Published online Sep 18, 2025. doi: 10.13105/wjma.v13.i3.107588
Revised: May 13, 2025
Accepted: September 1, 2025
Published online: September 18, 2025
Processing time: 167 Days and 20.8 Hours
Neural stem cells (NSCs) play a fundamental role in generating diverse neuronal populations that contribute to the formation of intricate neural circuitry. Dis
Core Tip: Neural stem cells (NSCs) are fundamental to the formation of neuronal circuitry, and their dysfunction can lead to systemic neural impairments. While intrinsic regulatory mechanisms of NSCs have been studied extensively, emerging evidence highlights the critical contributions of extrinsic factors, such as glial interactions, intertissue signaling, oxygen tension, and irradiation, to NSC development. This mini-review synthesizes current insights into these extrinsic regulators, leveraging the Drosophila model to elucidate their role in NSC dynamics and neural system integrity.
- Citation: Ren XM, Zhang Q, Zhang H, Zhang LJ, Yu Y, Chen QJ, An HP. Ambient effects on neural stem cells: Insights from Drosophila. World J Meta-Anal 2025; 13(3): 107588
- URL: https://www.wjgnet.com/2308-3840/full/v13/i3/107588.htm
- DOI: https://dx.doi.org/10.13105/wjma.v13.i3.107588
Understanding how to construct an integral neural system precisely remains a fundamental question in neuroscience. Neural stem cells (NSCs) or neural progenitors play a pivotal role in this process by dividing continuously to generate the diverse daughter cells required for forming intricate neural circuitry[1-5]. Critical functions of NCSs govern neural development under both physiological conditions and during responses to neurodegeneration or injury[6,7]. Elucidating the mechanisms that regulate NSC development spatiotemporally will deepen our understanding of the neural ontogeny and enhance therapeutic strategies for neurological disorders.
Regulatory mechanisms driving NSC development are broadly categorized into either intrinsic factors (inherited from progenitor cells) and extrinsic cues (derived from the cellular environment)[3,8,9]. Over the past decades, intrinsic regulatory networks-determining NSC identity, division patterns and spatiotemporal dynamics-have been characterized extensively in such model organisms as Caenorhabditis elegans, Drosophila, and mice[2,10-12]. Recent studies, however, have highlighted the indispensable role of extrinsic ambient signals in maintaining NSC identities, division modes and developmental timing[13-15]. These extrinsic causes synergize with intrinsic programs to ensure precise neural system assembly. For example, the steroid hormone ecdysone regulates neural temporal identities through Seven-up, the orphan nuclear receptor of ecdysone[16,17].
The extrinsic ambient regulatory landscape comprises three layers. The first is the external environment and includes such factors as irradiation, oxygen tension and viral infections[18-21]. The second is the systemic environment, especially intertissue interactions involving organs like the fat body, trachea, gut and heart[20,22-24]. The last is the microenvironment (niche), which encompasses local signals from the adjacent glia, sibling cells and niche component[15,25,26].
Model organisms, particularly Drosophila, play a pivotal role in elucidating the mechanisms underlying neurogenesis[27]. Studies on NSC behaviors, such as asymmetric division, temporal regulation, termination, and the reactivation of quiescent NSCs, have significantly advanced our understanding of their developmental processes in vertebrates[10,16,22,28]. Recent research in Drosophila has also provided critical insights into how extrinsic cues regulate NSC development.
This review synthesizes recent advances in understanding environmental systemic and niche-mediated regulation of NSCs in Drosophila. Such findings bridge foundational discoveries to clinical applications, offering novel perspectives for neural regeneration and disease intervention.
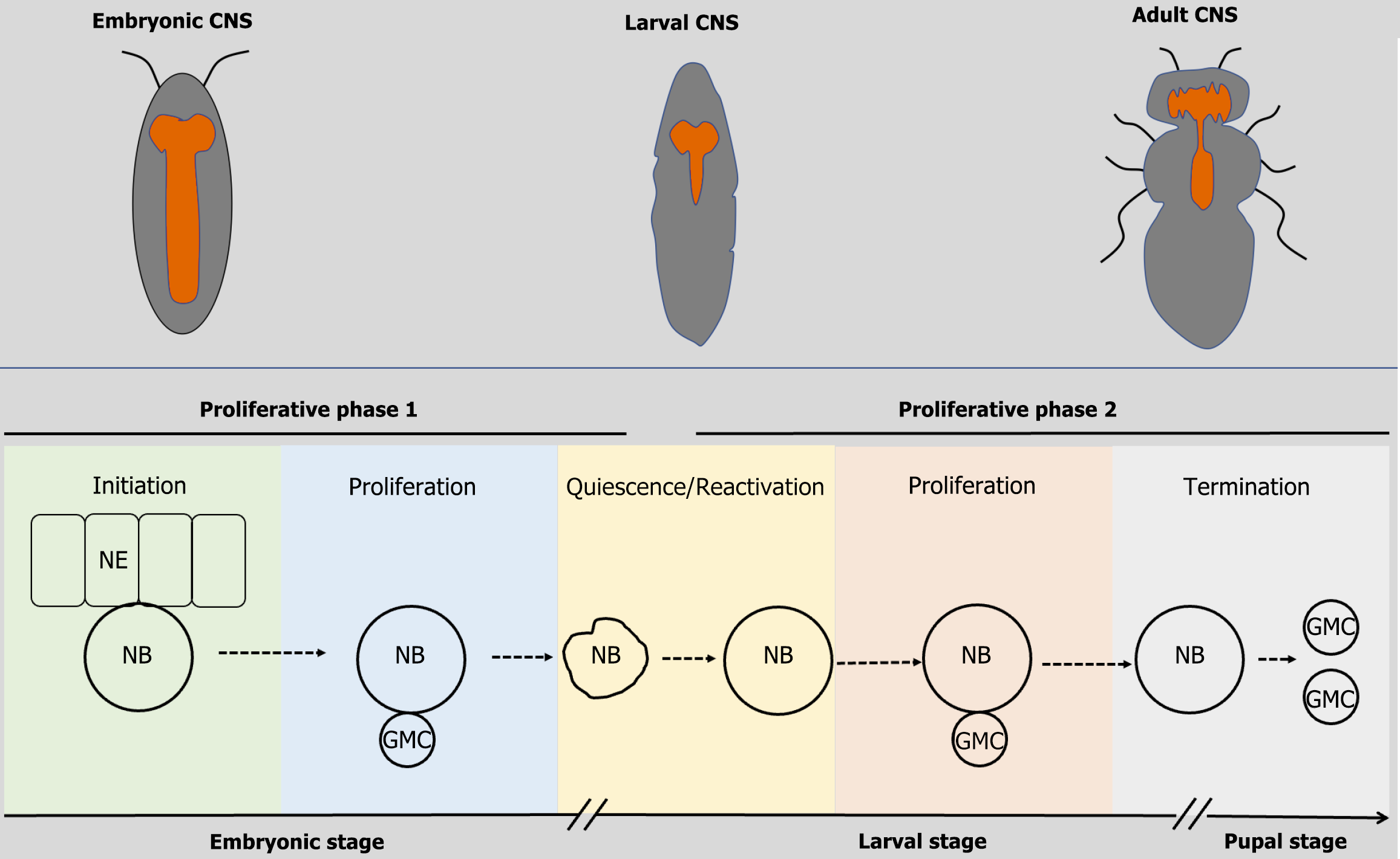
Drosophila NSCs, known as neuroblasts, generate neuronal progeny during development[28,29]. These NSCs arise during embryogenesis and cease activity by the early pupal stage (Figure 1). Their lifecycle comprises two proliferative phases separated by a mitotically quiescent stage[30,31]. Upon delamination from the neuroepithelium, embryonic NSCs initiate a phase of asymmetric divisions to produce differentiated cells while retaining stem cell identity[29]. By late embryogenesis, they enter a transient quiescent stage[25,32]. Reactivation occurs during the early second larval stage, triggering a second wave of asymmetric divisions reminiscent of embryonic proliferation[22,25,33,34]. NSCs ultimately exit the cell cycle and undergo decommissioning in the early pupal stage[35,36].
The asymmetric division process ensures self-renewal of the NSC pool while generating diverse neuronal progeny for circuit assembly[10,37]. Embryonic NSCs contribute primarily to larval behavior circuits, whereas larval and pupal division establish adult-specific neural networks[38].
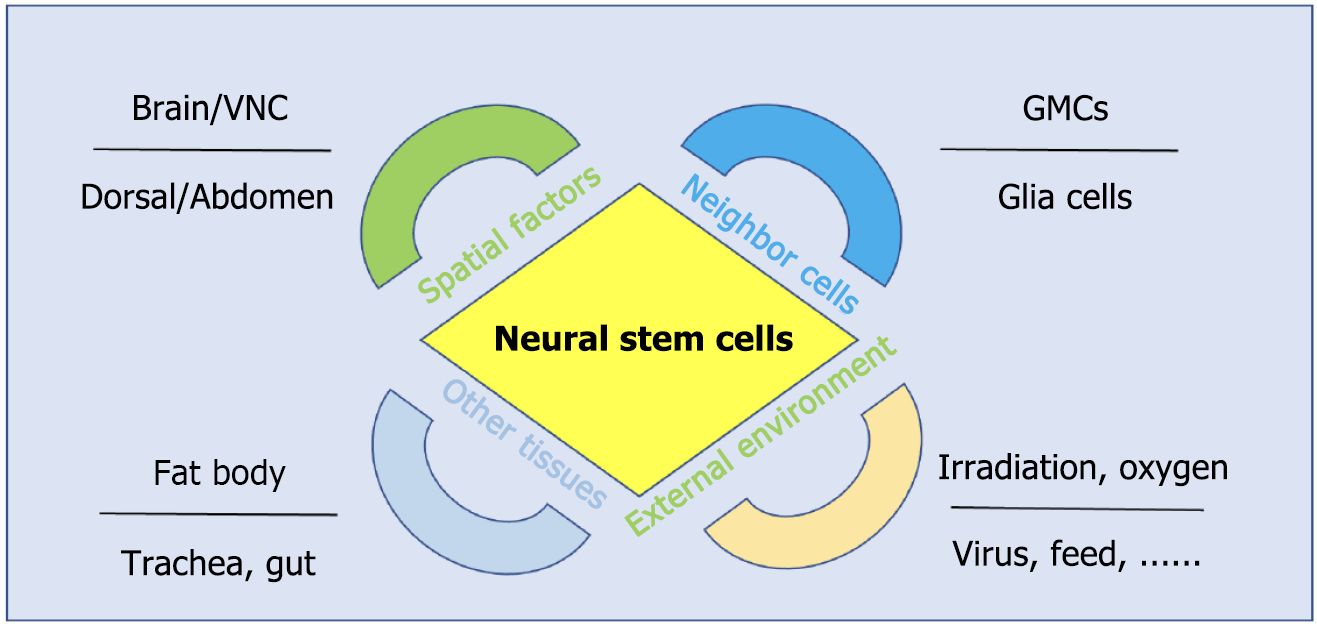
While asymmetric cell division and intrinsic temporal patterning confer autonomous properties to NSCs, accumulating evidence has underscored the critical role of ambient factors of NSCs in shaping their development[39-41]. For example, steroid hormones and dietary nutrients influence NSC temporal identities and development states[42]. These extrinsic ambient regulators of NSCs are broadly categorized into four groups (Figure 2). The first is the spatial patterning cues, especially body segregation, mediated by the gap genes, establishes regional identities of NSCs[43-45], leading to distinct lifespan and termination mechanism in brain, thoracic and abdominal NSCs[46,47]. Most of the ventral nerve cord NSCs undergo apoptosis, while brain NSCs are reactivated and undergo proliferation during the larval stage[32,48]. The second is the interaction between NSCs and their neighboring cells. The physical positioning of daughter cells relative to the niche influences NSC division orientation[49]. Additionally, surrounding glia cells modulate NSC reactivation and proliferation via nutrients and irons[15,50]. The third is intertissue communication. Systemic signals from organs such as the fat body, trachea and gut regulate NSC behavior[20,22,51]. For instance, the fat body integrates nutrient-derived energy status and relays growth-promoting signals to NSCs[16,22]. The last is external environmental inputs. External environmental factors, including irradiation, oxygen levels, virus infection, and dietary composition, directly or indirectly influence NSC dynamics[18-21,52,53]. Irradiation can induce NSC premature decommissioning in the third instar larval stage[21]. The interplay between these extrinsic ambient factors and intrinsic genetic programs ensures the precise spatiotemporal control of NSC development. Below, we detail mechanistic insights into how environmental cues orchestrate NSC behavior.
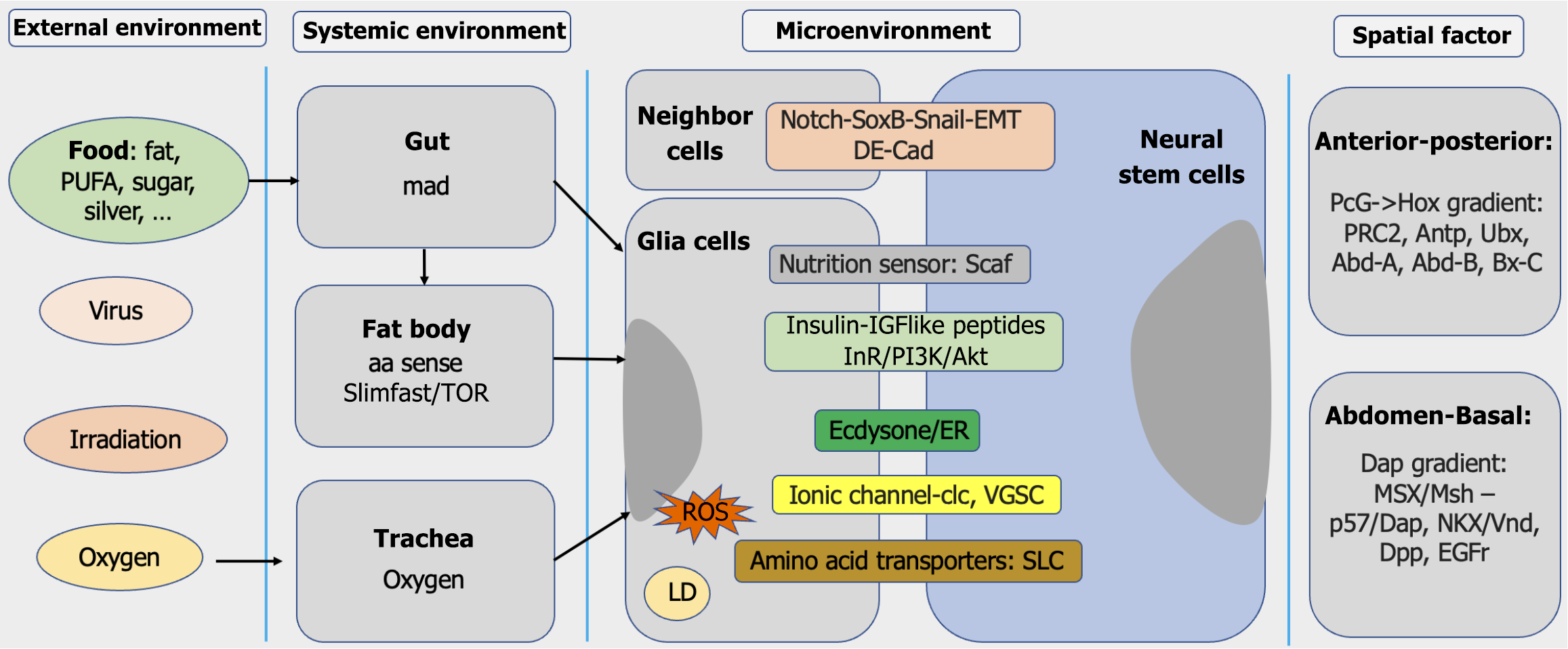
Intrinsic program and extrinsic cues jointly underpin NSC development. Ambient signals collaborate with intrinsic factors to regulate NSC division models, identity maintenance, and lifespan. These ambient signals originate from various sources, including the external environment, systemic environment, neighboring cells and spatial positioning cues (Figure 3). Recent studies have elucidated molecular mechanisms underlying these regulatory interactions. Key factors among these ambient cues are summarized in Table 1.
| Source | Factors | Observed effects | Ref. |
| Microenvironment | Snail, Notch, Sox, EMT | Altered cell number/division defect | [54,67,69,70] |
| Dap, Msh | Quiescent regulation (G0 or G2) | [44] | |
| PcG and Hox homeotic network | Anteroposterior stemness gradient | [47] | |
| DE-cad | Proliferation control | [71] | |
| ROS | Proliferation modulation | [65,81] | |
| GMC positioning | Division orientation | [49] | |
| Ecdysone | NCS termination | [16] | |
| Hh signaling | Proliferation promotion | [50] | |
| Insulin/IGF-like peptides | NSC reactivation | [25,40] | |
| Scaf protease | Proliferation regulation | [78] | |
| CLC, VGSC | Proliferation modulation | [39,76] | |
| Systemic environment | Fat body | NSC reactivation | [22] |
| Trachea | Proliferation regulation | [20] | |
| Gut | Proliferation modulation | [23] | |
| External environment | Irradiation | Premature differentiation | [18] |
| Oxygen | Proliferation suppression | [20] | |
| Virus infection | Differentiation defects | [19,80] | |
| High-sugar diet | Proliferation enhancement | [83] | |
| High-fat diet | Proliferation modulation | [84] | |
| Iron, silver ions | Nuclear deformation | [53] |
Localization critically determines NSC progeny positioning within the neural hierarchy. NSCs originate among the embryonic midline, guided by homeobox and proneural genes[54,55]. During segmentation, NSCs adopt segment-specific fates[47,56]. The NSCs along each segmentation present a specific pattern[57]. At the end of the embryonic stage, brain NSCs predominantly enter quiescence, while most of ventral nerve cord NSCs undergo apoptosis[33,58]. Post-embryonically, region-specific NSC behavior persists[59], basal and dorsal NSCs exhibit distinct developmental trajectories[44,60], and the polycomb group > Hox gene expression gradient controls brain expansion[47]. These spatial cues ensure regionally specialized neuronal outputs.
The neighboring cells of NSC serve as a microenvironment (niche) to govern NSC behavior. While early studies emphasized the intrinsic regulation via asymmetric division and temporal regulation[33,61,62], recent work has highlighted the microenvironment contributions[63-65].
Lateral inhibition is essential for the ‘birth’ of NSCs. Delta-expressing NSC precursors suppress pro-neural genes in neighboring cells via Notch[66,67]. Pro-neural genes and pan-neural genes work together to decide the central nervous system (CNS) fates[54]. Then, the CNS NSCs undergo delamination. During the first round of asymmetric division, the delaminating NSCs retain the apical stalk that is linked to the neuroepithelium, anchoring polarity[68]. After each round of division in the CNS, the newly ‘born’ NSCs adject to the neuroepithelia. Tight junctions, between the NSCs and epithelia, are essential for division orientation[69-71].
The larval brain NSC polarity axis lacks embryonic alignment from the epithelia to the inside[72,73] but orients division based on differentiated daughter positioning[49]. Neighboring glia regulate NSC metabolism via phosphoinositide 3-kinase/Akt/mammalian target of rapamycin signaling[74], secrete insulin-like peptides for reactivation[26,31,75], provide ions for proliferation[39,76], and buffer reactive oxygen species via unsaturated fatty acids[65,77]. Glia also form the protective blood-brain barrier[78,79].
During development, intertissue communication coordinates NSC activity. The fat body, the human liver homolog, integrates nutrient status to release insulin-like peptides, activating quiescent NSCs during the first larval stage[22,25]. The evidence indicates that dysfunctional trachea or gut impairs brain development[20,23,51,62], although the direct mechanistic links that underlie NSC development remain underexplored.
Global environmental inputs exert localized NSC effects. Irradiation induces NSC differentiation prematurely[18,21]. Virus infection impacts NSC development; for instance, Zika virus receptor mutants have been shown to disrupt NSC division[19,80]. Oxygen tension elevates reactive oxygen species, which in turn suppresses NSC proliferation[81]. Metabolic shifts, from glycolysis to oxidative phosphorylation, correlate with NSC termination[82]. Finally, dietary factors, such as high glucose, fats, or ions, modulate both NSC proliferation and differentiation[53,65,83,84]. However, how these external cues crosstalk within the NSC milieu remains unknown.
Adult brain neurogenesis represents a compelling area of study; however, in Drosophila, NSCs terminate activity during the early pupal stage and do not persist into adulthood[85]. Intriguingly, evidence from NSC-derived tumors or injury models has suggested that limited neurogenesis may occur in the adult-stage Drosophila brain[86].
No endogenous NSCs have been detected in the Drosophila adult brain under normal conditions. However, NSC-derived tumors retaining stem cell-like properties can persist in adult brains[72,87]. Additionally, the svp- or E93-mutant NSCs, which exhibit extended proliferation without tumorigenic traits, have been observed in adult brains[35,88]. These findings indirectly indicate that the adult Drosophila brain environment may support NSC survival under specific genetic perturbations.
While neuronal division is undetectable in the adult Drosophila brain under physiological conditions, injury-induced neurogenesis has been documented[85,89]. Advances in methodologies may further establish the adult Drosophila brain as a model for studying NSC regeneration and neurogenic plasticity.
The division patterns, temporal dynamics, and functional attributes of Drosophila NSCs are shaped by extrinsic environmental cues-encompassing niche signals, and external factors. These cues collectively safeguard NSCs from damage, deliver growth signals, and regulate division orientation and proliferation. For example, oxygen from the external environment, ecdysone from the systemic environment, nutrients from the neighboring glia cells and Hox protein from among the segmental factors collectively establish temporal regulation and facilitate NSC development during the larval stage[42]. Such precise coordination ensures the generation of specialized progeny and the maintenance of NSC identity, ultimately enabling the construction of refined neural circuitry.
However, several questions remain unanswered. How do these extrinsic ambient cues crosstalk? How do these extrinsic signals interact with intrinsic genetic factors? What are the responses of NSCs to the same extrinsic cue at different developmental stages? For instance, the Notch signaling pathway inhibits NSC birth at early stages[67] but promotes NSC proliferation at later stages[54]. Furthermore, how do other environmental inputs such as circadian rhythms, temperature, and microbial symbionts influence NSC behavior? Addressing these questions and validating these mechanisms functionally in vertebrate models (e.g., mouse/human NSCs) could inform therapeutic approaches directly, such as in mitigating irradiation-induced NSC damage or enhancing neurogenesis.
In summary, while the research in this field remains nascent, the insights reviewed here illuminate novel directions in further understanding NSC regulation and underscore the translational potential for clinical therapies targeting neural regeneration.
We acknowledge our collective global colleagues whose work could not be cited due to space constraints.
| 1. | Bond AM, Ming GL, Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 631] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 2. | Kang KH, Reichert H. Control of neural stem cell self-renewal and differentiation in Drosophila. Cell Tissue Res. 2015;359:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Holguera I, Desplan C. Neuronal specification in space and time. Science. 2018;362:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Berg DA, Su Y, Jimenez-Cyrus D, Patel A, Huang N, Morizet D, Lee S, Shah R, Ringeling FR, Jain R, Epstein JA, Wu QF, Canzar S, Ming GL, Song H, Bond AM. A Common Embryonic Origin of Stem Cells Drives Developmental and Adult Neurogenesis. Cell. 2019;177:654-668.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Turner NL, Macrina T, Bae JA, Yang R, Wilson AM, Schneider-Mizell C, Lee K, Lu R, Wu J, Bodor AL, Bleckert AA, Brittain D, Froudarakis E, Dorkenwald S, Collman F, Kemnitz N, Ih D, Silversmith WM, Zung J, Zlateski A, Tartavull I, Yu SC, Popovych S, Mu S, Wong W, Jordan CS, Castro M, Buchanan J, Bumbarger DJ, Takeno M, Torres R, Mahalingam G, Elabbady L, Li Y, Cobos E, Zhou P, Suckow S, Becker L, Paninski L, Polleux F, Reimer J, Tolias AS, Reid RC, da Costa NM, Seung HS. Reconstruction of neocortex: Organelles, compartments, cells, circuits, and activity. Cell. 2022;185:1082-1100.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 6. | Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2019;173:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 7. | Rajan A, Ostgaard CM, Lee CY. Regulation of Neural Stem Cell Competency and Commitment during Indirect Neurogenesis. Int J Mol Sci. 2021;22:12871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Guan W, Nie Z, Laurençon A, Bouchet M, Godin C, Kabir C, Darnas A, Enriquez J. The role of Imp and Syp RNA-binding proteins in precise neuronal elimination by apoptosis through the regulation of transcription factors. Elife. 2024;12:RP91634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Navarro Negredo P, Yeo RW, Brunet A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell. 2020;27:202-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 10. | Homem CC, Knoblich JA. Drosophila neuroblasts: a model for stem cell biology. Development. 2012;139:4297-4310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 11. | Obernier K, Alvarez-Buylla A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development. 2019;146:dev156059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 395] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 12. | Harterink M, Kim DH, Middelkoop TC, Doan TD, van Oudenaarden A, Korswagen HC. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development. 2011;138:2915-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Ramon-Cañellas P, Peterson HP, Morante J. From Early to Late Neurogenesis: Neural Progenitors and the Glial Niche from a Fly's Point of View. Neuroscience. 2019;399:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Li Y, Guo W. Neural Stem Cell Niche and Adult Neurogenesis. Neuroscientist. 2021;27:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Ma Z, Wang W, Yang X, Rui M, Wang S. Glial ferritin maintains neural stem cells via transporting iron required for self-renewal in Drosophila. Elife. 2024;13:RP93604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Homem CCF, Steinmann V, Burkard TR, Jais A, Esterbauer H, Knoblich JA. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell. 2014;158:874-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Syed MH, Mark B, Doe CQ. Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity. Elife. 2017;6:e26287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Wagle R, Song YH. Ionizing radiation reduces larval brain size by inducing premature differentiation of Drosophila neural stem cells. Biochem Biophys Res Commun. 2020;523:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Link N, Chung H, Jolly A, Withers M, Tepe B, Arenkiel BR, Shah PS, Krogan NJ, Aydin H, Geckinli BB, Tos T, Isikay S, Tuysuz B, Mochida GH, Thomas AX, Clark RD, Mirzaa GM, Lupski JR, Bellen HJ. Mutations in ANKLE2, a ZIKA Virus Target, Disrupt an Asymmetric Cell Division Pathway in Drosophila Neuroblasts to Cause Microcephaly. Dev Cell. 2019;51:713-729.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Baccino-Calace M, Prieto D, Cantera R, Egger B. Compartment and cell-type specific hypoxia responses in the developing Drosophila brain. Biol Open. 2020;9:bio053629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Xu X, An H, Wu C, Sang R, Wu L, Lou Y, Yang X, Xi Y. HR repair pathway plays a crucial role in maintaining neural stem cell fate under irradiation stress. Life Sci Alliance. 2023;6:e202201802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471:508-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Ding G, Xiang X, Hu Y, Xiao G, Chen Y, Binari R, Comjean A, Li J, Rushworth E, Fu Z, Mohr SE, Perrimon N, Song W. Coordination of tumor growth and host wasting by tumor-derived Upd3. Cell Rep. 2021;36:109553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Xu S, Scott K, Manshaii F, Chen J. Heart-brain connection: How can heartbeats shape our minds? Matter. 2024;7:1684-1687. [DOI] [Full Text] |
| 25. | Chell JM, Brand AH. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 2010;143:1161-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 317] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 26. | Bakshi A, Joshi R. Role of glial niche in regulating neural stem cell proliferation in Drosophila central nervous system. J Neurosci Res. 2020;98:2373-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 28. | Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 29. | Chia W, Yang X. Asymmetric division of Drosophila neural progenitors. Curr Opin Genet Dev. 2002;12:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Doe CQ. Temporal Patterning in the Drosophila CNS. Annu Rev Cell Dev Biol. 2017;33:219-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 31. | Rossi AM, Desplan C. Extrinsic activin signaling cooperates with an intrinsic temporal program to increase mushroom body neuronal diversity. Elife. 2020;9:e58880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Sood C, Justis VT, Doyle SE, Siegrist SE. Notch signaling regulates neural stem cell quiescence entry and exit in Drosophila. Development. 2022;149:dev200275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Tsuji T, Hasegawa E, Isshiki T. Neuroblast entry into quiescence is regulated intrinsically by the combined action of spatial Hox proteins and temporal identity factors. Development. 2008;135:3859-3869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | An H, Ge W, Xi Y, Yang X. Inscuteable maintains type I neuroblast lineage identity via Numb/Notch signaling in the Drosophila larval brain. J Genet Genomics. 2017;44:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 36. | An H, Yu Y, Ren X, Zeng M, Bai Y, Liu T, Zheng H, Sang R, Zhang F, Cai Y, Xi Y. Pipsqueak family genes dan/danr antagonize nuclear Pros to prevent neural stem cell aging in Drosophila larval brains. Front Mol Neurosci. 2023;16:1160222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 453] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 38. | Miyares RL, Lee T. Temporal control of Drosophila central nervous system development. Curr Opin Neurobiol. 2019;56:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Plazaola-Sasieta H, Zhu Q, Gaitán-Peñas H, Rios M, Estévez R, Morey M. Drosophila ClC-a is required in glia of the stem cell niche for proper neurogenesis and wiring of neural circuits. Glia. 2019;67:2374-2398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Harrison NJ, Connolly E, Gascón Gubieda A, Yang Z, Altenhein B, Losada Perez M, Moreira M, Sun J, Hidalgo A. Regenerative neurogenic response from glia requires insulin-driven neuron-glia communication. Elife. 2021;10:e58756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Spéder P, Brand AH. Systemic and local cues drive neural stem cell niche remodelling during neurogenesis in Drosophila. Elife. 2018;7:e30413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Sood C, Doyle SE, Siegrist SE. Steroid hormones, dietary nutrients, and temporal progression of neurogenesis. Curr Opin Insect Sci. 2021;43:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Doe CQ. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development. 1992;116:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 286] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Otsuki L, Brand AH. Dorsal-Ventral Differences in Neural Stem Cell Quiescence Are Induced by p57(KIP2)/Dacapo. Dev Cell. 2019;49:293-300.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Bayraktar OA, Doe CQ. Combinatorial temporal patterning in progenitors expands neural diversity. Nature. 2013;498:449-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 46. | Monedero Cobeta I, Salmani BY, Thor S. Anterior-Posterior Gradient in Neural Stem and Daughter Cell Proliferation Governed by Spatial and Temporal Hox Control. Curr Biol. 2017;27:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Bahrampour S, Jonsson C, Thor S. Brain expansion promoted by polycomb-mediated anterior enhancement of a neural stem cell proliferation program. PLoS Biol. 2019;17:e3000163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Tan Y, Yamada-Mabuchi M, Arya R, St Pierre S, Tang W, Tosa M, Brachmann C, White K. Coordinated expression of cell death genes regulates neuroblast apoptosis. Development. 2011;138:2197-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Berger C, Harzer H, Burkard TR, Steinmann J, van der Horst S, Laurenson AS, Novatchkova M, Reichert H, Knoblich JA. FACS purification and transcriptome analysis of drosophila neural stem cells reveals a role for Klumpfuss in self-renewal. Cell Rep. 2012;2:407-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Dong Q, Zavortink M, Froldi F, Golenkina S, Lam T, Cheng LY. Glial Hedgehog signalling and lipid metabolism regulate neural stem cell proliferation in Drosophila. EMBO Rep. 2021;22:e52130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Vaccaro A, Kaplan Dor Y, Nambara K, Pollina EA, Lin C, Greenberg ME, Rogulja D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 2020;181:1307-1328.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 52. | Endow SA, Miller SE, Ly PT. Mitochondria-enriched protrusions are associated with brain and intestinal stem cells in Drosophila. Commun Biol. 2019;2:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Basak AK, Chatterjee T, Chakravarty A, Ghosh SK. Silver nanoparticle-induced developmental inhibition of Drosophila melanogaster accompanies disruption of genetic material of larval neural stem cells and non-neuronal cells. Environ Monit Assess. 2019;191:497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Arefin B, Parvin F, Bahrampour S, Stadler CB, Thor S. Drosophila Neuroblast Selection Is Gated by Notch, Snail, SoxB, and EMT Gene Interplay. Cell Rep. 2019;29:3636-3651.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | von Ohlen T, Doe CQ. Convergence of dorsal, dpp, and egfr signaling pathways subdivides the drosophila neuroectoderm into three dorsal-ventral columns. Dev Biol. 2000;224:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Curt JR, Yaghmaeian Salmani B, Thor S. Anterior CNS expansion driven by brain transcription factors. Elife. 2019;8:e45274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Broadus J, Skeath JB, Spana EP, Bossing T, Technau G, Doe CQ. New neuroblast markers and the origin of the aCC/pCC neurons in the Drosophila central nervous system. Mech Dev. 1995;53:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 815] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 59. | Bakshi A, Sipani R, Ghosh N, Joshi R. Sequential activation of Notch and Grainyhead gives apoptotic competence to Abdominal-B expressing larval neuroblasts in Drosophila Central nervous system. PLoS Genet. 2020;16:e1008976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Otsuki L, Brand AH. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 61. | Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 62. | Cheng LY, Bailey AP, Leevers SJ, Ragan TJ, Driscoll PC, Gould AP. Anaplastic lymphoma kinase spares organ growth during nutrient restriction in Drosophila. Cell. 2011;146:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Harding K, White K. Decoupling developmental apoptosis and neuroblast proliferation in Drosophila. Dev Biol. 2019;456:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Mira H, Morante J. Corrigendum: Neurogenesis From Embryo to Adult - Lessons From Flies and Mice. Front Cell Dev Biol. 2020;8:686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, Postle AD, Gould AP. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell. 2015;163:340-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 521] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 66. | Park M, Yaich LE, Bodmer R. Mesodermal cell fate decisions in Drosophila are under the control of the lineage genes numb, Notch, and sanpodo. Mech Dev. 1998;75:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Zhao G, Skeath JB. The Sox-domain containing gene Dichaete/fish-hook acts in concert with vnd and ind to regulate cell fate in the Drosophila neuroectoderm. Development. 2002;129:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Schober M, Schaefer M, Knoblich JA. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature. 1999;402:548-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 307] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 69. | Ashraf SI, Ip YT. The Snail protein family regulates neuroblast expression of inscuteable and string, genes involved in asymmetry and cell division in Drosophila. Development. 2001;128:4757-4767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Cai Y, Chia W, Yang X. A family of snail-related zinc finger proteins regulates two distinct and parallel mechanisms that mediate Drosophila neuroblast asymmetric divisions. EMBO J. 2001;20:1704-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J Neurosci. 2003;23:3325-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 73. | Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 303] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 74. | Yuan X, Sipe CW, Suzawa M, Bland ML, Siegrist SE. Dilp-2-mediated PI3-kinase activation coordinates reactivation of quiescent neuroblasts with growth of their glial stem cell niche. PLoS Biol. 2020;18:e3000721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Gil-Ranedo J, Gonzaga E, Jaworek KJ, Berger C, Bossing T, Barros CS. STRIPAK Members Orchestrate Hippo and Insulin Receptor Signaling to Promote Neural Stem Cell Reactivation. Cell Rep. 2019;27:2921-2933.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 76. | Piggott BJ, Peters CJ, He Y, Huang X, Younger S, Jan LY, Jan YN. Paralytic, the Drosophila voltage-gated sodium channel, regulates proliferation of neural progenitors. Genes Dev. 2019;33:1739-1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Feng S, Zacharioudaki E, Millen K, Bray SJ. The SLC36 transporter Pathetic is required for neural stem cell proliferation and for brain growth under nutrition restriction. Neural Dev. 2020;15:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Contreras EG, Glavic Á, Brand AH, Sierralta JA. The Serine Protease Homolog, Scarface, Is Sensitive to Nutrient Availability and Modulates the Development of the Drosophila Blood-Brain Barrier. J Neurosci. 2021;41:6430-6448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Spéder P, Brand AH. Gap junction proteins in the blood-brain barrier control nutrient-dependent reactivation of Drosophila neural stem cells. Dev Cell. 2014;30:309-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 80. | Robinson BV, Faundez V, Lerit DA. Understanding microcephaly through the study of centrosome regulation in Drosophila neural stem cells. Biochem Soc Trans. 2020;48:2101-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (17)] |
| 81. | van den Ameele J, Brand AH. Neural stem cell temporal patterning and brain tumour growth rely on oxidative phosphorylation. Elife. 2019;8:e47887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Bonnay F, Veloso A, Steinmann V, Köcher T, Abdusselamoglu MD, Bajaj S, Rivelles E, Landskron L, Esterbauer H, Zinzen RP, Knoblich JA. Oxidative Metabolism Drives Immortalization of Neural Stem Cells during Tumorigenesis. Cell. 2020;182:1490-1507.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 83. | Kemppainen E, George J, Garipler G, Tuomela T, Kiviranta E, Soga T, Dunn CD, Jacobs HT. Mitochondrial Dysfunction Plus High-Sugar Diet Provokes a Metabolic Crisis That Inhibits Growth. PLoS One. 2016;11:e0145836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Nayak N, Mishra M. High fat diet induced abnormalities in metabolism, growth, behavior, and circadian clock in Drosophila melanogaster. Life Sci. 2021;281:119758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 85. | Li G, Hidalgo A. Adult Neurogenesis in the Drosophila Brain: The Evidence and the Void. Int J Mol Sci. 2020;21:6653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Crocker KL, Marischuk K, Rimkus SA, Zhou H, Yin JCP, Boekhoff-Falk G. Neurogenesis in the adult Drosophila brain. Genetics. 2021;219:iyab092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 87. | Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14:535-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 359] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 88. | Pahl MC, Doyle SE, Siegrist SE. E93 Integrates Neuroblast Intrinsic State with Developmental Time to Terminate MB Neurogenesis via Autophagy. Curr Biol. 2019;29:750-762.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 89. | Shepherd D, Sahota V, Court R, Williams DW, Truman JW. Developmental organization of central neurons in the adult Drosophila ventral nervous system. J Comp Neurol. 2019;527:2573-2598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/