Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1483
Peer-review started: November 14, 2020
First decision: December 3, 2020
Revised: December 16, 2020
Accepted: December 27, 2020
Article in press: December 27, 2020
Published online: February 26, 2021
Processing time: 84 Days and 5.1 Hours
Gastrointestinal (GI) hemangioma has a low incidence among systemic hemangiomas, and some GI hemangiomas occur in the intestine, stomach, and esophagus. Polidocanol has been increasingly used in sclerotherapy. However, this paper reports that minimally invasive treatment of multiple hemangiomas with large diameters can achieve satisfactory results by multipoint injection.
A 46-year-old female patient was hospitalized in another hospital for cough. We accidentally found thickening of the lower esophagus by chest computed tomography. The patient was eventually diagnosed with multiple GI hemangiomas and underwent a series of examinations including esophagogastroduodenoscopy (EGD), endoscopic ultrasound, and magnetic resonance imaging. We calculated the dose of polidocanol according to the volumes of the hemangiomas, fixed the target vein with the help of a transparent cap, and then administered polidocanol via multipoint injection into the hemangiomas under endoscopic guidance. EGD and endoscopic ultrasound showed that the hemangiomas disappeared. The color of the esophageal mucosa returned to normal 1 mo after sclerotherapy.
Sclerotherapy may be a safe and effective method for treating multiple hemangiomas of the alimentary canal.
Core Tip: Multiple gastrointestinal (GI) hemangiomas are rare in clinical practice, and the current treatment method for lager GI hemangiomas is surgery. In this case, multipoint injection of polidocanol was used to treat gastroesophageal hemangiomas. We used a reasonable dose of polidocanol and applied the treatment using a transparent cap. This sclerotherapy method successfully treated a patient with multiple hemangiomas without any complications.
- Citation: Yao H, Xie YX, Guo JY, Wu HC, Xie R, Shi GQ. Polidocanol sclerotherapy for multiple gastrointestinal hemangiomas: A case report. World J Clin Cases 2021; 9(6): 1483-1489
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1483.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1483
Hemangiomas can occur in various organs of the body. The incidence of oral and maxillofacial hemangioma accounts for 60% of the incidence of systemic hemangioma, followed by trunk hemangioma (25%) and limb hemangioma (15%). Gastrointestinal (GI) hemangioma is a rare benign vascular neoplasm and might be associated with congenital disorders such as Osler-Weber-Rendu disease, Maffucci’s syndrome, or the congenital blue rubber bleb nevus syndrome[1]. The main treatment for lager GI hemangioma is surgery. However, there are some disadvantages of surgical removal of GI hemangiomas, such as considerable trauma, long recovery period, and pain. Polidocanol injection sclerotherapy has been successfully applied for the treatment of cutaneous hemangiomas[2] and venous malformations[3]. In the treatment of GI hemangiomas, esophageal varices sclerotherapy, internal hemorrhoid sclerotherapy, and intestinal hemangioma sclerotherapy have been reported[4-6]. In a case of using sclerotherapy to control bleeding, it was mentioned that sclerotherapy with 3% polidocanol foam induces an inflammatory reaction with sclerosis of the submucosal tissue and consequent suspension of the hemorrhoidal tissue[7]. Markovic et al[8] reported that sclerotherapy in the treatment of low flow vascular malformations did not significantly change hemodynamic consequences and reduce complications. However, endoscopic multipoint injection of polidocanol is rarely reported in the treatment of lager cavernous hemangiomas of the digestive tract. Previous reports showed that most of hemangiomas larger than 3 cm in diameter are treated by surgical treatment. We report a rare case of multiple GI hemangiomas with multiple cavities. Satisfactory treatment results have been achieved by using multi-point sclerotherapy.
A 46-year-old woman was admitted to our hospital because of cough and intermittent swallowing discomfort.
The patient was hospitalized because of dysphagia in another hospital. Computed tomography (CT) examination showed that the lower esophagus was thickened. In order to get better treatment, the patient came to our hospital on July 8, 2019.
She had a previous history of thyroid sarcoidosis but did not receive treatment.
The patient had no significant family history.
On examination, the vital signs were normal with a respiratory rate of 20/min, heart rate of 89/min, and blood pressure of 98/62 mmHg.
The patient’s biochemical indicators were normal.
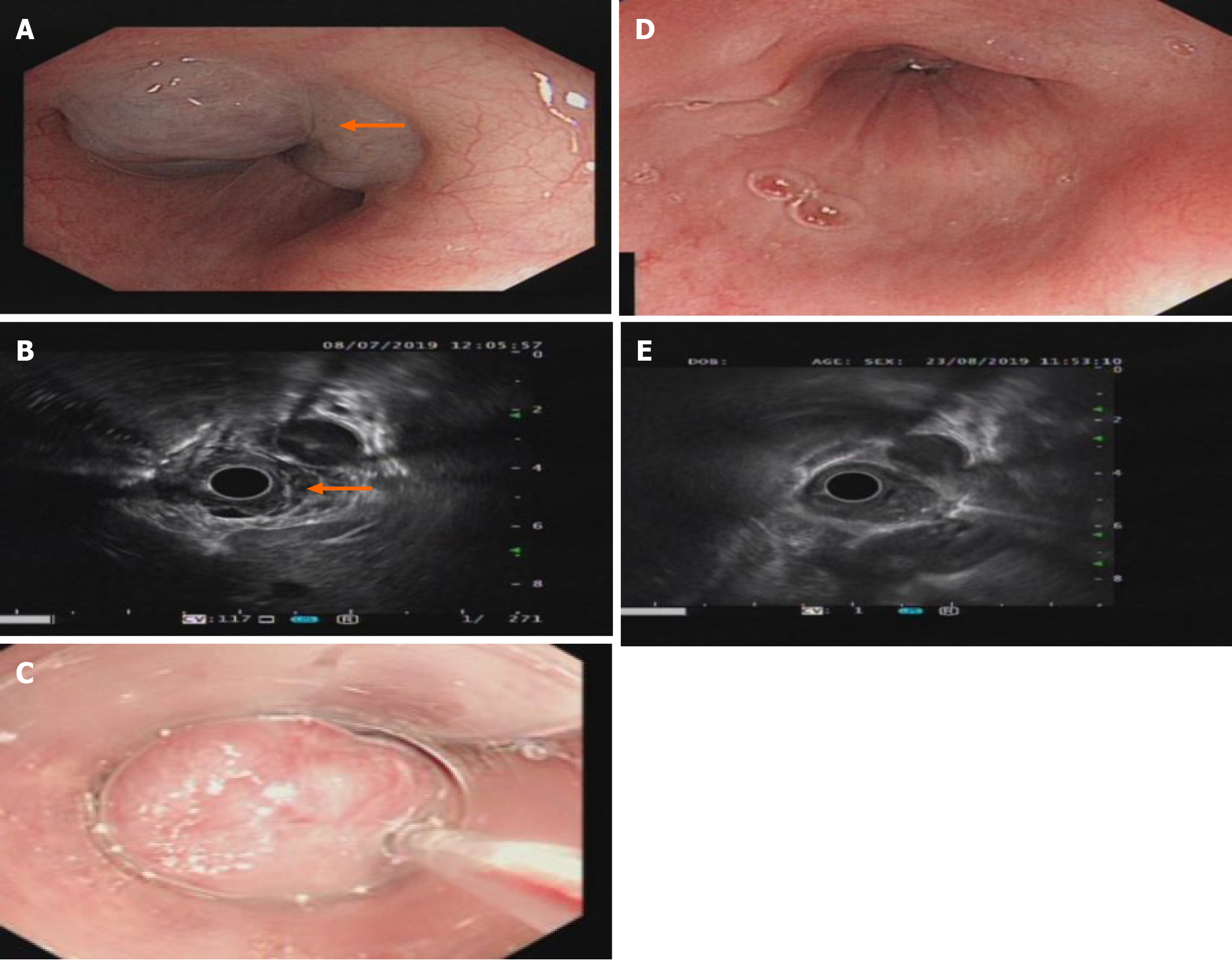
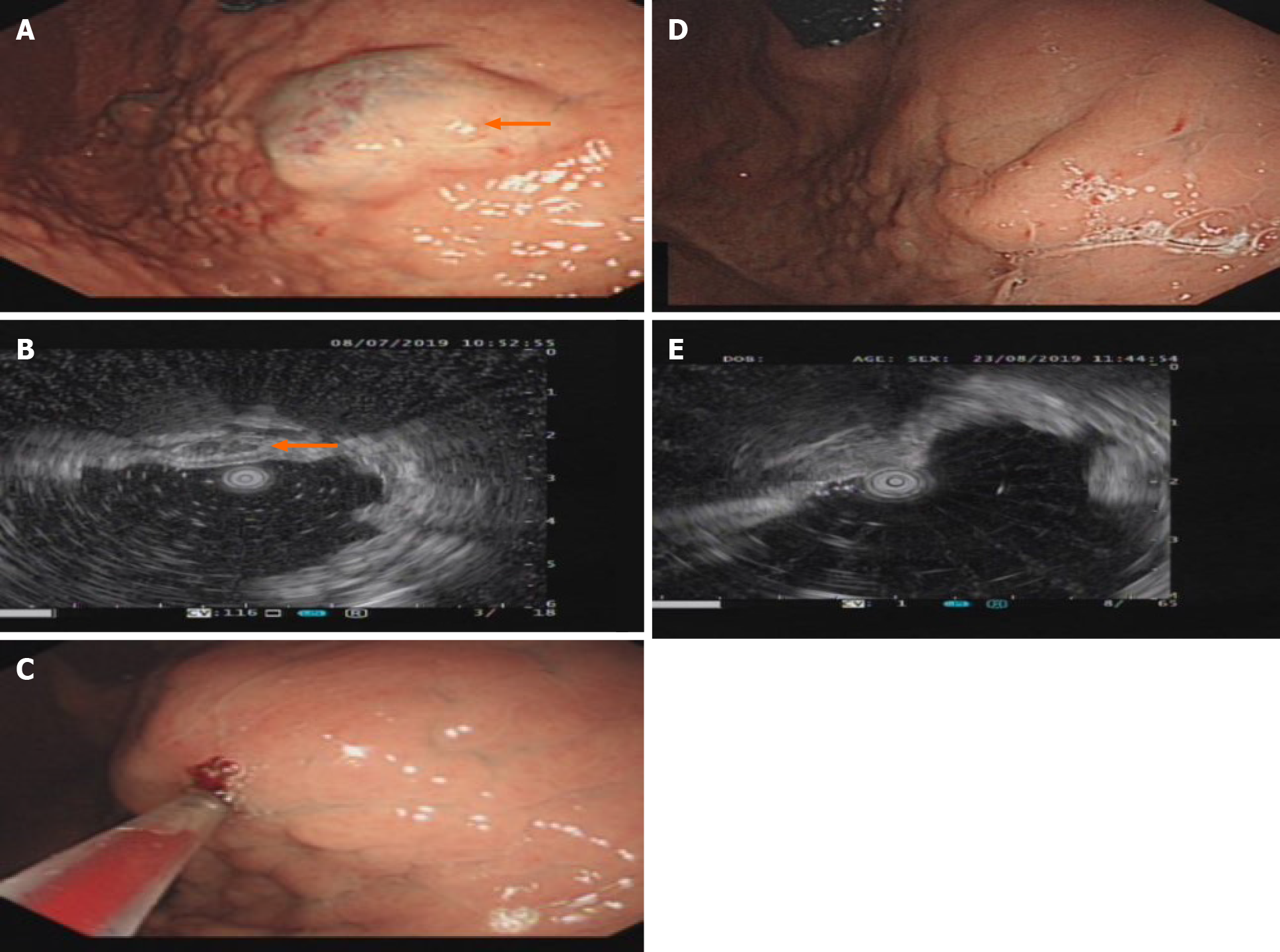
T2-weighted magnetic resonance imaging (MRI) showed hyperintensity of the lower esophagus and the cardia, no abnormal signal was observed on diffusion weighted-imaging (DWI), and nodular delayed enhancement was observed in the enhanced portal vein phase and delayed phase. Upper esophagogastroduodenoscopy (EGD) performed on July 8, 2019 revealed a large submucosal bulge 30-35 mm from the incisors. The surface mucosa was smooth and blue, and the bulge was approximately 4.0 cm × 4.5 cm × 2 cm in size and involved 1/2 of the tube wall (Figure 1A). A submucosal bulge of approximately 2.5 cm × 3.0 cm × 2 cm in size was observed on the posterior wall of the bottom of the stomach, the surface of the mucosa was smooth, and the color of the mucosa was pale purple (Figure 2A). Upper gastrointestinal endoscopic ultrasound performed on July 8, 2019 showed that the lesions in the lower esophagus and posterior wall of the stomach originated from the submucosa, which protruded into a cavity with a grid-like echoless structure. The lesion was considered a cavernous hemangioma (Figures 1B and 2B).
Multiple cavernous hemangiomas were finally diagnosed by esophagogastroduodenoscopy and endoscopic ultrasound.
The patient refused to undergo surgical treatment for her own reasons, and after detailed communication with her, she decided to undergo treatment for her multiple GI hemangiomas with an endoscopic polidocanol (10 mL:100 mg, China's Shaanxi Tianyu Pharmaceutical) injection according to the surgeon's experience with endoscopic polidocanol sclerotherapy for esophageal and gastric varices. The injection dose must be based on the volume of the tumor, and according to the formula for tumor volume, 36 mL of polidocanol was calculated for injection of the esophageal hemangioma (4.0 cm × 4.5 cm × 2 cm = 36 cm3 = 36 mL), and 15 mL of polidocanol was calculated for injection of the gastric hemangioma (2.5 cm × 3.0 cm × 2 cm = 15 cm3 = 15 mL). A 25G endoscopic injection needle (Boston Science Company) was used to puncture the hemangioma, and then polidocanol was injected after blood return was observed. The posterior wall of the stomach was injected with 15 mL of polidocanol at three points (5 mL per point). After the needle was removed, the minimal bleeding at the eye of the needle resolved spontaneously. The esophagus was injected with 36 mL of polidocanol at three points (12 mL per point). The eye of the needle oozed after the needle was withdrawn, and the bleeding stopped after 3-5 min of compression with a needle sheath (Figures 1C and 2C). No abnormal changes in the heart rhythm, breathing, or blood pressure were observed during the operation. The patient was fasted for 24 h, proton pump inhibitors were administered to inhibit gastric acid, and she was observed for 48 h after surgery. The patient was discharged without discomfort and continued to be treated with proton pump inhibitors after discharge.
The patient was discharged without any complications and was in good clinical condition 1 wk after the treatment. EGD reexamination found that gastric hemangioma tumors were absent and the local mucosa was smooth without erosion or ulcers (Figures 1E and 2E) 1 mo after the treatment. One month later, endoscopic ultrasound showed that the bodies of the hemangiomas had disappeared, and no blood flow echo was observed (Figures 1D and 2D).
Hemangiomas in the GI tract account for 0.05% of GI tumors[9]. Compared with primary or metastatic tumors in the GI tract, the incidence of GI hemangioma is very low. Although the gold standard for the diagnosis of GI hemangiomas is histopathology, with the development of medical technology, CT and MRI combined with GI ultrasound and endoscopy are also sufficient for the clinical diagnosis. The sensitivity of ultrasound is 96.9%, and the specificity is 60.3%; the sensitivity of MRI is 100%, and the specificity is 85.7% in noninvasive diagnostic studies of hepatic hemangioma[10]. Therefore, CT and MRI can obtain certain value in the diagnosis of GI hemangioma. Of course, EGD and endoscopic ultrasound have greater value in the diagnosis and treatment of submucosal hemangioma.
The main treatment methods for superficial hemangiomas include surgery, laser therapy, and sclerotherapy. For GI hemangiomas, most lager hemangiomas are treated by surgery or endoscopic mucosal dissection (ESD) (Table 1)[9,11-21]. Surgery is associated with considerable trauma, long recovery period, and significant pain. Complications of ESD treatment include bleeding, perforation, subcutaneous emphysema, and infection; thus, other feasible minimally invasive treatment methods should be actively attempted. In the treatment of GI hemangioma, Fernandes and Fonseca reported a clinical study on the treatment of internal hemorrhoids with a large dose of 2% polidocanol, which successfully reduced the rebleeding rate[5]. Igawa et al[6] reported a clinical case of a small intestinal hemangioma successfully treated with single-point injection of polidocanol, and the amount of polidocanol was calculated according to the surface area of the hemangioma. Polidocanol can disintegrate the tumor tissue, make the tumor necrosis and fall off, achieve a similar effect to surgical resection, and obtain satisfactory results. Polidocanol is an ether compound with a slight anesthetic effect on local tissues that can reduce the pain of local lesions in patients after surgery. Polidocanol is hypoallergenic and has low toxicity[2]. Polidocanol noninvasive treatment achieved satisfactory results. No adverse reactions such as fever, abdominal pain, or bleeding were observed during the follow-up period.
| No. | Ref. | Year | Age | Gender | Symptom | Site | Size (cm) | Treatment |
| 1 | Peng et al[11] | 2015 | 43 | F | Bloating, melena | Gastric | 7.5 ×3.5 × 2 | Surgery |
| 2 | Tai et al[12] | 2012 | 23 | F | Bloating, anemia | Gastric | 24 ×14 × 5 | Surgery |
| 3 | Feng et al[13] | 2008 | 14 | M | Hematemesis, melena | Gastric | 10 × 15 | Surgery |
| 4 | Shigemitsu et al[14] | 2000 | 39 | M | - | Esophagus | 11 × 10 | Laser |
| 5 | Shi et al[15] | 2019 | 52 | F | Dysphagia, thoracalgia | Esophagus | 0.7 ×1.0 | ESD |
| 6 | Gómez-Galán et al[16] | 2016 | 43 | F | Anemia | Duodenum, jejunum | 4 × 2 × 1 | Surgery |
| 7 | Mirioglu et al[17] | 2016 | 70 | M | Hematochezia, constipation | Rectum | Multiple | Surgery |
| 8 | Grgić et al[18] | 2019 | 73 | M | Anemia | Jejunum | 2 × 2 | Surgery |
| 9 | Grgić et al[18] | 2019 | 63 | M | Anemia | IleumJejunum | 8 | Surgery |
| 10 | Kitahama et al[19] | 2016 | 38 | F | Diarrhea | Colon | 3 | Surgery |
| 11 | Fernandes et al[9] | 2014 | 56 | F | Hematochezia, syncope | Ileum | 14 | Surgery |
| 12 | Aoyama et al[20] | 2020 | 58 | F | Anemia | Duodenum Jejunum | Multiple | Sclerotherapy |
| 13 | Xiao et al[21] | 2020 | 42 | M | Melena, dizziness, and fatigue | Jejunum | - | Sclerotherapy |
Compared with other reported cases, this case had the following characteristics: First, we report a case of multiple GI hemangiomas with multiple cavities. Multiple multicavity of GI hemangioma can be treated by injecting sclerosing agent into multiple sites. Multipoint injection can allow polidocanol to enter the entire tumor as much as possible. In addition, for conversion of the injection dose, we used the volume formula instead of the surface area formula, which can more accurately provide the specific dose required to eliminate the tumor. During the operation, the esophagus may be affected by peristalsis, respiration, the heartbeat, etc., resulting in unclear visualization and difficulty in fixing the puncture needle. Accordingly, puncture can be performed with the help of a transparent cap. Transparent cap-assisted sclerotherapy provides a clear field of vision that assists in fixing the targeted veins, significantly reducing the use of the salvage hemostasis method during sclerotherapy for injection hemorrhage[22].
For GI hemangioma, compared with gastroscopy and endoscopic ultrasound, CT or MRI has a certain value in the diagnosis of GI hemangioma. Multipoint polidocanol injection for the treatment of multiple GI hemangiomas under endoscopic guidance has the advantages of safety, efficacy, simplicity, cost effectiveness, and few adverse reactions.
| 1. | Levy AD, Abbott RM, Rohrmann CA Jr, Frazier AA, Kende A. Gastrointestinal hemangiomas: imaging findings with pathologic correlation in pediatric and adult patients. AJR Am J Roentgenol. 2001;177:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Grover C, Khurana A, Bhattacharya SN. Sclerotherapy for the treatment of infantile hemangiomas. J Cutan Aesthet Surg. 2012;5:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Gao Z, Zhang Y, Li W, Shi C. Effectiveness and safety of polidocanol for the treatment of hemangiomas and vascular malformations: A meta-analysis. Dermatol Ther. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Wu K, Song Q, Gou Y, He S. Sandwich method with or without lauromacrogol in the treatment of gastric variceal bleeding with liver cirrhosis: A meta-analysis. Medicine (Baltimore). 2019;98:e16201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Fernandes V, Fonseca J. Polidocanol Foam Injected at High Doses with Intravenous Needle: The (Almost) Perfect Treatment of Symptomatic Internal Hemorrhoids. GE Port J Gastroenterol. 2019;26:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 6. | Igawa A, Oka S, Tanaka S, Kunihara S, Nakano M, Chayama K. Polidocanol injection therapy for small-bowel hemangioma by using double-balloon endoscopy. Gastrointest Endosc. 2016;84:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Lobascio P, Laforgia R, Novelli E, Perrone F, Di Salvo M, Pezzolla A, Trompetto M, Gallo G. Short-Term Results of Sclerotherapy with 3% Polidocanol Foam for Symptomatic Second- and Third-Degree Hemorrhoidal Disease. J Invest Surg. 2020: 1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Markovic JN, Nag U, Shortell CK. Safety and efficacy of foam sclerotherapy for treatment of low-flow vascular malformations in children. J Vasc Surg Venous Lymphat Disord. 2020;8:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Fernandes D, Dionísio I, Neves S, Duarte P. Cavernous hemangioma of small bowel: a rare cause of digestive hemorrhage. Rev Esp Enferm Dig. 2014;106:214-215. [PubMed] |
| 10. | Toro A, Mahfouz AE, Ardiri A, Malaguarnera M, Malaguarnera G, Loria F, Bertino G, Di Carlo I. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol. 2014;13:327-339. [PubMed] |
| 11. | Peng X, Yang X, Fan CQ, Yang SM, Bai JY. A case of gastric cavernous hemangioma. Jujie Shoushu Xue Zazhi. 2015;24:698-699. [DOI] [Full Text] |
| 12. | Tai JD, Zhao PW, Li B. A case of giant cavernous hemangioma of stomach. Zhonghua Weichang Waike Zazhi. 2012;15. [DOI] [Full Text] |
| 13. | Feng HL, Li CY, Yin XP. A case of giant gastric hemangioma with multiple hemangioma in liver. Fangshe Xue Shijian Zazhi. 2008;23. [DOI] [Full Text] |
| 14. | Shigemitsu K, Naomoto Y, Yamatsuji T, Ono K, Aoki H, Haisa M, Tanaka N. Esophageal hemangioma successfully treated by fulguration using potassium titanyl phosphate/yttrium aluminum garnet (KTP/YAG) laser: a case report. Dis Esophagus. 2000;13:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Shi BP, Fu Y, Liu GB, Qu GD, Li XH, Wei L, Li J, Lu ML. A case report of esophageal cavernous hemangioma. Weichangbing Xue He Ganbing Xue Zazhi. 2019;8:959-960. |
| 16. | Gómez-Galán S, Mosquera-Paz MS, Ceballos J, Cifuentes-Grillo PA, Gutiérrez-Soriano L. Duodenal hemangiolymphangioma presenting as chronic anemia: a case report. BMC Res Notes. 2016;9:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Mirioglu S, Cavus B, Iliaz R, Besisik F. Diffuse Cavernous Hemangioma of the Colon. Acta Gastroenterol Belg. 2016;79:393-394. [PubMed] |
| 18. | Grgić D, Prijić R, Romić I, Augustin G, Markoš P, Korša L, Marušić Z, Rustemović N, Krznarić Ž. A Single Small Bowel Hemangioma Detected by Video Capsule Endoscopy in a Patient Presenting with Iron-deficiency Anemia - Two Case Reports. Prague Med Rep. 2019;120:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 19. | Kitahama A, Katayama Y, Sugamata Y, Tamano M. Large venous malformation of right colonic flexure. Korean J Intern Med. 2016;31:1194-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Aoyama T, Fukumoto A, Shigita K, Asayama N, Mukai S, Nagata S. Successful Endoscopic Sclerotherapy Using Polidocanol for Small Bowel Hemangioma. Intern Med. 2020;59:1727-1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Xiao NJ, Ning SB, Li T, Li BR, Sun T. Small intestinal hemolymphangioma treated with enteroscopic injection sclerotherapy: A case report and review of literature. World J Gastroenterol. 2020;26:1540-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ma L, Huang X, Lian J, Wang J, Chen S. Transparent cap-assisted endoscopic sclerotherapy in esophageal varices: a randomized-controlled trial. Eur J Gastroenterol Hepatol. 2018;30:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallo G S-Editor: Gao CC L-Editor: Wang TQ P-Editor:Liu JH