Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1359
Peer-review started: September 12, 2020
First decision: November 29, 2020
Revised: December 9, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 26, 2021
Processing time: 140 Days and 20.8 Hours
Central nervous system graft-vs-host disease (CNS-GVHD) is a rare cause of CNS disorders after allogeneic hematopoietic stem cell transplantation. Currently, establishing a diagnosis of CNS-GVHD is challenging because the diagnostic criteria and diagnostic methods are not well defined and many confounding factors need to be ruled out.
Here, we present two patients with CNS-GVHD. Both patients with a history of acute GVHD or chronic GVHD developed neurological symptoms that could not be explained by other causes, and had abnormal cerebrospinal fluid (CSF) studies as determined by CSF and blood immune biomarker examinations, suggestive of suspected CNS-GVHD. Due to the lack of specific magnetic resonance imaging abnormalities and the rapid clinical deterioration of the patients, we did not attempt to perform a brain biopsy, but prompted the initiation of empirical immunosuppressive therapy. In view of the rapid and favorable response to local and systematic immunosuppressive treatment and the aforementioned neurologic manifestations together with CSF abnormalities and other negative findings, a final diagnosis of CNS-GVHD was made.
CSF and blood immune biomarker examinations facilitated the diagnosis of CNS-GVHD, which are particularly suitable for patients who are critically ill and require urgent treatment and for those who are unsuitable for invasive diagnostic procedures.
Core Tip: We systematically report the diagnostic methods used for central nervous system graft-vs-host disease and present our own diagnostic criteria. Furthermore, we propose that non-invasive tools, especially cerebrospinal fluid and blood immune biomarker examinations, facilitated the diagnosis of central nervous system graft-vs-host disease, which are particularly suitable for patients who are critically ill and require urgent treatment and for those who are unsuitable for invasive diagnostic procedures.
- Citation: Lyu HR, He XY, Hao HJ, Lu WY, Jin X, Zhao YJ, Zhao MF. Noninvasive tools based on immune biomarkers for the diagnosis of central nervous system graft-vs-host disease: Two case reports and a review of the literature. World J Clin Cases 2021; 9(6): 1359-1366
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1359.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1359
Allogenic hematopoietic stem cell transplantation (allo-HSCT) is currently the only treatment strategy that has the potential to cure hematological malignancies. However, central nervous system (CNS) complications following transplantation pose a risk to patient survival[1-4]. Previous clinical studies reported that CNS complications occur in 11%-59% of patients after HSCT, including infections, drug-toxicity, disease relapse, secondary malignancies, vascular or metabolic abnormalities, as well as rare CNS graft-vs-host disease (CNS-GVHD)[5-7]. Two studies have reported that the incidence of immune-mediated neuropathy is 0.36% after stem cell transplantation and 1.04% after haploidentical HSCT, respectively[8,9]. Currently, establishing a diagnosis of CNS-GVHD is challenging because the diagnostic criteria and diagnostic methods are not well defined and many confounding factors need to be ruled out. Here, we report 2 cases of CNS-GVHD and review the literature on the currently available diagnostic methods for CNS-GVHD. We systematically report the diagnostic methods used for CNS-GVHD. It is suggested that the detection of immune biomarkers is necessary and has important clinical significance for the diagnosis of CNS-GVHD.
Case 1: A 15-year-old female complained of progressive vertigo, moderate headaches, nausea, vomiting, delusions, paroxysmal restlessness and insomnia. Simultaneously, she presented with a low fever.
Case 2: A 22-year-old male complained of vertigo, intermittent headache, impaired consciousness, confused speech, visual hallucinations, nausea, and vomiting.
Case 1: A 15-year-old female complained of progressive vertigo, moderate headaches, nausea, vomiting, delusions, paroxysmal restlessness and insomnia. Simultaneously, she presented with a low fever.
Case 2: A 22-year-old male complained of vertigo, intermittent headache, impaired consciousness, confused speech, visual hallucinations, nausea, and vomiting.
Case 1: The patient underwent a 5/10 human leukocyte antigen (HLA)-matched haploidentical donor peripheral blood stem cell transplant from her father in September 2018 for severe aplastic anemia with ASXL1 mutation. Approximately 4 mo after transplantation, the patient developed chronic GVHD (cGVHD) with lichen planus-like changes in the oral mucosa, which was treated with oral triamcinolone and topical dexamethasone mouthwashes.
Case 2: The patient underwent a 5/10 HLA-matched haploidentical peripheral blood stem cell transplant from his father in August 2018 for acute lymphoblastic leukemia with BCR/ABL P210 positive. The blood concentration of cyclosporine was maintained at a low level due to the presence of minimal residual disease. Approximately 2.5 mo after transplantation, he developed grade II acute GVHD (aGVHD) with rash and diarrhea based on the Glucksberg classification. He was treated with cyclosporine, sirolimus, and intravenous methylprednisolone (120 mg/d) for three days, and quickly achieved complete remission. The corticosteroid was then tapered and finally stopped.
No relevant personal and family history.
Case 1: Physical examination revealed bradypsychia, posterior cord track syndrome and ataxia.
Case 2: Physical examination revealed impaired consciousness, confused speech and visual hallucinations.
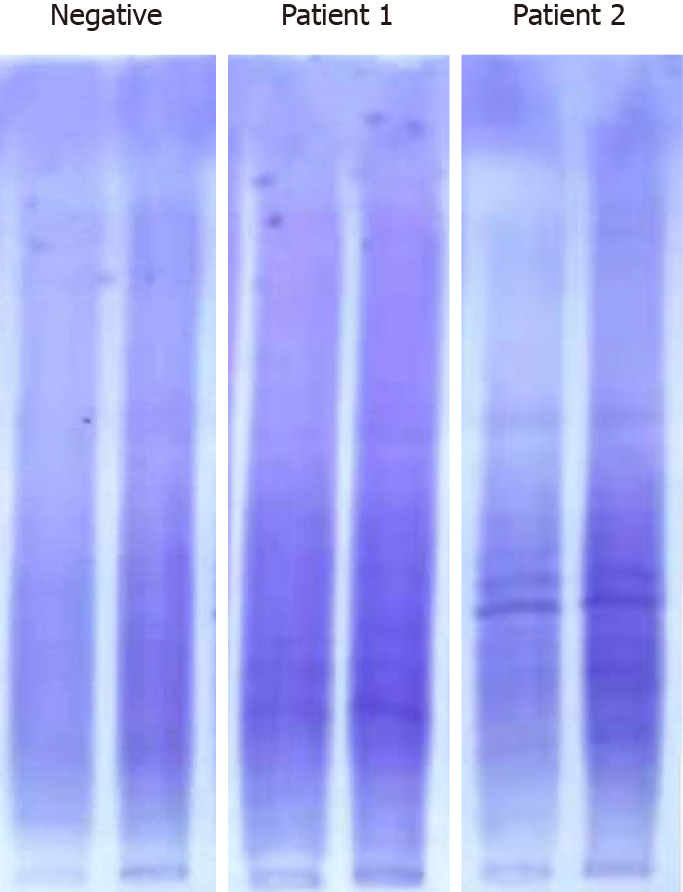
Case 1: CSF examination revealed no pleocytosis or abnormal glucose level, but showed elevated immunoglobulin G [IgG, 8.69 mg/dL (normal range 0.48-5.86 mg/dL)]. Then CSF and blood immune biomarker examinations were performed, including blood-brain barrier (BBB) permeability, IgG index, IgG synthesis rate (IgG-Syn), CSF and blood myelin basic protein (MBP), CSF and blood anti-myelin basic protein antibody (MBP.Ab), and CSF and blood anti-myelin oligodendrocyte glycoprotein antibody (MOG.Ab), which showed oligoclonal band type IV in blood and CSF (isoelectric focusing) and elevated IgG index (Figure 1). There was no evidence of hemolysis suggesting ongoing microangiopathy, and no serious kidney or liver dysfunction.
Case 2: CSF studies revealed pleocytosis [32 leucocytes/µL (normal range 0-5/µL)], lymphocytosis (89%), protein elevation [84.4 mg/dL (normal range 15-45 mg/dL)] and normal glucose level. There was no evidence of leukemia cells when the immuno-phenotype of CSF cells was detected. The CSF and blood immune biomarker examination showed positive oligoclonal band type IV in blood and CSF, elevated MBP and MBP.Ab in blood, and increased BBB permeability, IgG index and CSF IgG-Syn (Figure 1).
Case 1: A brain magnetic resonance imaging (MRI) ruled out the possibility of bleeding complications or post-transplantation lymphoproliferative disorders. A cervical MRI showed mild protrusion of C3-4, 4-5 and 5-6 intervertebral discs.
Case 2: A brain MRI did not reveal any abnormal lesions.
The final diagnosis of the presented two cases was CNS-GVHD.
Tacrolimus was replaced by oral rapamycin and intravenous dexamethasone (15 mg/d) to control the neurological symptoms. The patient's clinical symptoms quickly improved. The dose of dexamethasone was then tapered and decreased to an oral dose of 5.25 mg/d. After 20 d of treatment, her symptoms were relieved, and the patient was discharged. However, her neurological symptoms reappeared after 7 d, with progressive confusion, vertigo, delirium, visual hallucinations, suicidal tendency and temporarily impaired consciousness. Her performance status dropped to 4 points according to the Eastern Cooperative Oncology Group scoring criteria. All other findings were very similar to those at the time of initial diagnosis, including a repeat MRI. She was treated with 20 mg/d intravenous dexamethasone, 500 mg/d MMF and 20 g/d immunoglobulin for 5 d, and 10 mg/d oral ruxolitinib, in addition to anti-psychotic therapy with risperidone, and significant improvement was observed within 8 d. The dose of corticosteroid was then tapered. After 20 d of hospitalization, the patient was discharged and given oral triamcinolone (16 mg/d), MMF (500 mg/d) and ruxolitinib (10 mg/d). Unfortunately, she developed vertigo and diplopia again two months later. She received treatment with intravenous dexamethasone (20 mg/day for 5 d), CY (400 mg/m2 every 2 wk) and rituximab (375 mg/m2, once a week, 2 doses).
He received empiric therapy with intrathecal dexamethasone (10 mg) once a week for 4 wk and achieved rapid improvement of neurological symptoms within 48 h.
The patient has been followed up for three and a half months and her neurological symptoms have not reappeared. However, the CSF and blood immune biomarker examination still displayed an increase in the level of BBB permeability, neuron-specific enolase (NSE), S-100β, MBP and MBP.Ab. Therefore, she is currently receiving maintenance treatment with triamcinolone (24 mg/d), sirolimus, and ruxolitinib. In line with the clinical manifestations, laboratory findings, radiology, microbiology, and treatment response to immunosuppressive agents, the patient was diagnosed with CNS-GVHD.
The patient has been followed up for three months and has been in good condition without recurrence of abnormal neurological symptoms.
GVHD is one of the most serious complications after allo-HSCT and occurs when donor T cells recognize and target alloantigens on healthy recipient tissues. aGVHD mainly targets the skin, gut, and liver, whereas cGVHD can affect most organs, including the CNS in rare cases. In the past, CNS involvement of GVHD was controversial, but more animal and human cases were histologically confirmed and revealed that there was frequent T cell infiltration, supporting the hypothesis of an immune-mediated CNS disease after allo-HSCT[10-12]. However, CNS-GVHD remains very rare, and only a few cases have been reported. The clinical diagnosis of CNS-GVHD is extremely challenging for clinicians. In 2010, the neurological manifestations of cGVHD were described as a distinct entity in the Consensus Conference on Clinical Practice in cGVHD. The authors proposed the following mandatory criteria: the occurrence of neurological symptoms with cGVHD affecting other organs and CNS involvement without other explanations (i.e., without any infectious, vascular, drug toxicity, or metabolic etiologies). Other criteria were facultative: (1) Consistent brain MRI abnormalities; (2) CSF abnormalities (pleocytosis, elevated protein or IgG oligoclonal bands); (3) Pathological brain biopsy or postmortem examinations revealing GVHD lesions; and (4) A response to immunosuppressive therapy. The diagnosis of chronic CNS-GVHD can be made when both the mandatory and 2 facultative criteria are met[13]. In the consensus conference, the occurrence of cGVHD affecting other organs is one of the mandatory criteria for diagnosing chronic CNS-GVHD. No diagnostic criteria for aGVHD have been defined in the literature. However, several case reports only had an aGVHD history without extra-CNS chronic GVHD during neurological symptoms[14,15], which was similar to our case 2. A study also demonstrated that CNS can be a direct target of alloreactive T cells following allo-HSCT in mice[16]. These results suggest that early encephalitis after allo-HSCT may be a clinical presentation of CNS involvement of aGVHD. Thus, more clinical evidence and diagnostic methods are needed to further improve the diagnostic criteria for CNS-GVHD.
The Consensus Conference delineated 3 types of chronic CNS-GVHD, including cerebrovascular disease, CNS demyelinating disease, and immune-mediated encephalitis. Cerebrovascular disease can affect medium and large vessels, causing stroke-like episodes, or can involve CNS small vessels, inducing vasculitis. CNS demyelinating disease is described as having a relapsing-remitting course that resembles multiple sclerosis. Diagnosis is based on the white-matter lesions with gadolinium enhancement in MRI and CSF abnormalities[17,18]. Immune-mediated encephalitis is the most difficult to diagnose due to negative imaging findings. The two cases reported here showed negative imaging, which made the diagnosis more difficult. The most common histological feature was the infiltration of CD3-positive T cell-dominant inflammatory cells in the perivascular space or within the vessel wall, whereas only scattered infiltrates were observed in the brain parenchyma[19]. Most of these inflammatory cells were CD8-positive cytotoxic T cells[20]. The infiltration of CD68-positive monocytes/microglia and HLA-DR-positive microglia has also been reported[21]. However, brain biopsy is a painful and traumatic operation, and there may be no positive imaging results, making it impossible to determine the biopsy position, as in our cases. Therefore, we considered whether there are other valuable detection methods to assist the diagnosis of CNS-GVHD in the case of negative imaging and an inability to carry out brain biopsy.
Numerous studies have documented that the IgG index in the CSF is associated with many neurologic disorders. IgG-Syn is used to diagnose neurological diseases, such as multiple sclerosis. Bonnan et al[22] reported that IgG-Syn is a robust marker of persistent intrathecal inflammation[22], and its complete normalization should be one of the goals of future therapeutic strategies. Zhang et al[23] found that BBB permeability, CSF IgG-Syn and MOG.Ab were related to the occurrence of CNS demyelination[23]. Another study described the presence of anti-neuronal antibodies directed against contactin-associated protein-like 2, a protein associated with voltage-gated potassium neurological channels, in patients with CNS-GVHD[24]. Altogether, the CSF and blood immune biomarker examinations may act as another promising approach for diagnosing CNS-GVHD. CSF and blood immune biomarker examinations were performed in both our cases, including the oligoclonal band (isoelectric focusing), BBB permeability, IgG index, IgG synthesis rate (IgG-Syn), CSF and blood myelin basic protein (MBP), CSF and blood anti-myelin basic protein antibody (MBP.Ab), and CSF and blood anti-myelin oligodendrocyte glycoprotein antibody (MOG.Ab). In case 1, type IV oligoclonal band was positive and IgG index increased at the time of onset. After three and a half months, the immune markers were reexamined, the results showed that BBB permeability, NSE, S-100β, MBP and MBP.Ab increased, which did not appear at the time of onset. In case 2, the immune biomarker examination showed positive oligoclonal band type IV, elevated MBP and MBP.Ab in blood, and increased BBB permeability, IgG index and IgG-Syn. Therefore, it is necessary to detect immune biomarkers assessing neuronal, myelin and glial cell damage to diagnose CNS-GVHD, and multiple detection of immune biomarkers can improve the positive rate of the results and increase the accuracy of diagnosis.
Most CNS-GVHD cases had multiple hyperintense lesions in brain MRI, showing signs of healing, that were predominantly located in the white matter[18,25,26]. The MRI of leukoencephalopathy involves symmetric, high-intensity lesions in the white matter on T2-weighted imaging and fluid-attenuated inversion recovery. Punctate and curvilinear gadolinium enhancement can be seen along the path of the perforating medullary arteries[11,19]. We know that leukoencephalopathy can also result from many immunosuppressants, radiation therapy, and opportunistic infections after HSCT[5]. The MRI appearance of these forms of toxic leukoencephalopathy involves symmetric hyperintense lesions in the white matter on T2-weighted imaging and fluid-attenuated inversion recovery, but there is no punctate and curvilinear gadolinium enhancement[27]. It is worth noting that abnormal brain MRI findings do not appear in all CNS-GVHD cases. There are also several reports of patients with CNS-GVHD who presented only brain atrophy or who had no abnormalities in brain MRI[17,28], similar to our cases. However, some reports without obvious lesions on MRI revealed diffuse alterations in brain activity on 18F-fluorodeoxyglucose (18F-FDG) PET-CT imaging in patients with CNS-GVHD. Brain 18F-FDG PET-CT demonstrated diffuse cortical and subcortical hypometabolism that completely normalized following immunosuppressive therapy[17]. Therefore, brain 18F-FDG PET-CT can provide supplemental information to facilitate the diagnosis of CNS-GVHD, especially in patients with normal MRI, and more research is warranted in the future. In addition, we believe that NGS is a time-saving and highly sensitive diagnostic method[29,30]. It can be used for the detection of pathogens and tumor cells, and plays an important role in the exclusion of CNS-GVHD.
In our cases, both patients with a history of aGVHD or cGVHD developed neurological symptoms that could not be explained by other causes, and had abnormal CSF studies as determined by CSF and blood immune biomarker examinations, suggestive of suspected CNS-GVHD. Due to the lack of specific MRI abnormalities and the rapid clinical deterioration of the patients, we did not attempt to perform a brain biopsy, but prompted the initiation of empirical immunosuppressive therapy. In view of the rapid and favorable response to local and systematic immunosuppressive treatment and the aforementioned neurologic manifestations together with CSF abnormalities and other negative findings, a final diagnosis of CNS-GVHD was made. Of note, all diagnostic methods related to CNS-GVHD used in this work are safe and fast.
Due to the rarity of CNS-GVHD after allo-HSCT, this complication has not been well recognized, leading to imperfect diagnostic criteria and diagnostic methods. Therefore, the diagnosis of CNS-GVHD by clinicians is challenging. Our research team proposed the following diagnostic criteria for CNS-GVHD: Prerequisites are the occurrence of cGVHD or a history of aGVHD leading to central nervous system involvement as the main manifestation, with no other explanation of CNS abnormalities (no infectious, vascular, drug toxicity or metabolic etiology, etc.). Required conditions include: (1) Brain MRI suggests white matter demyelinating lesions; (2) CSF abnormalities, including pleocytosis, elevated protein or abnormalities in CSF biomarkers (positive IgG oligoclonal bands type IV or V, increased IgG index, IgG-Syn, MBP, MBP.Ab, S100β, NSE, etc.); (3) Pathological brain biopsy or postmortem examinations revealing GVHD lesions; and (4) Immunosuppressive therapy is effective. A definite diagnosis of CNS-GVHD can be made by satisfying these prerequisites and three of the required conditions, and possible diagnosis by satisfying the prerequisites and two of the required conditions.
This report systematically describes the diagnostic methods for CNS-GVHD and presents our own diagnostic criteria. Furthermore, non-invasive tools, especially CSF and blood immune biomarker examinations, are proposed to facilitate the diagnosis of CNS-GVHD, which is particularly suitable for patients who are critically ill and require urgent treatment and for those who are unsuitable for invasive diagnostic procedures. All clinical cases should be documented to better define this entity and improve the diagnostic criteria and diagnostic methods.
We thank our patients for participating in this study.
| 1. | An K, Wang Y, Li B, Luo C, Wang J, Luo C, Chen J. Prognostic factors and outcome of patients undergoing hematopoietic stem cell transplantation who are admitted to pediatric intensive care unit. BMC Pediatr. 2016;16:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, de Medeiros CR. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000;25:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lee YJ, Yum MS, Kim EH, Kim MJ, Kim KM, Im HJ, Kim YH, Park YS, Ko TS. Clinical Characteristics of Transplant-associated Encephalopathy in Children. J Korean Med Sci. 2017;32:457-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Maffini E, Festuccia M, Brunello L, Boccadoro M, Giaccone L, Bruno B. Neurologic Complications after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Dulamea AO, Lupescu IG. Neurological complications of hematopoietic cell transplantation in children and adults. Neural Regen Res. 2018;13:945-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Colombo AA, Marchioni E, Diamanti L, Di Matteo AM, Baldanti F, Furione M, Cazzola M, Ferretti VV, Pascutto C, Alessandrino EP. Neurological Complications Involving the Central Nervous System After Allogeneic Hematopoietic Stem Cell Transplantation During a Period of Evolution in Transplant Modalities: A Cohort Analysis. Transplantation. 2017;101:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Cordelli DM, Masetti R, Zama D, Toni F, Castelli I, Ricci E, Franzoni E, Pession A. Central Nervous System Complications in Children Receiving Chemotherapy or Hematopoietic Stem Cell Transplantation. Front Pediatr. 2017;5:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Karam C, Mauermann ML, Johnston PB, Lahoria R, Engelstad JK, Dyck PJ. Immune-mediated neuropathies following stem cell transplantation. J Neurol Neurosurg Psychiatry. 2014;85:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ren XY, Liu X, Huang QS, Wang QM, He Y, Zhu XL, Han W, Chen H, Chen YH, Wang FR, Wang JZ, Zhang YY, Mo XD, Chen Y, Wang Y, Fu HX, Chang YJ, Xu LP, Liu KY, Huang XJ, Zhang XH. Incidence, Risk Factors, and Outcome of Immune-Mediated Neuropathies (IMNs) following Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019;25:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Padovan CS, Gerbitz A, Sostak P, Holler E, Ferrara JL, Bise K, Straube A. Cerebral involvement in graft-versus-host disease after murine bone marrow transplantation. Neurology. 2001;56:1106-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Sostak P, Padovan CS, Eigenbrod S, Roeber S, Segerer S, Schankin C, Siegert S, Saam T, Theil D, Kolb HJ, Kretzschmar H, Straube A. Cerebral angiitis in four patients with chronic GVHD. Bone Marrow Transplant. 2010;45:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Saad AG, Alyea EP 3rd, Wen PY, Degirolami U, Kesari S. Graft-versus-host disease of the CNS after allogeneic bone marrow transplantation. J Clin Oncol. 2009;27:e147-e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Grauer O, Wolff D, Bertz H, Greinix H, Kühl JS, Lawitschka A, Lee SJ, Pavletic SZ, Holler E, Kleiter I. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain. 2010;133:2852-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Harvey CM, Gottipati R, Schwarz S, Auer D, O'Donoghue M, Russell NH, Fox CP. Acute disseminated encephalomyelitis following allo-SCT: central nervous system manifestation of GVHD. Bone Marrow Transplant. 2014;49:854-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Yamamoto H, Uchida N, Ishiwata K, Araoka H, Takagi S, Tsuji M, Kato D, Matsuhashi Y, Seo S, Matsuno N, Masuoka K, Wake A, Yoneyama A, Makino S, Taniguchi S. Possible graft-versus-host disease involving the central nervous system soon after cord blood transplantation. Am J Hematol. 2009;84:764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Hartrampf S, Dudakov JA, Johnson LK, Smith OM, Tsai J, Singer NV, West ML, Hanash AM, Albert MH, Liu B, Toth M, van den Brink MR. The central nervous system is a target of acute graft vs host disease in mice. Blood. 2013;121:1906-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Ruggiu M, Cuccuini W, Mokhtari K, Meignin V, Peffault de Latour R, Robin M, Fontbrune FS, Xhaard A, Socié G, Michonneau D. Case report: Central nervous system involvement of human graft vs host disease: Report of 7 cases and a review of literature. Medicine (Baltimore). 2017;96:e8303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Min GJ, Park S, Park SS, Yoon JH, Lee SE, Cho BS, Eom KS, Lee S, Kim HJ, Min CK, Cho SG, Kim DW, Lee JW, Kim YJ. A case of central nervous system graft-versus-host disease following allogeneic stem cell transplantation. Int J Hematol. 2019;110:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Terada M, Nakamagoe K, Obara N, Ogawa S, Sakamoto N, Sato T, Nohara S, Chiba S, Tamaoka A. Chronic Graft-versus-host Disease Presenting with Multiple Punctate Intracranial Lesions on Contrast-enhanced Magnetic Resonance Imaging. Intern Med. 2017;56:363-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Kaliyaperumal S, Watkins B, Sharma P, Furlan S, Ramakrishnan S, Giver C, Garcia A, Courtney C, Knight H, Strobert E, Elder E, Crenshaw T, Blazar BR, Waller EK, Westmoreland S, Kean LS. CD8-predominant T-cell CNS infiltration accompanies GVHD in primates and is improved with immunoprophylaxis. Blood. 2014;123:1967-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Iwasaki Y, Sako K, Ohara Y, Miyazawa M, Minegishi M, Tsuchiya S, Konno T. Subacute panencephalitis associated with chronic graft-versus-host disease. Acta Neuropathol. 1993;85:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Bonnan M, Gianoli-Guillerme M, Courtade H, Demasles S, Krim E, Marasescu R, Dréau H, Debeugny S, Barroso B. Estimation of intrathecal IgG synthesis: simulation of the risk of underestimation. Ann Clin Transl Neurol. 2018;5:524-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Zhang XH, Zhao X, Wang CC, Han W, Chen H, Chen YH, Wang FR, Wang JZ, Zhang YY, Mo XD, Chen Y, Wang Y, Fu HX, Chang YJ, Xu LP, Liu KY, Huang XJ. IgG synthesis rate and anti-myelin oligodendrocyte glycoprotein antibody in CSF may be associated with the onset of CNS demyelination after haplo-HSCT. Ann Hematol. 2018;97:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Pirotte M, Forte F, Lutteri L, Willems E, Duran U, Belle L, Baron F, Beguin Y, Maquet P, Bodart O, Servais S. Neuronal surface antibody-mediated encephalopathy as manifestation of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. J Neuroimmunol. 2018;323:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Padovan CS, Bise K, Hahn J, Sostak P, Holler E, Kolb HJ, Straube A. Angiitis of the central nervous system after allogeneic bone marrow transplantation? Stroke. 1999;30:1651-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kamble RT, Chang CC, Sanchez S, Carrum G. Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant. 2007;39:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27:1071-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Ma M, Barnes G, Pulliam J, Jezek D, Baumann RJ, Berger JR. CNS angiitis in graft vs host disease. Neurology. 2002;59:1994-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Guan H, Shen A, Lv X, Yang X, Ren H, Zhao Y, Zhang Y, Gong Y, Ni P, Wu H, Zhu Y, Cui L. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol. 2016;22:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect. 2018;76:225-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jaing TH S-Editor: Zhang L L-Editor: Webster JR P-Editor: Yuan YY