of using the past and recent articles and studies found and highlighted with the use of Medical Subject Headings terminologies in platforms such as PubMed Central, Google Scholar, Scopus, Web of Science, and Research Gate.
- Citation: Ashwin W, Deva R, Girish C. Paradigm shifts in hepatic encephalopathy: Review of recent therapeutic breakthroughs. World J Meta-Anal 2025; 13(3): 108018
- URL: https://www.wjgnet.com/2308-3840/full/v13/i3/108018.htm
- DOI: https://dx.doi.org/10.13105/wjma.v13.i3.108018
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome occurring due to liver insufficiency and/or portosystemic shunting, with a spectrum of manifestations ranging from subclinical neurological alterations to severe coma. This condition is commonly associated with chronic liver diseases such as alcohol-related liver disease, nonalcoholic fatty liver disease, viral hepatitis, and primary biliary cholangitis[1]. In patients with cirrhosis, this continues to be a major cause of hospitalizations, readmissions, and mortality, resulting in a substantial economic and healthcare burden[2].
HE is classified into three types based on its etiology: Type A (associated with acute liver failure), Type B (caused by portosystemic shunts without underlying liver disease), and Type C (associated with cirrhosis). The condition is further categorized by severity into minimal HE (MHE), overt HE (grades I–IV), and by its time course as episodic, recurrent, or persistent MHE, although subclinical, profoundly impacts quality of life and cognitive function, whereas overt HE serves as a critical indicator of decompensated cirrhosis, often indicating a poor prognosis[3].
Recent advances have significantly deepened our understanding of the pathophysiology of HE, revealing a complex interaction of factors including ammonia toxicity, systemic and neuroinflammation, oxidative stress, blood-brain barrier dysfunction, disruptions in cerebral metabolism, neurotransmission, and cellular communication. Despite its historical classification as a reversible syndrome, emerging evidence suggests that HE episodes can cause irreversible damage due to neuroinflammation and neuronal cell death, which may potentially lead to persistent neurological deficits even after liver transplantation[4].
The treatment landscape of HE has evolved significantly since the publication of the American Association for the Study of Liver Diseases-European Association for the Study of the Liver clinical guidelines in 2014[3]. Ongoing research into interorgan ammonia metabolism and the role of systemic inflammation in HE has identified novel therapeutic targets and strategies. This review provides a comprehensive overview of the recent breakthroughs in HE treatment. By exploring these developments, we shed light on the evolving therapeutic approaches and highlight avenues for future research in managing this challenging condition.
Ammonia, a neurotoxic byproduct of bacterial urease activity, protein digestion[5], and amino acid deamination, is normally detoxified by the liver through the urea cycle and conversion to glutamine[6]. In liver diseases like cirrhosis, the liver's ability to detoxify ammonia is reduced, leading to hyperammonemia, which contributes to astrocyte swelling, brain edema, and altered neurotransmission due to increased intracellular glutamine and osmolarity. The body tries to compensate by using skeletal muscles to remove ammonia, which is often insufficient. This cycle of rising ammonia levels and increased glutamine production worsens HE and affects muscle and brain function[7].
Neuroinflammation is a critical component in the pathogenesis of HE, with brain cell damage acting both as a result and a contributing factor[8]. Astroglial cells during inflammatory conditions release tumor necrosis factor alpha (TNF-α), followed by glutamate, while also initiating microglial activation[9] leading to the proliferation and the release of pro-inflammatory cytokines, including TNF-α, interleukin 1 beta (IL-1β), and IL-6, which collectively contribute to neuronal death in both in vitro and in vivo models[10]. This inflammatory cascade is often triggered by systemic inflammation associated with alterations in the gut-liver-brain axis[9].
Evidence from clinical and experimental studies indicates that hyperammonemia induces HE only in the presence of systemic inflammatory response syndrome[8,10]. The CANONIC study underscores the association among systemic inflammation, HE severity, and mortality in advanced cases[8]. At the cellular level, TNF-α exposure increases the capacity of human cerebrovascular endothelial cells to transport ammonia, while astroglial cells exposed to ammonia and recombinant pro-inflammatory cytokines exhibit upregulated expression of genes implicated in HE[11].
Oxidative stress plays a pivotal role in the pathogenesis of HE, driven by an imbalance between reactive oxygen species, reactive nitrogen species and the body's antioxidant defenses[12]. This imbalance, exacerbated by elevated ammonium levels due to liver dysfunction, leads to systemic and central nervous system (CNS) oxidative stress[13]. The resultant neuroinflammation, mitochondrial dysfunction, and oxidative damage to lipids, proteins, and nucleic acids contribute to neuronal and astrocytic injury[14].
Key mechanisms include the depletion of antioxidants such as glutathione and catalase, impaired detoxification processes[15] and additionally, the formation of toxic compounds, like peroxynitrite from the reaction of nitric oxide with superoxide these contributes to cellular and tissue damage, neurotoxicity, and the progression of HE[16].
Alterations in neurotransmission systems, particularly the glutamatergic and GABAergic systems, contribute to energy disturbances and neuronal dysfunction[17]. Astrocytes detoxify plasma ammonia via glutamine synthetase, disrupting potassium buffering leading to elevated potassium and ammonium levels[8,17]. Additionally, hyperammonemia impairs hemichannel function and reduces lactate release, further limiting energy supply to neurons[17]. These findings highlight the complex interaction between ammonia detoxification, energy metabolism, and neurotransmitter systems in HE.
Systemic inflammation, oxidative stress, and elevated levels of blood manganese, bile acids, and lactate contribute to pathological changes in HE. These factors disrupt the blood-brain barrier (BBB), allowing an increased influx of molecules like ammonia, which typically do not cross the BBB in significant amounts[1,10,17].
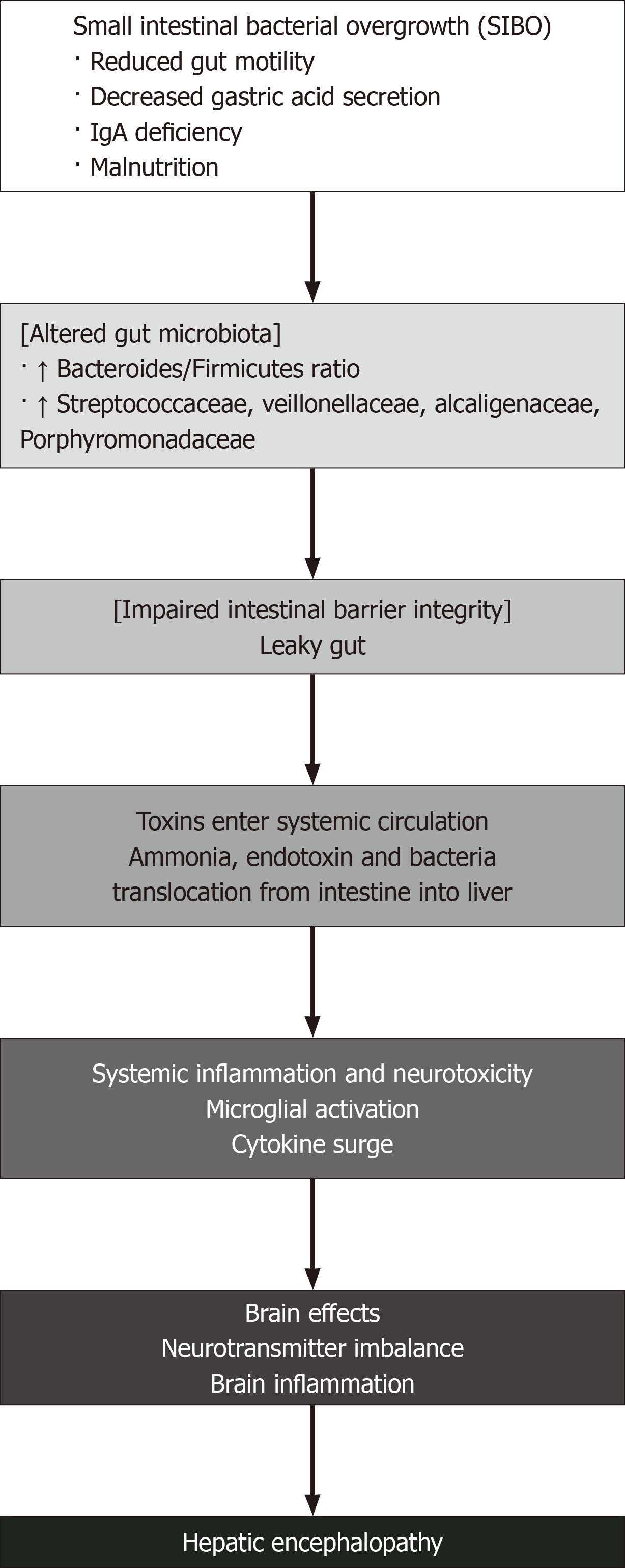
HE is intrinsically linked to dysfunction of the gut-liver axis, wherein alterations in gut microbiota and impaired intestinal barrier integrity exacerbate the neurotoxic effects on the brain (Figure 1), allowing gut-derived toxins, such as ammonia and endotoxins, to enter the systemic circulation[5,18,19]. Small intestinal bacterial overgrowth frequently accompanies cirrhosis due to factors such as reduced gut motility, gastric acid secretion, luminal immunoglobulin A deficiency, and malnutrition, further compromising intestinal barrier integrity and increasing bacterial translocation from intestine into the liver[20,21].
Manganese accumulation has been implicated in the pathogenesis of HE, with elevated plasma levels resulting from impaired hepatic excretion, leading to its deposition in the basal ganglia[1]. Elevated manganese concentrations in blood, cerebrospinal fluid, and basal ganglia tissue of patients with cirrhosis, along with dopaminergic alterations, further emphasize its role[9,10]. Animal studies also suggest that manganese exacerbates ammonia and glutamine accumulation in the brain, highlighting its contribution to HE pathogenesis[8].
Hyperammonemia in HE induces mitochondrial dysfunction and disrupts cellular bioenergetics, which leads to altered pH levels that contribute to neuronal depolarization and asterixis via increased neuronal resting membrane potential and inhibition of outward chloride pumps[8]. While definitive evidence linking HE primarily to brain energy failure is lacking, positron emission tomography studies indicate decreased glucose uptake in the anterior cingulate cortex of patients with cirrhosis and HE, correlating with psychometric impairments[9]. Furthermore, reduced cerebral oxygen metabolism has been noted in both episodic HE linked to cirrhosis and that associated with acute liver failure[8]. This suggests that hyperammonemia increases GABAergic tone via astrocytic glutamine synthesis, leading to diminished neuronal activity and energy demand, which further aggravates metabolic dysfunction in the brain during HE episodes[17].
Bile acids play a significant role in the pathophysiology of HE, as evidenced by recent studies. Cirrhotic individuals with HE have been shown to exhibit enormous concentrations of bile acids in their cerebrospinal fluid[9]. Animal models further validate this finding; in a study, rats with acute galactosamine-induced liver failure develop regional cerebral edema, indicating a partial loss of the BBB function[22]. Similarly, bile duct ligation models reveal increased circulating bile acids and compromised BBB integrity[23]. Thus, the role of bile acids and bilirubin in the development of HE warrants further research in light of these findings.
Lactulose is a nonabsorbable disaccharide. It is the first-line therapy for managing HE[24]. Its therapeutic effects are based on its ability to reduce ammonia levels in the gastrointestinal tract. Lactulose functions by promoting the excretion of nitrogen-containing substances through its laxative effects, which enhance bowel movements and increase fecal nitrogen excretion[24-26]. Upon reaching the colon, lactulose is metabolized by colonic microbiota into short-chain organic acids, such as lactic and acetic acid[27]. This conversion acidifies the colonic contents, creating an environment unfavorable for ammonia-producing bacteria and facilitates the conversion of ammonia to nonabsorbable ammonium ions[25,27]. Although lactulose has been used for decades, its consistent benefits in comparative studies remain unproven[24,26]. Nonetheless, it is believed to effectively decrease ammonia absorption and production in the intestinal lumen, thereby alleviating symptoms associated with HE[27,28].
Polyethylene glycol is an effective treatment for HE. It primarily works as an osmotic agent that enhances bowel clearance and thereby reduces the systemic ammonia levels[28].
Rifaximin is a nonabsorbable antibiotic primarily used in the management of HE[29]. It works by inhibiting urease-producing bacteria in the gut, which reduces the absorption of ammonia thereby improving hyperammonemia and cognitive function in affected patients[30]. Additionally, rifaximin modulates the gut microbiota, enhancing metabolic pathways that could further mitigate HE symptoms without significantly altering the overall diversity of gut bacteria or inducing antimicrobial resistance[31]. However, caution is advised in patients with severe hepatic impairment due to increased systemic exposure[30,32].
Metronidazole is presented as a potential alternative or adjunctive treatment to neomycin for HE. The rationale behind its use lies in its activity against anaerobic bacteria, which play a significant role in ammonia production in the gut. By targeting these bacteria, metronidazole aims to reduce the endogenous formation of ammonia, a key factor in the pathogenesis of HE. A clinical trial compared the effects of metronidazole and neomycin in patients with varying degrees of HE. The study found that metronidazole was as effective as neomycin in improving clinical and biochemical criteria, including mental state and EEG readings. This suggests that metronidazole's mechanism of action, targeting anaerobic ammonia production, offers a viable approach to managing HE[33].
L-ornithine L-aspartate (LOLA) is a salt composed of L-ornithine and L-aspartate, utilized in treating HE by effectively lowering blood ammonia levels[34]. Its mechanism of action involves supplying critical substrates for the urea cycle, where L-ornithine acts as an activator of carbamoyl phosphate synthetase, enhancing ammonia detoxification into urea in periportal hepatocytes[35]. Upon administration, LOLA is rapidly absorbed and dissociates into its constituent amino acids, which participate in transamination reactions to form glutamate, a precursor for glutamine synthesis in the liver and other tissues[35]. LOLA's ability to enhance both ureagenesis and glutamine synthesis explains its role in managing hyperammonemia associated with liver dysfunction[36] (Table 1).
| Treatment option | Proposed mechanism of action | Ref. | Year | Title of study | Study details | Effect |
| Lactulose | Nonabsorbable disaccharide that reduces ammonia levels in the gastrointestinal tract by promoting excretion of nitrogen-containing substances through laxative effects and acidifying colonic contents | Sharma et al[25] | 2013 | A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy | Randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt HE | Decreases ammonia absorption and production in the intestinal lumen, alleviating symptoms associated with HE |
| Polyethylene glycol | Osmotic agent that enhances bowel clearance, thereby reducing systemic ammonia levels | Rahimi et al[28] | 2014 | Lactulose vs polyethylene glycol 3350-electrolyte solution for treatment of overt hepatic encephalopathy: The HELP randomized clinical trial | 50 cirrhotic patients with HE 2 groups polyethylene glycol (PEG) 3350-electrolyte solution (n = 25) or standard-of-care lactulose (n = 25) | HELP Study: 91% of PEG-treated patients showed improvement of 1 or more in HE grade at 24 hours compared to only 52% in the lactulose group (P < 0.01) |
| Rifaximin | Nonabsorbable antibiotic that inhibits urease-producing bacteria in the gut and modulates gut microbiota | Sharma et al[25] | 2013 | A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy | Randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt HE | Decreases ammonia absorption and production in the intestinal lumen, alleviating symptoms associated with HE |
| Metronidazole | Reduces ammonia production by inhibiting urease-producing bacteria | Morgan et al[33] | 1982 | Treatment of hepatic encephalopathy with metronidazole | Randomized trial in 18 patients with cirrhosis comparing metronidazole vs neomycin | Equal effectiveness to neomycin in treating HE |
| L-ornithine-L-aspartate | Stable salt that dissociates into L-ornithine and L-aspartate amino acids.L-ornithine activates carbamoyl phosphate synthetase (rate-limiting urea cycle enzyme). Both amino acids undergo transamination to glutamate for glutamine synthesis | Blanco Vela et al[34] | 2011 | Efficacy of oral L-ornithine L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy | Efficacy study in patients with cirrhosis with hyperammonemic HE | Effectively lowers blood ammonia levels through enhanced ureagenesis and glutamine synthesis |
| Glycerol phenylbutyrate | Nitrogen-binding agent providing alternative pathway for ammonia elimination. Hydrolysed by pancreatic lipases to phenylbutyric acid, which forms phenylacetylglutamine (PAGN) for urinary excretion, bypassing compromised urea cycle | Zacharias et al[37] | 2019 | Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis | Phase II RCT: 178 patients with cirrhosis with ≥ 2 HE episodes, 6 mL twice daily for 16 weeks | Reduced HE events (21% vs 36%, P = 0.02), decreased hospitalizations (13 vs 25), enhanced efficacy in non-rifaximin patients (10% vs 32% events, P < 0.01). Safety profile similar to placebo |
| Sodium phenylbutyrate | Provides alternative nitrogen excretion pathway by β-oxidation to phenylacetate, which conjugates with glutamine to form phenylacetylglutamine for urinary elimination. Inhibits LPS-induced pro-inflammatory cytokines | Weiss et al[39] | 2019 | Treating hepatic encephalopathy in patients with cirrhosis admitted to ICU with sodium phenylbutyrate: A preliminary study | The study suggests that sodium phenylbutyrate may help treat HE in patients with cirrhosis in the ICU, but results are preliminary | Reverses dysfunctional bile acid synthesis, mitigates CNS abnormalities, and lowers ammonia levels through alternative nitrogen elimination while providing anti-inflammatory effects |
| Sodium benzoate | Conjugation with glycine to form hippuric acid, which is then excreted in urine, thereby reducing the ammonia load | Sushma et al[39], van Zoest et al[44] | 1992, 2025 | Sodim Benzoate in the Treatment of Acute Hepatic Encephalopathy: A Double-blind Randomized Trial[39]. Sodium benzoate for the treatment of hepatic encephalopathy in humans and animals: A systematic review and meta-analysis[44] | Double-blind randomized trial comparing sodium benzoate (5 g twice daily) vs lactulose in 74 patients with acute HE. Meta-analysis (2025) study: Systematic review of 16 studies including 314 subjects | 80% recovery rate with sodium benzoate vs 81% with lactulose (similar efficacy, P > 0.1). Cost was 30 times lower than lactulose. Similar side effect profile. Meta-analysis (2025) study: Standardized mean difference of ammonia-lowering effect was 0.89 (95%CI: 0.27-1.51) in clinical trials, indicating effective ammonia reduction |
| L-Ornithine Phenylacetate | L-ornithine acts as substrate for glutamine synthesis in skeletal muscle to detoxify ammonia, while phenylacetate facilitates excretion of ornithine-derived glutamine as phenylacetylglutamine in urine | Safadi et al[47] | 2022 | Pharmacokinetics/pharmacodynamics of L-ornithine phenylacetate in overt hepatic encephalopathy and the effect of plasma ammonia concentration reduction on clinical outcomes | Pharmacokinetics/pharmacodynamics study examining plasma ammonia concentration reduction and clinical outcomes | L-ornithine phenylacetate (OP) significantly reduced plasma ammonia levels at 3 hours post-infusion compared to placebo (P = 0.014) and accelerated the time to achieve normal ammonia levels (P = 0.028). Most importantly, the study established that clinical response based on HE stage was directly associated with greater reduction in mean plasma ammonia levels (P = 0.009), confirming that OP's ammonia-lowering effect translates into meaningful clinical improvements in HE severity |
| Probiotics | Bacterial adhesion inhibition, mucosal barrier enhancement, immune system modulation, bioactive metabolite secretion, nervous system regulation, improved metabolism of amino acids/vitamins/bile acids, reduced ammonia production | Dalal et al[51] | 2017 | Probiotics for people with hepatic encephalopathy | Cochrane Systematic Review study analysed 21 randomized clinical trials with 1,420 participants to determine the beneficial and harmful effects of probiotics for HE | Probiotics probably improve recovery and may lead to improvements in the development of overt HE, quality of life, and plasma ammonia concentrations, but may lead to little or no difference in mortality |
| Zinc | Enhances ornithine transcarbamylase activity (key enzyme in urea cycle) for improved ammonia detoxification. Maintains intestinal barrier integrity to reduce absorption of gut-derived toxins involved in HE pathogenesis | Reding et al[52], Shen et al[53] | 1984, 2019 | Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial[52]. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: A systematic review and meta-analysis[53] | RCT involving 22 patients with cirrhosis with chronic encephalopathy who received either zinc acetate 600 mg daily or placebo for 7 days. Systematic Review and Meta-Analysis examined the effects of zinc supplementation on HE treatment in patients with cirrhosis including four trials with 247 patients | The study demonstrated significant improvements in HE as measured by trail-making tests on day 8 in the zinc-supplemented group compared to placebo. The meta-analysis revealed that combination treatment of zinc supplementation and lactulose over 3 to 6 months significantly improved performance in the number connection test (SMD: -0.97; 95%CI: -1.75 to |
| Albumin | Binds and transports toxins (bilirubin, bile acids, ammonia) reducing their concentrations in blood and brain Anti-inflammatory and antioxidant properties | Sharma et al[25] | 2017 | Randomized controlled trial comparing lactulose plus albumin vs lactulose alone for treatment of HE | Randomized controlled trial comparing lactulose plus albumin vs lactulose alone for treatment of HE consisting of 120 patients | Combination of lactulose plus albumin is more effective than lactulose alone in treatment of overt HE |
| Flumazenil | Antagonizes the GABA/benzodiazepine receptor complex, which is overactivated in HE | Goh et al[60] | 2017 | Flumazenil vs placebo or no intervention for people with cirrhosis and HE | Meta analysis study 14 randomized clinical trials with 867 participants | Short-term clinical improvement (RR 0.75, 95%CI: 0.71-0.80); No significant effect on mortality (RR 0.75, 95%CI: 0.48-1.16) |
| Naloxone | Opioid receptor antagonist that targets elevated levels of opioid peptides in patients with liver disease, which are potentially involved in HE manifestations | Jiang et al[61] | 2010 | Naloxone in the management of HE | Meta-analysis study, 15 RCT studies involving 1054 patients with HE | Naloxone use was associated with a significant improvement in HE (RR 1.46, 95%CI: 1.27–1.67; P = 0.0005). Subgroup analysis showed that parenteral administration by intermittent or continuous infusion was most effective (RR 1.34, 95%CI: 1.17–1.53; P < 0.0001), and infusion-only trials showed an RR of 1.42 (95%CI: 1.19–1.69; P < 0.0001) |
| Acetyl L carnitine | Enhances ureagenesis leading to decreased blood and brain ammonia levels | Malaguarnera et al[62] | 2011 | Oral acetyl-L-carnitine therapy reduces fatigue in overt hepatic encephalopathy: A randomized, double-blind, placebo-controlled study | Randomized, double-blind, placebo-controlled design with 121 patients with overt HE with treatment duration of 90 days | Malaguarnera et al[62] Study showed significant reductions in blood ammonia levels across treatment groups receiving ALC, along with improvements in cognitive functions including attention, learning, psychomotor speed, and visual function-related domains |
| Branched-chain amino acids (BCAAs) | Improvement of muscle mass, BCAAs compete with aromatic amino acids for transport across the blood-brain barrier, potentially reducing the influx of false neurotransmitters that contribute to HE symptoms | Marrone et al[64] | 2023 | Branched chain amino acids in hepatic encephalopathy and sarcopenia in liver cirrhosis: Evidence and uncertainties | 16 randomized clinical trials, including 827 patients with overt HE (12 trials) or minimal HE (4 trials) | The study shown the restoration of normal amino acid levels with BCAA supplementation may improve the clinical course of HE and sarcopenia with few side effects. BCAA administration alone improves HE manifestation and reduces HE recurrence but has no significant improvement in mortality |
| Artificial liver support | Remove protein-bound and water-soluble toxins from circulation. Reduction in serum bilirubin levels, decrease in phenolic aromatic amino acids levels, improvement of systemic hemodynamics by removing vasoactive substances, reduction in neurotoxic substances reaching the brain | Kanjo et al[69] | 2021 | Efficacy and safety of liver support devices in acute and hyperacute liver failure: A systematic review and network meta-analysis | Meta-analysis of 11 randomized controlled trials comparing liver support systems to standard medical therapy, published between 1973 and 2016, including 479 patients in acute and hyperacute liver failure | Shown benefit in HE improvement and decreasing mortality |
| Golexanolone | GABA-A receptor-modulating steroid antagonist involves reducing the potentiation of GABA-A receptor activation by neurosteroids such as allopregnanolone which is present in higher concentration in the brains of patients with HE. | Montagnese et al[74] | 2021 | A pilot study of golexanolone, a new GABA-A receptor-modulating steroid antagonist, in patients with covert hepatic encephalopathy | A pilot study involving 3 weeks dosing with 33 patients to golexanolone (10, 40, or 80 mg BID) or 12 patients to placebo | Golexanolone was well tolerated and associated with improvement in cognitive performance |
| Embolization | Occlusion of large spontaneous portosystemic shunts thereby reducing the amount of ammonia and other toxins bypassing hepatic filtration. | Ke et al[78] | 2024 | Safety and efficacy of interventional embolization in cirrhotic patients with refractory hepatic encephalopathy associated with spontaneous portosystemic shunts | Study involves interventional embolization in 123 patients with cirrhosis with refractory HE associated with large spontaneous portosystemic shunts (34 in the embolization group and 89 in the control group) | Interventional embolization was found to be associated with prolonged HE-free survival and improved liver function in cirrhotic patients with refractory HE related to SPSS |
| Faecal microbiota transplantation | Restoring the gut microbiota balance to reduce ammonia synthesis, improve intestinal barrier integrity, and enhance ammonia clearance by improving liver function | Gao et al[82] | 2023 | A meta-analysis of microbiome therapies for hepatic encephalopathy | Meta-analysis study comprising 21 randomized controlled trials and 1746 patients with cirrhosis | FMT significantly: Reversed minimal HE (OR: 0.41, 95%CI: 0.19-0.90, P = 0.03); Reduced development of overt HE (OR: 0.41; 95%CI: 0.28-0.61, P < 0.00001). Decreased serious adverse events (OR: 0.14, 95%CI: 0.04-0.47, P = 0.001). Reduced ammonia levels, inflammatory markers, and hospitalization rate |
Glycerol phenylbutyrate (GPB) is a nitrogen-binding agent indicated for the management of urea cycle disorders[37]. Its mechanism of action involves the hydrolysis of GPB by pancreatic lipases, which releases phenylbutyrate, a prodrug that subsequently undergoes β-oxidation to form phenylacetate (PAA)[38]. PAA then conjugates with glutamine in the liver and kidneys to produce phenylacetylglutamine, an alternative pathway for nitrogen excretion that mimics urea, effectively reducing toxic ammonia levels in the bloodstream. This metabolic conversion has potential in reducing the CNS effects associated with hyperammonemia in HE[37,38].
Sodium phenylbutyrate (NaPB) has emerged as a potential therapeutic agent, demonstrating the ability to reverse dysfunctional bile acid synthesis and mitigate CNS abnormalities associated with HE[39,40]. Specifically, NaPB has been shown to inhibit lipopolysaccharide (LPS)-induced expression of pro-inflammatory cytokines such as IL-6, IL-1β, cyc
Sodium phenylacetate has emerged as a promising novel therapy for HE, targeting the reduction of ammonia levels in the body[40]. This works by conjugating with glutamine to form phenylacetylglutamine, which is then excreted in the urine, effectively removing excess nitrogen from the bloodstream[42]. By enhancing the elimination of conjugated nitrogen, sodium phenylacetate helps to lower blood ammonia levels improving HE[43].
Sodium benzoate has emerged as a promising alternative therapy for HE, offering a novel approach to managing this serious neuropsychiatric complication of liver disease[44,45]. The mechanism of action involves conjugation with glycine to form hippuric acid, which is then excreted in urine, thereby reducing the ammonia load in the body improving HE[40,44,46].
L-ornithine phenylacetate (OP) helps in HE by its ammonia detoxification potential[47,48]. The mechanism of action of OP involves two key components: L-ornithine acting as a substrate for glutamine synthesis in skeletal muscle, effectively detoxifying ammonia, while phenylacetate facilitates the excretion of ornithine-derived glutamine as phenylacetylglutamine in the urine[48]. This synergistic action results in a sustained reduction of plasma ammonia levels improving the disease condition[47].
Probiotics are a promising novel therapy for HE in patients with liver disorder[49]. These beneficial bacteria, when administered orally, can disrupt the pathogenesis of HE through multiple mechanisms of action like inhibiting bacterial adhesion, enhancing mucosal barrier function, modulating the innate and adaptive immune systems, secreting bioactive metabolites, and regulating the enteric and central nervous systems[50]. These changes in the gut microbiome and metabolome correlate with improvements in amino acid, vitamin, and secondary bile acid metabolism, potentially reducing the production of ammonia and other gut-derived toxins contributing to the therapeutic effects of probiotics in HE[49,50,51].
Zinc supplementation is a potential therapy for HE[52,53]. The mechanism of action of zinc in HE is multifaceted: It enhances the activity of ornithine transcarbamylase, a key enzyme in the urea cycle, thereby improving ammonia detoxification[54]. Additionally, zinc plays a crucial role in maintaining the integrity of the intestinal barrier, potentially reducing the absorption of gut-derived toxins involved in HE pathogenesis[55].
Albumin has emerged as a promising novel therapy for HE due to its multifaceted mechanisms of action. The primary mechanism of action involves albumin's ability to bind and transport toxins like bilirubin, bile acids, ammonia which are implicated in HE pathogenesis thereby effectively reducing their concentrations in the blood and brain. Additionally, albumin exhibits potent anti-inflammatory and antioxidant properties, which help mitigate the systemic inflammatory state associated with HE[56,57].
Flumazenil, a benzodiazepine receptor antagonist, has shown potential as a novel therapy for patients with HE. Its mechanism of action involves antagonizing the GABA/benzodiazepine receptor complex, which is overactivated in HE. Studies have demonstrated that flumazenil can induce rapid improvements in mental status and electroencephalographic activity in some patients with HE[58,59]. While flumazenil has shown short-term benefits in improving symptoms, its effects are often transient, lasting only 1-2 hours[59,60].
Elevated levels of opioid peptides in patients with liver disease suggest their potential involvement in HE manifestations. Naloxone, an opioid receptor antagonist, has shown promise in ameliorating HE symptoms in both animal models and some clinical reports. A meta-analysis of randomized controlled trials indicates that naloxone use is associated with a significant improvement in HE, particularly when administered parenterally via intermittent or continuous infusions. These findings suggest naloxone as a potential therapeutic candidate for HE, though further research is needed to confirm its effectiveness and safety[61].
AST-120 is a promising novel therapy for HE. It is an oral adsorbent that efficiently adsorbs ammonia in the gastrointestinal tract, thereby lowering circulating ammonia levels and reducing its toxic effects on the brain[40].
Acetyl-L-carnitine (ALC) has emerged as a promising novel therapy for HE, demonstrating significant benefits in reducing ammonia levels and improving cognitive functions[62,63]. The mechanism of action of ALC involves enhancing ureagenesis, which leads to decreased blood and brain ammonia levels, thereby addressing a key pathogenic factor in HE[63]. While a meta-analysis by Jiang et al[63] confirmed the efficacy of ALC across multiple studies, highlighting its role as a safe and effective adjunct therapy in HE management, a randomized controlled trial by Malaguarnera et al[62] found that ALC significantly improves cognitive test scores and reduces serum ammonia levels compared to placebo.
Branched-chain amino acids (BCAAs) have emerged as a promising novel therapy for HE[64-67]. The mechanism of action of BCAAs in HE involves their role in ammonia detoxification, improvement of muscle mass, and modulation of neurotransmitter balance in the brain. BCAAs compete with aromatic amino acids for transport across the blood-brain barrier, potentially reducing the influx of false neurotransmitters that contribute to HE symptoms. Studies have shown that long-term BCAA supplementation can improve minimal HE, enhance cognitive function, and increase mid-arm muscle circumference in patients with cirrhosis and a history of HE[64-68].
Artificial liver support systems have emerged as a promising therapy for HE. These systems, particularly albumin dialysis, aim to remove protein-bound and water-soluble toxins[69,70]. Artificial liver support systems, particularly albumin dialysis, demonstrate varying efficacy in toxin removal and management of HE[69,71,72]. These systems consistently achieve significant reductions in serum bilirubin levels and effectively decrease levels of phenolic aromatic amino acids, both of which are implicated in HE[71,73]. However, results for ammonia removal are mixed, with some studies reporting significant reductions while others found no substantial changes[72,74]. The combined effects of toxin removal improved systemic hemodynamics (by removing vasoactive substances), and modulation of inflammatory responses contributes to the observed improvements in HE, likely due to the reduction in neurotoxic substances reaching the brain and enhanced cerebral blood flow[69,71,72].
Golexanolone is a promising novel therapy for HE, currently under investigation in preclinical and clinical studies. As a GABA-A receptor-modulating steroid antagonist, its mechanism of action involves reducing the potentiation of GABA-A receptor activation by neurosteroids such as allopregnanolone which is present in higher concentration in the brains of patients with HE. This compound has shown efficacy in restoring spatial learning and motor coordination in animal models of HE, as well as mitigating the effects of intravenous allopregnanolone in healthy adults[73,74]. In a pilot study involving patients with cirrhosis and abnormal continuous reaction time, golexanolone demonstrated satisfactory safety and pharmacokinetics, with directionally favourable changes in measures of sleepiness, attention span, and brain wave activity[73]. Furthermore, golexanolone has been found to reduce peripheral inflammation and neuroinflammation in hyperammonemic rats, which are key factors in the pathogenesis of HE[75].
Embolization has emerged as a promising novel therapy for HE, particularly those with large spontaneous portosystemic shunts (SPSS)[76-79]. The mechanism of action involves occluding these abnormal vascular connections, which redirect blood flow back to the liver, reducing the amount of ammonia and other toxins bypassing hepatic filtration. This procedure has shown significant efficacy in reducing HE symptoms and recurrence rates[76-79].
Faecal microbiota transplantation (FMT) has emerged as a promising novel therapy for HE[80]. This is typically performed by delivering a fecal suspension from a healthy donor into the patient's gastrointestinal tract. The delivery methods can include upper gastrointestinal routes such as esophagogastroduodenoscopy, nasogastric or nasojejunal tube, and oral capsules, with the procedure often repeated multiple times to achieve therapeutic success[81]. The mechanism of action of FMT in HE involves altering the gut microbiota composition to reduce ammonia synthesis, improve intestinal barrier integrity, and enhance ammonia clearance by improving liver function. FMT aims to restore gut microbiota balance, addressing factors such as ammonia toxicity, gut-brain communication disruption, and inflammation in HE[80-82].
The article outlines several promising approaches to treating HE, yet moving these discoveries from the laboratory into actual patient care presents substantial obstacles. Researchers face a complex web of regulatory requirements, methodological challenges, safety questions, and significant cost considerations leading to practical difficulties of implementing new treatments in real-world clinical settings. What's particularly challenging is that overcoming these barriers requires unprecedented collaboration between research teams, practicing clinicians, regulatory bodies, and pharmaceutical companies. The future success of these therapeutic advances ultimately hinges on our ability to navigate these obstacles while keeping patients and their actual clinical needs at the center of our efforts.
HE remains a significant neurological complication of liver dysfunction, with complex pathophysiological mechanisms involving ammonia toxicity, systemic and neuroinflammation, oxidative stress, neurotransmission disturbances, and gut-liver-brain axis dysregulation. Advances in research have deepened our understanding of these mechanisms, revealing that HE is not merely a transient and reversible condition but may lead to persistent neurological deficits. Therapeutic strategies for HE has evolved, with traditional treatments such as lactulose and rifaximin forming the cornerstone of management. However, novel approaches—including nitrogen-scavenging agents, probiotics, zinc supplementation, and artificial liver support systems—offer promising avenues for more targeted and effective intervention. Emerging therapies, such as golexanolone, fecal microbiota transplantation, and embolization, further highlight the potential for personalized and precision medicine in HE management. Despite these advancements, significant gaps remain in optimizing treatment efficacy, predicting disease progression, and mitigating long-term neurological consequences. Future research should focus on refining diagnostic tools, identifying biomarkers for early intervention, and exploring combination therapies to improve patient outcomes. A multidisciplinary approach integrating hepatology, neurology, and microbiome research will be critical in addressing the complexities of HE and improving the quality of life for affected individuals.
| 1. | Swaminathan M, Ellul MA, Cross TJ. Hepatic encephalopathy: current challenges and future prospects. Hepat Med. 2018;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Chirapongsathorn S, Poovorawan K, Soonthornworasiri N, Pan-Ngum W, Phaosawasdi K, Treeprasertsuk S. Thirty-Day Readmission and Cost Analysis in Patients With Cirrhosis: A Nationwide Population-Based Data. Hepatol Commun. 2020;4:453-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1487] [Article Influence: 123.9] [Reference Citation Analysis (1)] |
| 4. | Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, Vilstrup H, Jalan R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73:1526-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 5. | Chen Z, Ruan J, Li D, Wang M, Han Z, Qiu W, Wu G. The Role of Intestinal Bacteria and Gut-Brain Axis in Hepatic Encephalopathy. Front Cell Infect Microbiol. 2020;10:595759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | van Hall G, van der Vusse GJ, Söderlund K, Wagenmakers AJ. Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J Physiol. 1995;489 (Pt 1):251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Sørensen M, Andersen JV, Bjerring PN, Vilstrup H. Hepatic encephalopathy as a result of ammonia-induced increase in GABAergic tone with secondary reduced brain energy metabolism. Metab Brain Dis. 2024;40:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Butterworth RF. Hepatic Encephalopathy in Cirrhosis: Pathology and Pathophysiology. Drugs. 2019;79:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12:135-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Sharma K, Akre S, Chakole S, Wanjari MB. Hepatic Encephalopathy and Treatment Modalities: A Review Article. Cureus. 2022;14:e28016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Bosoi CR, Rose CF. Oxidative stress: a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2013;28:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Seyan AS, Hughes RD, Shawcross DL. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress. World J Gastroenterol. 2010;16:3347-3357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 14. | Simicic D, Cudalbu C, Pierzchala K. Overview of oxidative stress findings in hepatic encephalopathy: From cellular and ammonium-based animal models to human data. Anal Biochem. 2022;654:114795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Allameh A, Niayesh-Mehr R, Aliarab A, Sebastiani G, Pantopoulos K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants (Basel). 2023;12:1653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 16. | Sangeetha K, Krishnasamy N, Padma K, Rajendran K. Evaluation of Oxidative Stress in Liver Cirrhosis Patients to Early Diagnosis of Minimal Hepatic Encephalopathy. Int Neuropsychiatr Dis J. 2016;5:1-9. [DOI] [Full Text] |
| 17. | Fallahzadeh MA, Rahimi RS. Hepatic Encephalopathy: Current and Emerging Treatment Modalities. Clin Gastroenterol Hepatol. 2022;20:S9-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Wang J, Wang X, Zhuo E, Chen B, Chan S. Gutliver axis in liver disease: From basic science to clinical treatment (Review). Mol Med Rep. 2025;31:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 19. | Tenderenda M, Buczkowski B, Oleksy P, Klecza A, Broniak A, Reclik M, Zieliński K, Chourasia A, Nowakowski K, Chraścina M. Liver Disease and Central Nervous System Dysfunction: Linking the Two - A Narrative Review. J Educ Health Sport. 2024;75:56180. [DOI] [Full Text] |
| 20. | Ghosh G, Jesudian AB. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. J Clin Exp Hepatol. 2019;9:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Won SM, Oh KK, Gupta H, Ganesan R, Sharma SP, Jeong JJ, Yoon SJ, Jeong MK, Min BH, Hyun JY, Park HJ, Eom JA, Lee SB, Cha MG, Kwon GH, Choi MR, Kim DJ, Suk KT. The Link between Gut Microbiota and Hepatic Encephalopathy. Int J Mol Sci. 2022;23:8999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | McClung HJ, Sloan HR, Powers P, Merola AJ, Murray R, Kerzner B, Pollack JD. Early changes in the permeability of the blood-brain barrier produced by toxins associated with liver failure. Pediatr Res. 1990;28:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328:1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Khungar V, Poordad F. Hepatic encephalopathy. Clin Liver Dis. 2012;16:301-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Mortensen PB, Rasmussen HS, Holtug K. Lactulose detoxifies in vitro short-chain fatty acid production in colonic contents induced by blood: implications for hepatic coma. Gastroenterology. 1988;94:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Zacharias HD, Kamel F, Tan J, Kimer N, Gluud LL, Morgan MY. Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2023;7:CD011585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Ponziani FR, Gerardi V, Pecere S, D'Aversa F, Lopetuso L, Zocco MA, Pompili M, Gasbarrini A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322-12333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 32. | Bajaj JS, Riggio O. Drug therapy: rifaximin. Hepatology. 2010;52:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Blanco Vela CI, Poo Ramírez JL. Efficacy of oral L-ornithine L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Ann Hepatol. 2011;10 Suppl 2:S55-S59. [PubMed] [DOI] [Full Text] |
| 35. | Kircheis G, Lüth S. Pharmacokinetic and Pharmacodynamic Properties of L-Ornithine L-Aspartate (LOLA) in Hepatic Encephalopathy. Drugs. 2019;79:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Sidhu SS. L-Ornithine L-Aspartate is Effective and Safe for the Treatment of Hepatic Encephalopathy in Cirrhosis. J Clin Exp Hepatol. 2018;8:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Zacharias HD, Zacharias AP, Gluud LL, Morgan MY. Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. Cochrane Database Syst Rev. 2019;6:CD012334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | McGuire BM, Zupanets IA, Lowe ME, Xiao X, Syplyviy VA, Monteleone J, Gargosky S, Dickinson K, Martinez A, Mokhtarani M, Scharschmidt BF. Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology. 2010;51:2077-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Weiss N, Tripon S, Lodey M, Guiller E, Junot H, Monneret D, Mayaux J, Brisson H, Mallet M, Rudler M, Imbert-Bismut F, Thabut D; Brain-Liver Pitié-Salpêtrière Study Group (BLIPS). Treating hepatic encephalopathy in cirrhotic patients admitted to ICU with sodium phenylbutyrate: a preliminary study. Fundam Clin Pharmacol. 2018;32:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Rockey DC, Vierling JM, Mantry P, Ghabril M, Brown RS Jr, Alexeeva O, Zupanets IA, Grinevich V, Baranovsky A, Dudar L, Fadieienko G, Kharchenko N, Klaryts'ka I, Morozov V, Grewal P, McCashland T, Reddy KG, Reddy KR, Syplyviy V, Bass NM, Dickinson K, Norris C, Coakley D, Mokhtarani M, Scharschmidt BF; HALT-HE Study Group. Randomized, double-blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy. Hepatology. 2014;59:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 41. | Hasan LZ, Wu GY. Novel Agents in the Management of Hepatic Encephalopathy: A Review. J Clin Transl Hepatol. 2021;9:749-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | De Las Heras J, Aldámiz-Echevarría L, Martínez-Chantar ML, Delgado TC. An update on the use of benzoate, phenylacetate and phenylbutyrate ammonia scavengers for interrogating and modifying liver nitrogen metabolism and its implications in urea cycle disorders and liver disease. Expert Opin Drug Metab Toxicol. 2017;13:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Mendenhall CL, Rouster S, Marshall L, Weesner R. A new therapy for portal systemic encephalopathy. Am J Gastroenterol. 1986;81:540-543. [PubMed] |
| 44. | van Zoest D, Gal B, Agha AH, den Hoed CM, Langendonk JG, Wagenmakers MAEM, Peltenburg C. Sodium benzoate for the treatment of hepatic encephalopathy in humans and animals: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2025;37:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 121] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol. 2000;95:3574-3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Safadi R, Rahimi RS, Thabut D, Bajaj JS, Ram Bhamidimarri K, Pyrsopoulos N, Potthoff A, Bukofzer S, Wang L, Jamil K, Devarakonda KR. Pharmacokinetics/pharmacodynamics of L-ornithine phenylacetate in overt hepatic encephalopathy and the effect of plasma ammonia concentration reduction on clinical outcomes. Clin Transl Sci. 2022;15:1449-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Jalan R, Wright G, Davies NA, Hodges SJ. L-Ornithine phenylacetate (OP): a novel treatment for hyperammonemia and hepatic encephalopathy. Med Hypotheses. 2007;69:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (3)] |
| 49. | Bloom PP, Tapper EB, Young VB, Lok AS. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021;75:1452-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 50. | Dazıroğlu MEÇ, Yıldıran H. Intestinal dysbiosis and probiotic use: its place in hepatic encephalopathy in cirrhosis. Ann Gastroenterol. 2023;36:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 51. | Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Reding P, Duchateau J, Bataille C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet. 1984;2:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Shen YC, Chang YH, Fang CJ, Lin YS. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J. 2019;18:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Riggio O, Merli M, Capocaccia L, Caschera M, Zullo A, Pinto G, Gaudio E, Franchitto A, Spagnoli R, D'Aquilino E. Zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology. 1992;16:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Ullah MI, Alameen AAM, Al-Oanzi ZH, Eltayeb LB, Atif M, Munir MU, Ejaz H. Biological Role of Zinc in Liver Cirrhosis: An Updated Review. Biomedicines. 2023;11:1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 56. | Murtaza F, Mathew M, Fagbamila O, Subramani S, Nimal S, Nyshita VN, Priya V, Sany AT, Kumar Y, Cicani L, Ehsan M, Kandel K. Efficacy and safety of albumin for the treatment of hepatic encephalopathy: an updated systematic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond). 2024;86:3416-3422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Is B, Bombassaro IZ, Tovo CV, de Mattos ÂZ, Ahlert M, Chiesa T, de Mattos AA. Albumin in the management of hepatic encephalopathy: A systematic review and meta-analysis. Ann Hepatol. 2021;26:100541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Als-Nielsen B, Gluud LL, Gluud C. Benzodiazepine receptor antagonists for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;CD002798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Bansky G, Meier PJ, Riederer E, Walser H, Ziegler WH, Schmid M. Effects of the benzodiazepine receptor antagonist flumazenil in hepatic encephalopathy in humans. Gastroenterology. 1989;97:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Goh ET, Andersen ML, Morgan MY, Gluud LL. Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy. Cochrane Database Syst Rev. 2017;7:CD002798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Jiang Q, Jiang G, Welty TE, Zheng M. Naloxone in the management of hepatic encephalopathy. J Clin Pharm Ther. 2010;35:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Malaguarnera M, Vacante M, Motta M, Giordano M, Malaguarnera G, Bella R, Nunnari G, Rampello L, Pennisi G. Acetyl-L-carnitine improves cognitive functions in severe hepatic encephalopathy: a randomized and controlled clinical trial. Metab Brain Dis. 2011;26:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Jiang Q, Jiang G, Shi KQ, Cai H, Wang YX, Zheng MH. Oral acetyl-L-carnitine treatment in hepatic encephalopathy: view of evidence-based medicine. Ann Hepatol. 2013;12:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Marrone G, Serra A, Miele L, Biolato M, Liguori A, Grieco A, Gasbarrini A. Branched chain amino acids in hepatic encephalopathy and sarcopenia in liver cirrhosis: Evidence and uncertainties. World J Gastroenterol. 2023;29:2905-2915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (4)] |
| 65. | Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 66. | Dam G, Aamann L, Vistrup H, Gluud LL. The role of Branched Chain Amino Acids in the treatment of hepatic Encephalopathy. J Clin Exp Hepatol. 2018;8:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 67. | Btaiche IF. Branched-chain amino acids in patients with hepatic encephalopathy. 1982. Nutr Clin Pract. 2003;18:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 68. | Stadlbauer V, Wright GA, Jalan R. Role of artificial liver support in hepatic encephalopathy. Metab Brain Dis. 2009;24:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Kanjo A, Ocskay K, Gede N, Kiss S, Szakács Z, Párniczky A, Mitzner S, Stange J, Hegyi P, Molnár Z. Efficacy and safety of liver support devices in acute and hyperacute liver failure: a systematic review and network meta-analysis. Sci Rep. 2021;11:4189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Parés A, Deulofeu R, Cisneros L, Escorsell A, Salmerón JM, Caballería J, Mas A. Albumin dialysis improves hepatic encephalopathy and decreases circulating phenolic aromatic amino acids in patients with alcoholic hepatitis and severe liver failure. Crit Care. 2009;13:R8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Martí-carvajal AJ, Gluud C, Gluud LL, Pavlov CS, Mauro E, Monge Martín D, Liu JP, Nicola S, Comunián-carrasco G, Martí-amarista CE. Liver support systems for adults with acute liver failure. Cochrane Database Syst Rev. 2022;2022. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 72. | Boonsrirat U, Tiranathanagul K, Srisawat N, Susantitaphong P, Komolmit P, Praditpornsilpa K, Tungsanga K, Eiam-Ong S. Effective bilirubin reduction by single-pass albumin dialysis in liver failure. Artif Organs. 2009;33:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Gupta S, Fenves AZ, Hootkins R. The Role of RRT in Hyperammonemic Patients. Clin J Am Soc Nephrol. 2016;11:1872-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 74. | Montagnese S, Lauridsen M, Vilstrup H, Zarantonello L, Lakner G, Fitilev S, Zupanets I, Kozlova I, Bunkova E, Tomasiewicz K, Berglund JE, Rorsman F, Hagström H, Kechagias S, Ocklind CE, Mauney J, Thunarf F, Mokhatarani M, Bäckström T, Doverskog M, Lins LE, Månsson M, Samuelson P, Nilsson D, Schalling M, Johansson M, Arlander E, Scharschmidt BF. A pilot study of golexanolone, a new GABA-A receptor-modulating steroid antagonist, in patients with covert hepatic encephalopathy. J Hepatol. 2021;75:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Mincheva G, Gimenez-Garzo C, Izquierdo-Altarejos P, Martinez-Garcia M, Doverskog M, Blackburn TP, Hällgren A, Bäckström T, Llansola M, Felipo V. Golexanolone, a GABA(A) receptor modulating steroid antagonist, restores motor coordination and cognitive function in hyperammonemic rats by dual effects on peripheral inflammation and neuroinflammation. CNS Neurosci Ther. 2022;28:1861-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Leonard H, O'Beirne J, Yu D, Tsochatzis E. Embolization of porto-systemic shunt as treatment for recurrent hepatic encephalopathy. Ann Hepatol. 2014;13:555-557. [PubMed] [DOI] [Full Text] |
| 77. | Lynn AM, Singh S, Congly SE, Khemani D, Johnson DH, Wiesner RH, Kamath PS, Andrews JC, Leise MD. Embolization of portosystemic shunts for treatment of medically refractory hepatic encephalopathy. Liver Transpl. 2016;22:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Ke Q, He J, Cai L, Lei X, Huang X, Li L, Liu J, Guo W. Safety and efficacy of interventional embolization in cirrhotic patients with refractory hepatic encephalopathy associated with spontaneous portosystemic shunts. Sci Rep. 2024;14:14848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M, Jalan R, Brookes J, Thalassinos E, Burroughs AK, Cordoba J, Nevens F; EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 80. | Karimi M, Shirsalimi N, Hashempour Z, Salehi Omran H, Sedighi E, Beigi F, Mortezazadeh M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: a comprehensive literature review. Front Immunol. 2024;15:1439176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 81. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 842] [Article Influence: 93.6] [Reference Citation Analysis (1)] |
| 82. | Gao J, Nie R, Chang H, Yang W, Ren Q. A meta-analysis of microbiome therapies for hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2023;35:927-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/