Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1037
Peer-review started: July 22, 2020
First decision: November 3, 2020
Revised: November 20, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 16, 2021
Processing time: 190 Days and 20.4 Hours
The prevalence of perineal endometriosis (PEM) is low among women with endometriosis (EM) treated by surgery. It manifests as hard or cystic nodules with pain in the perineal wounds and surrounding areas. Implantation theory is regarded as the main pathogenesis of PEM. There are few clinical studies on the incidence and clinical characteristics of PEM. This study aims to summarize the clinical data of 14 PEM cases and analyze the factors that may be related to the incubation period and pain.
To analyze the medical history, clinical manifestations, diagnosis, treatment and treatment effect of PEM.
The present study is a case series. We collected the clinical data and follow-up data of 14 patients with PEM who visited The International Peace Maternal and Child Health Hospital Affiliated to Shanghai Jiaotong University from January 2009 to December 2019. Paired t test and Pearson correlation analysis were used for statistical analysis. P < 0.05 was considered statistically significant.
The 14 patients included had a history of vaginal delivery. All patients underwent PEM lesion resection. Three patients were treated by levator ani muscle repair at the same time and 1 patient underwent extensive PEM lesion resection and anal sphincter repair. Body mass index (BMI) at delivery and BMI within 1 mo after delivery were negatively correlated with the latent period, respectively (R2 = 0.53/0.86, P < 0.05). The average visual analog scale score in lesions at the third month after surgery was 0.57 ± 1.28 for all patients, which was significantly lower than that prior to surgery (P < 0.05). One patient relapsed during the sixth month after surgery, and to date, no recurrence occurred after the second surgery.
The higher the BMI during delivery and within 1 mo after delivery, the shorter the incubation period of PEM. It is very important to evaluate the location of lesions before surgery. Surgical resection of the lesion is the best treatment for PEM and results in significant alleviation of symptoms. Therefore, following the diagnosis of PEM, immediate surgery is recommended.
Core Tip: The overall incidence of perineal endometriosis (PEM) is low, accounting for only 0.31% in women with endometriosis treated by surgery. At present, there are few clinical studies on the incidence and clinical characteristics of PEM. This study summarizes the clinical data of 14 PEM cases who visited The International Peace Maternal and Child Health Hospital Affiliated to Shanghai Jiaotong University during the past 11 years. Furthermore, we analyze the incubation period-related factors and pain-related factors of PEM to provide suggestions for the prevention and treatment of PEM.
- Citation: Liang Y, Zhang D, Jiang L, Liu Y, Zhang J. Clinical characteristics of perineal endometriosis: A case series. World J Clin Cases 2021; 9(5): 1037-1047
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1037.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1037
Endometriosis (EM) is a common disease among women of childbearing age with an incidence of 2% to 11% in asymptomatic women and 5% to 50% in infertile women[1,2]. More than 90% of endometriotic lesions are in the pelvic organs, but can also invade organs outside the pelvic cavity such as the liver, lung, kidney, abdominal wall, perineal wounds, etc.[1,2] The overall incidence of perineal endometriosis (PEM) is low, accounting for only 0.31% among women with EM treated by surgery[3]. Similar to EM in the abdominal wall, implantation theory is regarded as the main pathogenesis. Furthermore, changes in local inflammatory response, growth factors and immune function may strengthen the effect of estrogen on endometrial cells[4]. PEM manifests as hard or cystic nodules with pain in the perineal wounds and surrounding areas including menstrual-related pain, varying degrees of pain in the lesions, radiation pain, dyspareunia, and defecation pain, which seriously affects the quality of life and physical and mental health of patients[5,6]. At present, there are few clinical studies on the incidence and clinical characteristics of PEM. This study summarizes the clinical data of 14 PEM cases who visited The International Peace Maternal and Child Health Hospital Affiliated to Shanghai Jiaotong University over the past 11 years. Further-more, we analyze the factors that may be related to the incubation period and pain to provide suggestions for the prevention and treatment of PEM.
By searching the electronic medical record system of The International Peace Maternity and Child Health Hospital Affiliated to Shanghai Jiaotong University, we included patients who were admitted to the hospital for surgical treatment and were diagnosed with PEM by pathology from January 2009 to December 2019. We retrospectively collected patient clinical data (including demographic data, medical history, fertility history, information on the last delivery, symptoms, diagnosis basis, treatment and outcome) and follow-up data (including postoperative treatment, pain relief, whether relapse occurred and treatment for relapse). This study focused on the relationships between the incubation period of PEM and other factors including age at onset, delivery age, body mass index (BMI) at delivery, BMI within 1 mo after delivery, breastfeeding period, and the time of return to menses. The incubation period was defined as the period from the last delivery to the time when symptoms appeared. The visual analog scale (VAS, 1-10) was applied to evaluate the degree of pain in the perineal lesion before surgery, in the third month after surgery, and the last follow-up date. Pain-related factors were also analyzed. Data were analyzed with IBM SPSS statistics ver. 25.0 (IBM Corp., Armonk, NY, United States). Paired t test, and Pearson correlation analysis were used for statistical analysis. P < 0.05 was considered statistically significant.
A total of 13558 patients with EM underwent surgeries from January 2009 to December 2019, including 14 PEM cases, accounting for 0.10% of EM patients. The average gravidity of the 14 patients was 1.43 ± 0.85 (1-4), and the average parity was 1.14 ± 0.36 (1-2). Four patients were treated for laceration of the perineum and the other 10 patients were treated for lateral episiotomy following vaginal delivery.
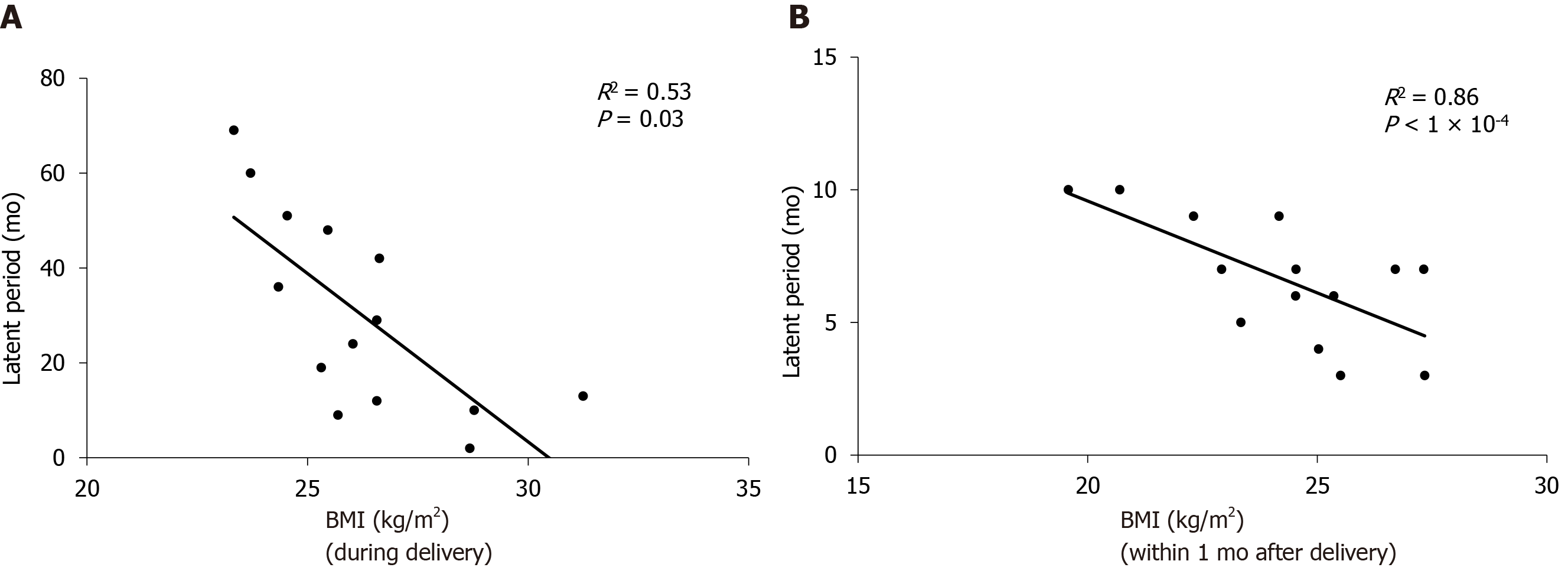
The average age at onset was 30.05 ± 4.07 years (24-39 years), the average delivery age was 27.14 ± 3.16 years (21-32 years), the average BMI at delivery was 26.21 ± 2.17 kg/m2 (23.33-31.25 kg/m2), and the average BMI within one month after delivery was 24.24 ± 2.31 kg/m2 (19.58-27.34 kg/m2). The average breastfeeding period after delivery was 6.11 ± 4.06 mo (1-16 mo), and the average time of return to menses was 5.93 ± 3.47 mo (1-12 mo). The average incubation period was 30.29 ± 21.05 mo (2-69 mo). Correlation analysis showed that the incubation period of PEM was unrelated to the age of onset, delivery age, breastfeeding period, and the time of return to menses, but it was negatively correlated with BMI at delivery and BMI within 1 mo after delivery (R2 = 0.53/0.86, P < 0.05) (Figure 1).
Two of the 14 patients underwent prior surgeries, one patient underwent PEM lesion resection combined with 3 cycles of gonadotropin-releasing hormone agonist (GnRH-a) treatment and Mirena intrauterine device in another hospital due to PEM lesions (40 mm) involving the perianal muscles and she relapsed at the sixth month after surgery. The other patient was treated by puncture and drainage of the lesion and relapsed at the third month after treatment. Five patients received medical treatment before admission, including 2 patients with 6 cycles GnRH-a treatment, 2 patients with oral contraceptives for 3 mo, and 1 patient with gestrinone for 3 mo. Following drug therapy, the lesions diminished and pain was alleviated in 4 patients, but the symptoms relapsed after medication was stopped. One patient who took an oral contraceptive did not have symptom relief.
All 14 patients presented with hard or cystic nodules around the perineal scars, accompanied by various degrees of menstruation-related pain. Twelve patients had lesions located underneath the left perineal scars, and two underneath the perineum and vaginal epithelium. In one patient, the local skin of the lesion was purple-blue, and no ulceration or bleeding was observed among the other patients. Two patients (14.3%) had pelvic EM, one patient was diagnosed with unilateral ovarian endometrioma (OE) 26 mo after delivery, and one patient was diagnosed with bilateral OE by pathology at 47 mo after delivery. Five patients (35.7%) had mild dysmenorrhea, including 1 patient with bilateral OE, and no pelvic EM was detected by ultrasound among the other 4 patients.
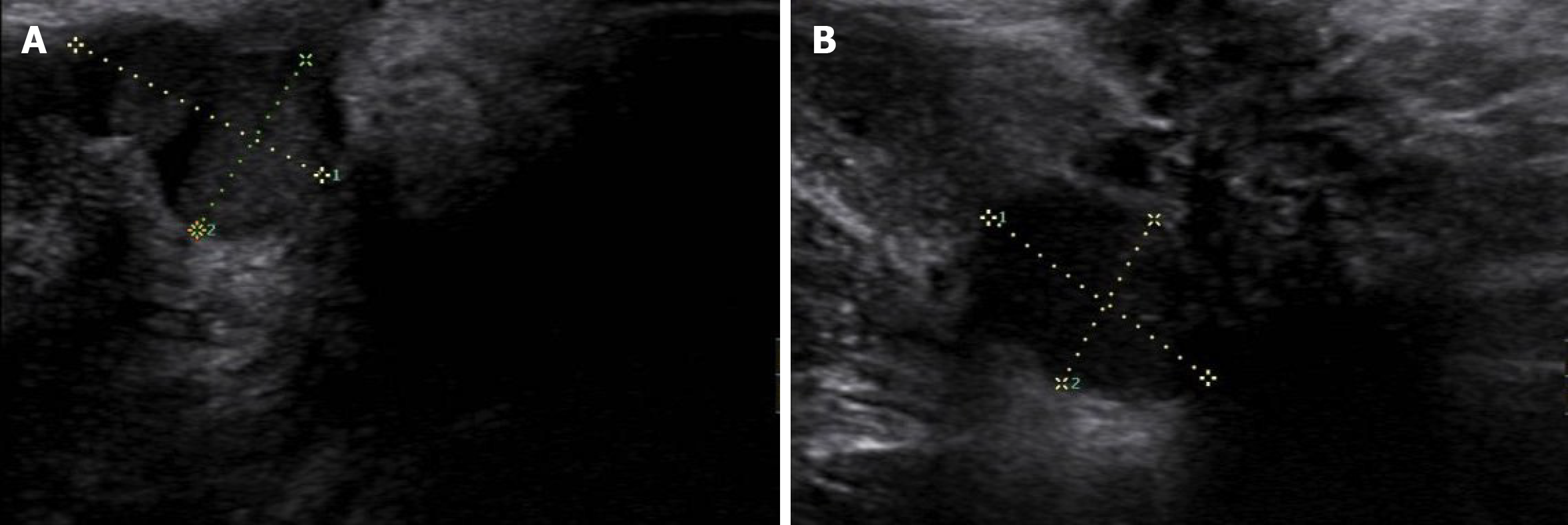
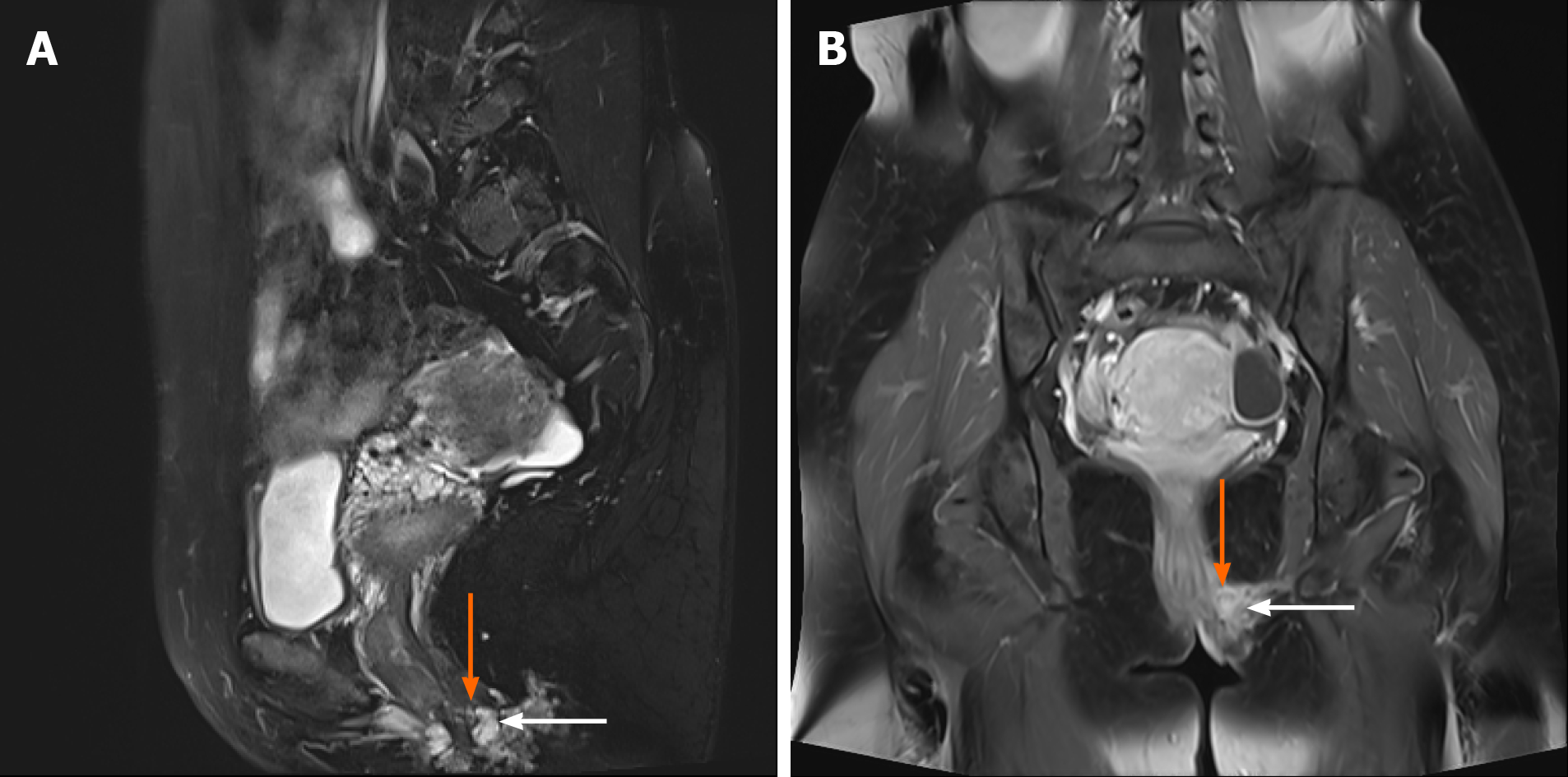
After admission, perineal ultrasonic examination was performed to assess the size, number, and location of the perineal lesions and showed that 10 cases had single lesions and 4 cases had multiple lesions (2-4). The average size of the lesions was 23.2 ± 10.10 mm (5-40 mm). Lesions were smaller than 30 mm in 8 cases, and were larger than 30 mm in 6 cases. Ultrasonic examination of PEM presented as a hypoecho combined with mixed echo mass with a blurred edge beneath the perineum which was surrounded by blood flow signals (Figure 2). Gynecological and rectal examination showed that the lesion invaded the perianal muscles in 4 patients. Thus, further magnetic resonance imaging (MRI) examinations were performed and showed that the lesions in 3 patients involved the levator ani and 1 involved the external anal sphincter (Figure 3). On MR images, PEM lesions presented as a low signal on T1-weighted imaging, equi signal on T2-weighted imaging and cystic abnormal signals or patchy higher signals if with cysts or bleeding lesions.
Menstruation-related pain may be manifested as pain in the lesions, radiating pain, dyspareunia and perianal pain before, during or after the menstrual period. The average duration of pain among the 14 patients was 14.86 ± 7.01 d (7-28 d). The average VAS score was 6.64 ± 2.34 (3-10). Correlation analysis showed that the VAS score evaluating pain in the lesions was unrelated to the duration of pain, the size and number of nodules, involvement of the perianal muscles, and pelvic pain (P > 0.05). Nine patients (VAS score = 7-10) suffered from severe dyspareunia which seriously affected the quality of their sexual life. One patient (VAS score = 9) had combined perianal pain during menstruation and change in stool. Five patients (VAS score 7-10) required pain medication, of which 2 patients’ symptoms (VAS score = 10) could not be alleviated by pain medications.
Not all patients underwent preoperative medical therapy. Colorectal surgeons were invited to assist with the operation in 4 patients whose lesions were thought to invade the perianal muscles as evaluated by MRI and bowel preparation was performed before surgery. On the day before surgery, the patients were required to have a liquid-only diet and to drink 2-3 L sulfate-free polyethylene glycol electrolyte solution (PEG-ELS). Cleansing enema was performed before surgery in some patients with unsatisfactory bowel preparation after taking PEG-ELS. In these 4 patients, three patients’ lesions involved the perianal muscles, thus we resected the nodules and surrounding tissue at a range of 5 mm and crossly sutured the incision to reinforce the levator ani muscle, and then sutured the perineal incision layer by layer. In another case the lesions involved the superficial layer of the external anal sphincter where we resected the lesion and the surrounding tissue and muscle within a radius of 10 mm and repaired the external anal sphincter. In these 4 patients, antibiotics were administered for 3 d following the operation to prevent infection and a liquid diet was prescribed for 3 d. The other cases were treated with resection of the perineal lesions and surrounding tissue with a radius of 5 mm. For patients with multiple lesions or small lesions around the main lesions, we radically removed the lesions with the aid of preoperative imaging and careful palpation during surgery to avoid residual lesions. The average size of the lesions resected during the operation was 25.00 ± 10.86 mm which was not different to the size evaluated preoperatively (23.2 ± 10.10 mm, P = 0.48). If the lesion was deep or the range of resection was large, a strip or tube was placed in the incision for adequate drainage. Following surgery, the patients were asked to clean the perineum twice daily and rinse the wound with an iodine complex after defecation.
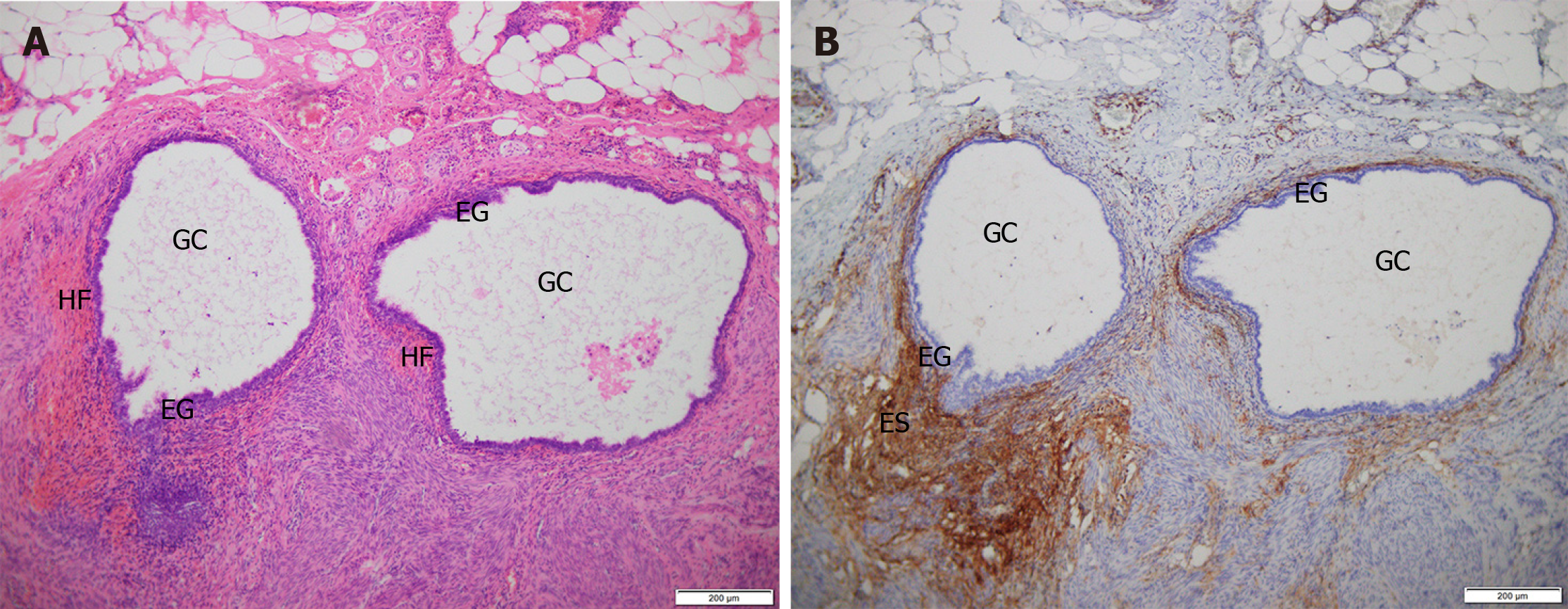
No surgical complications such as rectal or anal injury and heavy bleeding occurred in the 14 patients. The average volume of blood loss was 18.57 ± 13.79 mL (5-50 mL), and the average surgical time was 37.14 ± 13.97 min (15-65 min). The incisions in 13 cases were primary healing and 1 case was secondary healing after debridement and suturing for poor healing and local Staphylococcus aureus infection suggested by secretion culture. One patient complained of loss of local cutaneous sensation in the incision after surgery and gradually recovered without treatment several months later. Defecation function was normal in all patients following surgery and no complications such as fecal incontinence and anal fistula occurred. Pathology showed that the lesions were composed of endometrial glands and stroma, CD10 positivity was seen on immunohistochemistry and hemorrhage was observed in some lesions, which confirmed benign PEM (Figure 4). In a patient with recurrent PEM after the first surgery, we applied GnRH-a for three cycles after the second surgery as she had multiple lesions (20, 25 mm) invading the external anal sphincter with an unclear boundary and obvious scarring. The other 13 patients were not treated with medication after surgery.
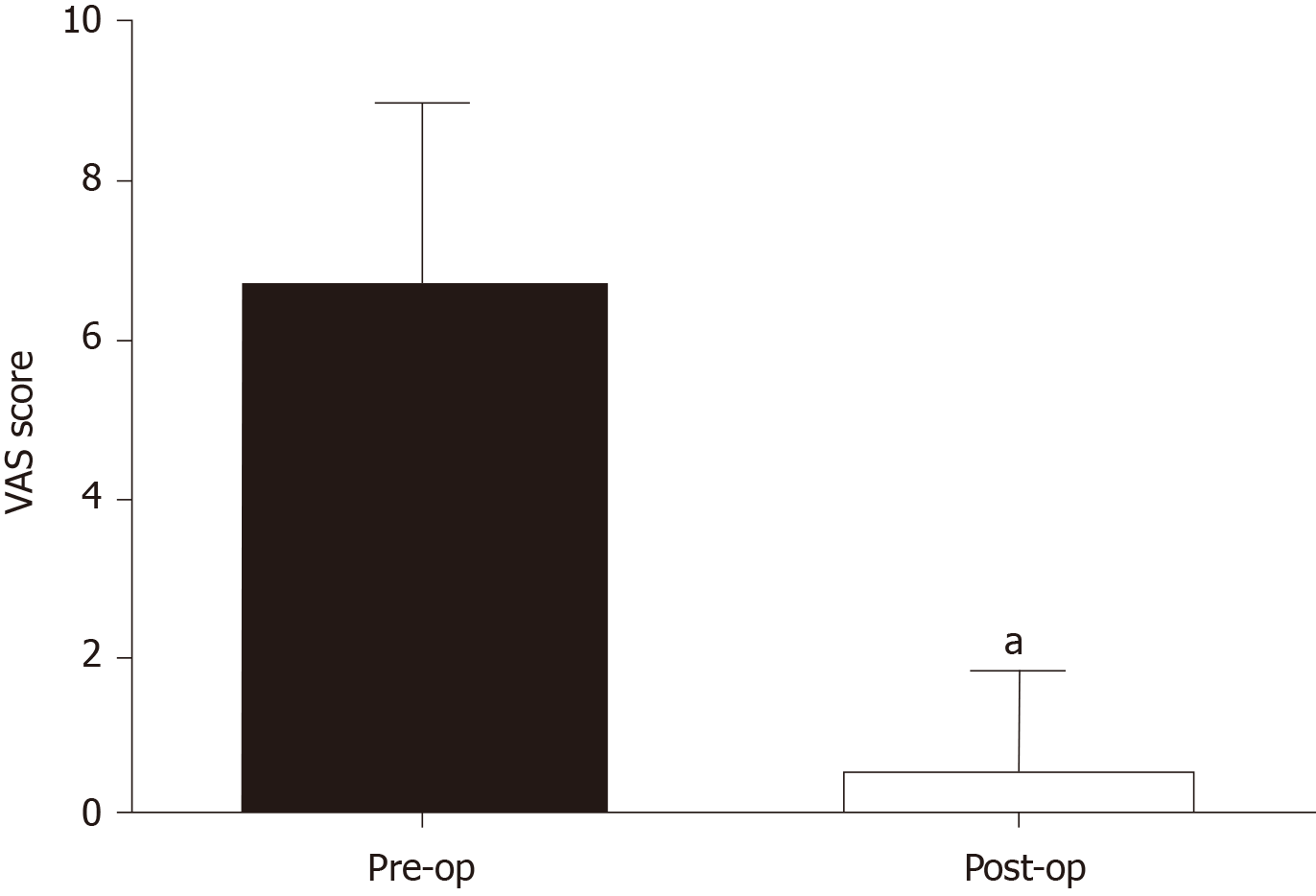
The follow-up period ranged from 4 mo to 129 mo. One patient relapsed at the sixth month after the operation, and to date, 13 patients have not relapsed. At the third month after surgery, the average VAS score in the perineum was 0.57 ± 1.28, which was significantly lower than that prior to surgery (P < 0.05) (Figure 5). Eleven of 14 patients reported a VAS score of 0 in the perineal site, the other three patients reported a VAS score of 1, 3, and 4, respectively. Up to the last follow-up day, the VAS score in all patients remained the same as the third month after surgery except for one patient who relapsed after surgery. The diameter of that patient’s lesion was 25 mm with a VAS score of 9 before surgery, and the VAS score dropped to 3 at the third month after surgery. At the sixth month, a lesion was found in the perineum, along with the VAS score rising to 5, which suggests the relapse of PEM. Thus, she attended our hospital for a second surgery and was proved to have PEM by pathology and now (28 mo) has recovered with no pain (VAS score 0) and no recurrence (Table 1, case No. 3). The patient had a 30 mm lesion involving the levator ani with a VAS score of 9 before surgery, and ultrasound examination was normal 6 mo after surgery. The patient now has no lesions and a VAS score of 1 that may be caused by stimulation of local nerves due to scar contracture (Table 1, case No. 4). Another patient had 2 lesions (20/25 mm) invading the levator ani and reported a VAS score of 10 before surgery and her incision healed poorly due to a Staphylococcus aureus infection (Table 2). She was then treated by re-suturing after debridement and had stage II healing. No recurrence was observed on ultrasound at the sixth month after surgery, and she now has a VAS score of 4, which may be due to nerve stimulation by scar contracture (Table 1, case No. 5).
| Case No. | Age (yr) | Gravidity and parity | Vaginal delivery | BMI at delivery | The duration of breastfeeding (mo) | Interval between delivery and menstruation (mo) | Latent period (mo) | Pain nodules/menstruation-related |
| 1 | 29 | G1P1 | 1 | 23.33 | 1 | 2 | 69 | +/+ |
| 2 | 27 | G1P1 | 1 | 26.57 | 6 | 8 | 29 | +/+ |
| 3 | 33 | G2P2 | 2 | 25.69 | 8 | 6 | 9 | +/+ |
| 4 | 30 | G1P1 | 1 | 23.71 | 1 | 2 | 60 | +/+ |
| 5 | 30 | G1P1 | 1 | 25.31 | 2 | 3 | 19 | +/+ |
| 6 | 30 | G1P1 | 1 | 28.68 | 6 | 1 | 2 | +/+ |
| 7 | 33 | G1P1 | 1 | 26.03 | 6 | 8 | 24 | +/+ |
| 8 | 24 | G1P1 | 1 | 24.54 | 10 | 12 | 51 | +/+ |
| 9 | 37 | G1P1 | 1 | 25.46 | 8 | 8 | 48 | +/+ |
| 10 | 27 | G1P1 | 1 | 26.57 | 6 | 6 | 12 | +/+ |
| 11 | 29 | G4P2 | 2 | 28.78 | 3 | 4 | 10 | +/+ |
| 12 | 39 | G2P1 | 1 | 26.63 | 16 | 6.5 | 42 | +/+ |
| 13 | 28 | G1P1 | 1 | 31.25 | 9 | 12 | 13 | +/+ |
| 14 | 32 | G1P1 | 1 | 24.34 | 3.5 | 4.5 | 36 | +/+ |
| Case No. | Size of lesions (mm) | Involvement of perianal muscles | Preoperative VAS score | Surgery | VAS score at the 3rd month after surgery | Follow-up time (mo) | Follow-up outcome |
| 1 | 20, 25 | Sphincter ani externus | 10 | c | 0 | 7 | Normal |
| 2 | 20, 35 | - | 6 | a | 0 | 14 | Normal |
| 3 | 10 | - | 3 | a | 0 | 14 | Normal |
| 4 | 30 | Levator ani | 10 | b | 4 | 22 | Normal |
| 5 | 25 | - | 4 | a | 0 | 34 | Relapse |
| 6 | 25 | - | 7 | a | 0 | 35 | Normal |
| 7 | 20, 25 | Levator ani | 9 | b | 1 | 41 | Normal |
| 8 | 20 | - | 9 | a | 3 | 52 | Normal |
| 9 | 30 | - | 5 | a | 0 | 68 | Normal |
| 10 | 15 | - | 6 | a | 0 | 101 | Normal |
| 11 | 30 | - | 7 | a | 0 | 129 | Normal |
| 12 | 5, 5, 10, 20 | - | 7 | a | 0 | 4 | Normal |
| 13 | 30 | - | 3 | a | 0 | 12 | Normal |
| 14 | 40 | Levator ani | 7 | b | 0 | 6 | Normal |
PEM is an estrogen-dependent, chronic disease that can be divided into primary and secondary PEM. The pathogenesis is currently unclear, but it is generally recognized that active endometrial cells planted at perineal incision are the main mechanism of secondary PEM. The occurrence of primary PEM is considered to be related to hematogenous spread, lymphatic dissemination, coelomic metaplasia theory or composite mechanisms[7,8]. All 14 patients in this study had PEM secondary to a lateral episiotomy or laceration and the incubation period ranged from 2 mo to 69 mo after surgery. Current studies indicate that the occurrence and severity of pelvic EM are negatively correlated with BMI and pelvic EM[9,10]. However, due to the lag in diagnosis of pelvic EM, there are no reports on the incubation period of pelvic EM and BMI. The current study shows that the higher the BMI during delivery, and that within 1 mo after delivery, the shorter the incubation period of PEM. This may be attributed to the fact that for patients with a high BMI, more fat in the perineum and lochia may lead to blood accumulation in the vagina and perineum, which leads to endometrial cells implanted in the perineal incision. Apart from the ovary, adipocytes are an important source of estrogen, which mainly exists as a form of sulfated steroid, which is an inactive form of estrogen and has low affinity to estrogen receptors. Steroid sulfatase and 17 beta-hydroxysteroid dehydrogenases in ectopic endometrial cells can convert estrogen sulfates into biologically active estrogens, which increase the local estrogen effect and build a positive feedback and ultimately stimulate the growth of endometrial cells in the perineum[11].
Menstruation-related pain is a typical symptom of PEM and is also the main factor seriously affecting the quality of life. The preoperative VAS score in PEM patients in this study was as high as 10 points and the degree of pain was unrelated to the duration of pain, the size and number of lesions, the involvement of perianal muscles, and pelvic pain. This is similar to the relationship between pain and ovarian or peritoneal EM[12]. However, studies by Leng et al[13] showed that deep infiltrating EM, especially lesions located in the posterior pelvic cavity, are closely related to pain. Apart from the anatomy of the pelvic plexus, the distribution of nerve fibers in the lesion, the production and release of nerve growth factor and inflammatory factors are also closely related to pain[14-16]. Additionally, Anaf et al[17] found that the density of nerve fibers and the number of mastocytes around the nerve fibers in the rectal vaginal septum nodules increased. Trypsin, tumor necrosis factor-alpha, etc., directly released by mastocytes act on the nerves to increase pain and hyperpathia. Interestingly, the study also found activation and degranulation of mastocytes in and around the perineum. The sample size in future studies needs to be expanded to examine the existence and density of nerve fibers in PEM lesions and whether nerve fibers are related to the degree of pain in the perineum.
Secondary PEM is characterized by certain symptoms, however, few PEM cases are lacking in typical symptoms[18]. Additionally, it is necessary to confirm the size, number, and location of lesions and their relationships to the perianal muscles by digital rectal examination, anal ultrasound and MRI[18-20], especially the anal sphincter, before surgery to ensure the safety and effectiveness of surgery. Wang et al[21] reported that 6 of 30 PEM patients who had been treated surgically experienced recurrence due to incomplete removal of the lesions involving the anal sphincter resulting from a lack of preoperative evaluation.
Once PEM is diagnosed, surgically removing the lesion as soon as possible is recommended to prevent the lesion from invading normal tissues. It is necessary to remove the lesion completely and radically. During surgery, we suggest that a 5 mm radius of tissue from the lesion should also be resected to prevent residual lesions and that cystic lesions be removed completely to prevent cyst fluid from contaminating and implanting normal tissue.
For PEM involving the anal sphincter, many doctors recommend extensive lesion resection (removal of the lesions and surrounding normal tissues and muscles) and repair of the anal sphincter. However, some researchers believe that to obviate long-term medical treatment or recurrence, extensive lesion resection and anal sphincter reconstruction is more suitable for younger patients. It is considered, particularly in older patients, that lesion resection (resection along the edge of the lesion) should be performed to avoid serious complications such as fecal incontinence[22]. To sum up, a surgical procedure for PEM should be decided after considering age, fertility desire and the patient’s expectation for surgical outcome. Additionally, in order to maintain normal anal sphincter muscle function and avoid complications, it is necessary to ask an experienced colorectal surgeon to assist during surgery.
Medical treatment of PEM includes pseudo-pregnancy therapy and pseudo-menopausal therapy. However, due to the long treatment cycle involved and severe side effects[23], medication is generally used for preoperative and postoperative treatment, perimenopausal patients, those who are unable to tolerate surgery, or those who need to relieve symptoms temporarily. For large lesions, or lesions invading the anal sphincter or rectum, preoperative medical treatment can reduce the lesion to decrease the surgical risk and complications, and to improve surgical outcome. However, for those with multiple small lesions, postoperative treatment should be chosen carefully. There is no consensus on whether postoperative medical treatment can reduce the recurrence rate of PEM and most researchers believe that it can help to control or delay the relapse of PEM instead of reduce the recurrence[24,25]. According to our study, we suggest that postoperative medication is unnecessary if lesions are completely resected.
As a particular type of EM, PEM has unique characteristics although it has features in common with pelvic EM. The current study analyzed the unique factors of PEM and their relationship with treatment outcome; however, there are still some limitations such as a small sample size and recall bias due to the retrospective nature of this study.
The higher the BMI during delivery and within 1 mo after delivery, the shorter the incubation period of PEM. It is very important to evaluate the location of lesions before surgery. Surgical resection of PEM lesions is the best treatment option. Therefore, when PEM is diagnosed, immediate surgery is recommended.
Perineal endometriosis (PEM) seriously affects the quality of life and physical and mental health of patients due to pain including menstrual-related pain, varying degrees of pain in the lesions, radiation pain, dyspareunia, and defecation pain. However, there are few clinical studies on the incidence and clinical characteristics of PEM. The prevalence of PEM is low among women with endometriosis treated by surgery. PEM manifests as hard or cystic nodules with pain in the perineal wounds and surrounding areas. Implantation theory is regarded as the main pathogenesis of PEM. There are few clinical studies on the incidence and clinical characteristics of PEM. This study aimed to summarize the clinical data of 14 PEM cases and analyze the factors that may be related to the incubation period and pain.
We analyzed the factors that may be related to the incubation period and pain to provide suggestions for the prevention and treatment of PEM.
To analyze the medical history, clinical manifestations, diagnosis, treatment and treatment effect of PEM.
This is a case series. We collected the clinical data of 14 patients with PEM who visited The International Peace Maternal and Child Health Hospital Affiliated to Shanghai Jiaotong University from January 2009 to December 2019 who were followed up after treatment. Paired t test and Pearson correlation analysis were used for statistical analysis.
Body mass index (BMI) at delivery and BMI within 1 mo after delivery were negatively correlated with the latent period, respectively (R2 = 0.53/0.86, P < 0.05). The average visual analog scale score in lesions at the third month after surgery was 0.57 ± 1.28 for all patients, which was significantly lower than that prior to surgery (P < 0.05).
The higher the BMI during delivery and within 1 mo after delivery, the shorter the incubation period of PEM. It is very important to evaluate the location of lesions before surgery. Surgical resection of the lesion is the best treatment for PEM which significantly alleviates the symptoms.
When PEM is diagnosed, immediate surgery is recommended. The prevention and occurrence of PEM requires further study.
| 1. | Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 1376] [Article Influence: 229.3] [Reference Citation Analysis (0)] |
| 2. | Winkel CA. Evaluation and management of women with endometriosis. Obstet Gynecol. 2003;102:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Zhu L, Lang J, Wang H, Liu Z, Sun D, Leng J, Zhou H, Cui Q, Wong F. Presentation and management of perineal endometriosis. Int J Gynaecol Obstet. 2009;105:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Carsote M, Terzea DC, Valea A, Gheorghisan-Galateanu AA. Abdominal wall endometriosis (a narrative review). Int J Med Sci. 2020;17:536-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Su HY, Chen WH, Chen CH. Extra-pelvic endometriosis presenting as a vulvar mass in a teenage girl. Int J Gynaecol Obstet. 2004;87:252-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Buda A, Ferrari L, Marra C, Passoni P, Perego P, Milani R. Vulvar endometriosis in surgical scar after excision of the Bartholin gland: report of a case. Arch Gynecol Obstet. 2008;277:255-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Nasu K, Okamoto M, Nishida M, Narahara H. Endometriosis of the perineum. J Obstet Gynaecol Res. 2013;39:1095-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Bacher H, Schweiger W, Cerwenka H, Mischinger HJ. Use of anal endosonography in diagnosis of endometriosis of the external anal sphincter: report of a case. Dis Colon Rectum. 1999;42:680-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Yi KW, Shin JH, Park MS, Kim T, Kim SH, Hur JY. Association of body mass index with severity of endometriosis in Korean women. Int J Gynaecol Obstet. 2009;105:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Zolbin MM, Mamillapalli R, Nematian SE, Goetz L, Taylor HS. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Piccinato CA, Neme RM, Torres N, Sanches LR, Derogis PBMC, Brudniewski HF, Rosa E Silva JC, Ferriani RA. Effects of steroid hormone on estrogen sulfotransferase and on steroid sulfatase expression in endometriosis tissue and stromal cells. J Steroid Biochem Mol Biol. 2016;158:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 182] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Leng JH, Lang JH, Dai Y, Li HJ, Li XY. [Relationship between pain symptoms and clinico-pathological features of pelvic endometriosis]. Zhonghua Fuchan Ke Zazhi. 2007;42:165-168. [PubMed] |
| 14. | Zhang X, Yao H, Huang X, Lu B, Xu H, Zhou C. Nerve fibres in ovarian endometriotic lesions in women with ovarian endometriosis. Hum Reprod. 2010;25:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Kobayashi H, Yamada Y, Morioka S, Niiro E, Shigemitsu A, Ito F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch Gynecol Obstet. 2014;289:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod. 2009;24:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, Noël JC. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril. 2006;86:1336-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Watanabe M, Kamiyama G, Yamazaki K, Hiratsuka K, Takata M, Tsunoda A, Shibusawa M, Kusano M. Anal endosonography in the diagnosis and management of perianal endometriosis: report of a case. Surg Today. 2003;33:630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Rodrigues BD, Alves MC, da Silva AL, Reis IG. Perianal endometriosis mimicking recurrent perianal abscess: case report and literature review. Int J Colorectal Dis. 2016;31:1385-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Shanmuga Jayanthan S, Shashikala G, Arathi N. Perineal scar endometriosis. Indian J Radiol Imaging. 2019;29:457-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Wang HB, Zhu L, Lang JH, Liu ZF, Sun DW, Leng JH, Fan QB. [Clinical analysis of 30 patients with perineal endometriosis]. Zhonghua Yixue Zazhi. 2007;87:1181-1183. [PubMed] |
| 22. | Dougherty LS, Hull T. Perineal endometriosis with anal sphincter involvement: report of a case. Dis Colon Rectum. 2000;43:1157-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Barisic GI, Krivokapic ZV, Jovanovic DR. Perineal endometriosis in episiotomy scar with anal sphincter involvement: report of two cases and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Chen N, Zhu L, Lang J, Liu Z, Sun D, Leng J, Fan Q, Zhang H, Cui Q. The clinical features and management of perineal endometriosis with anal sphincter involvement: a clinical analysis of 31 cases. Hum Reprod. 2012;27:1624-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Li J, Shi Y, Zhou C, Lin J. Diagnosis and treatment of perineal endometriosis: review of 17 cases. Arch Gynecol Obstet. 2015;292:1295-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aslam MF S-Editor: Huang P L-Editor: Webster JR P-Editor: Wang LL