Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11448
Peer-review started: August 24, 2021
First decision: September 29, 2021
Revised: October 8, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 26, 2021
Processing time: 121 Days and 6.3 Hours
It is relatively rare for schwannomas to invade bone, but it is very rare for a large mass to form concurrently in the paravertebral region. Surgical resection is the only effective treatment. Because of the extensive tumor involvement and the many important surrounding structures, the tumor needs to be fully exposed. Most of the tumors are completely removed by posterior combined open-heart surgery to relieve spinal cord compression, restore the stability of the spine and maximize the recovery of nerve and spinal cord function. The main objective of this article is to present a schwannoma that had invaded the T5 and T6 vertebral bodies and formed a large paravertebral mass with simultaneous invasion of the spinal canal and compression of the spinal cord.
A 40-year-old female suffered from intermittent chest and back pain for 8 years. Computed tomography and magnetic resonance imaging scans showed a paravertebral tumor of approximately 86 mm × 109 mm × 116 mm, where the adjacent T5 and T6 vertebral bodies were invaded by the tumor, the right intervertebral foramen was enlarged, and the tumor had invaded the spinal canal to compress the thoracic medulla. The preoperative puncture biopsy diagnosed a benign schwannoma. Complete resection of the tumor was achieved by a two-step operation. In the first step, the thoracic surgeon adopted a lateral approach to separate the thoracic tumor from the lung. In the second step, a spine surgeon performed a posterior midline approach to dissect the tumor from the vertebral junction through removal of the tumor from the posterior side and further resection of the entire T5 and T6 vertebral bodies. The large bone defect was reconstructed with titanium mesh, and the posterior root arch was nail-fixed. Due to the large amount of intraoperative bleeding, we performed tumor angioembolization before surgery to reduce and avoid large intraoperative bleeding. The postoperative diagnosis of benign schwannoma was confirmed by histochemical examination. There was no sign of tumor recurrence or spinal instability during the 2-year follow-up.
Giant schwannoma is uncommon. In this case, a complete surgical resection of a giant thoracic nerve sheath tumor that invaded part of the vertebral body and compressed the spinal cord was safe and effective.
Core Tip: The main objective of this article is to present a schwannoma that invaded the T5 and T6 vertebral bodies and formed a large paravertebral mass with simultaneous invasion of the spinal canal and compression of the spinal cord. This large thoracic schwannoma was treated by thoracic surgeons and spinal surgeons in combination to provide more experience for clinical treatment.
- Citation: Zhou Y, Liu CZ, Zhang SY, Wang HY, Nath Varma S, Cao LQ, Hou TT, Li X, Yao BJ. Giant schwannoma of thoracic vertebra: A case report. World J Clin Cases 2021; 9(36): 11448-11456
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11448.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11448
Schwannoma, also called Schwann cell tumor, The main sites of disease are the relatively superficial areas of the head and neck, trunk and extremities. The spine is also the most common clinical site, accounting for approximately 25% of spinal tumors. The tumor is usually located in the epidural area and rarely invades the subdural area along the nerve roots. It forms a "dumbbell" growth with intervertebral foramen expansion and oppression of adjacent bone and is often accompanied by hardened zone formation. The boundary is relatively clear, and tumor growth is relatively slow[1,2]. The main symptom is segmental pain.
Literature research found that due to the lack of sensitivity to radiation and chemotherapy, removal of the tumors completely is key to achieve a good treatment outcome. A good surgical treatment outcome is related to the choice of surgical approach and good prognosis after surgical treatment of the disease; there is less recurrence after complete resection, and the chance of malignancy is small[3]. However, there is no unified surgical approach standard for spinal schwannoma at present, especially for large schwannoma and the defect reconstruction following the removal of schwannoma. We recently successfully treated a patient with giant schwannoma. The main purpose of this paper is to report a case of large thoracic schwannoma treated by thoracic surgeons and spinal surgeons in combination in order to provide reference for clinical treatment.
A 40-year-old female suffered from intermittent chest and back pain for 8 years.
Intermittent chest and back pain discomfort for 8 years.
She reported that her chest and back pain had progressively worsened over the past 8 years but was not taken seriously.
There was nothing special in her medical history, and she had no family history of the disease.
The specialist examination revealed right chest and back tenderness and percussion pain. Skin sensation was significantly reduced below the umbilical level. The muscle strength of both lower limbs was weakened, and the muscle tension was slightly increased. The bilateral hoffman sign was negative, while the babinski reflex was positive. The bilateral knee tendon reflex was hyperactive. Other physical examinations were normal.
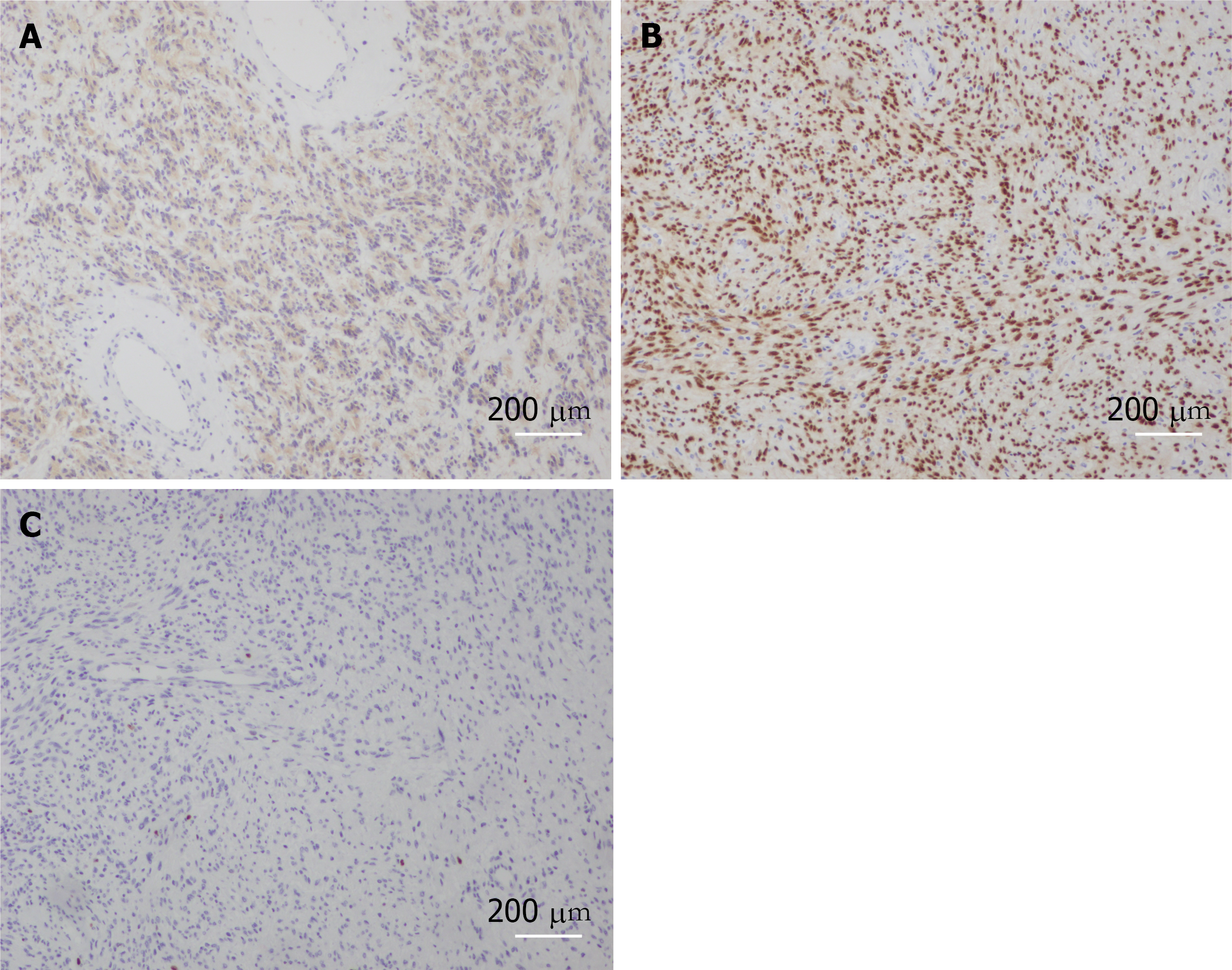
The results of routine blood tests and biochemical tests were normal. The pathological findings from other hospitals included (from tissue of a right lung puncture biopsy) soft tissue tumor, considered to be neurogenic, with relatively active focal cell proliferation, not excluding malignant potential. Immunohistochemical results: cytokeratin (-), vimentin (+), smooth muscle antibody (-), S-100 (+), Desmin (-), Ki67 (hot spot 10%), Bcl-2 (+), CD117 (-), discovered on gastrointestinal stromal tumors 1 (-), WT-1 plasma (+), and Calretinin (-). Given the aforementioned details, the preoperative diagnosis was benign schwannoma.
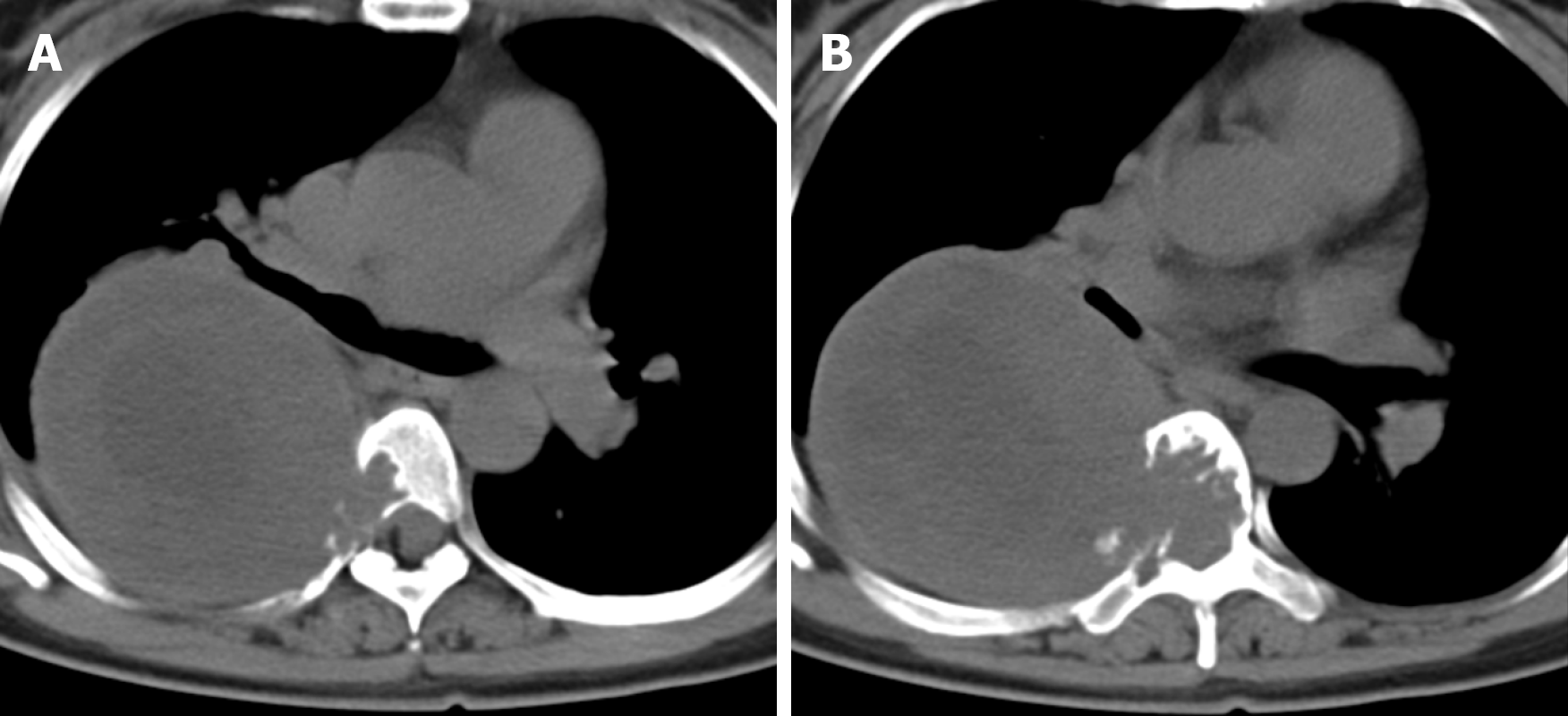
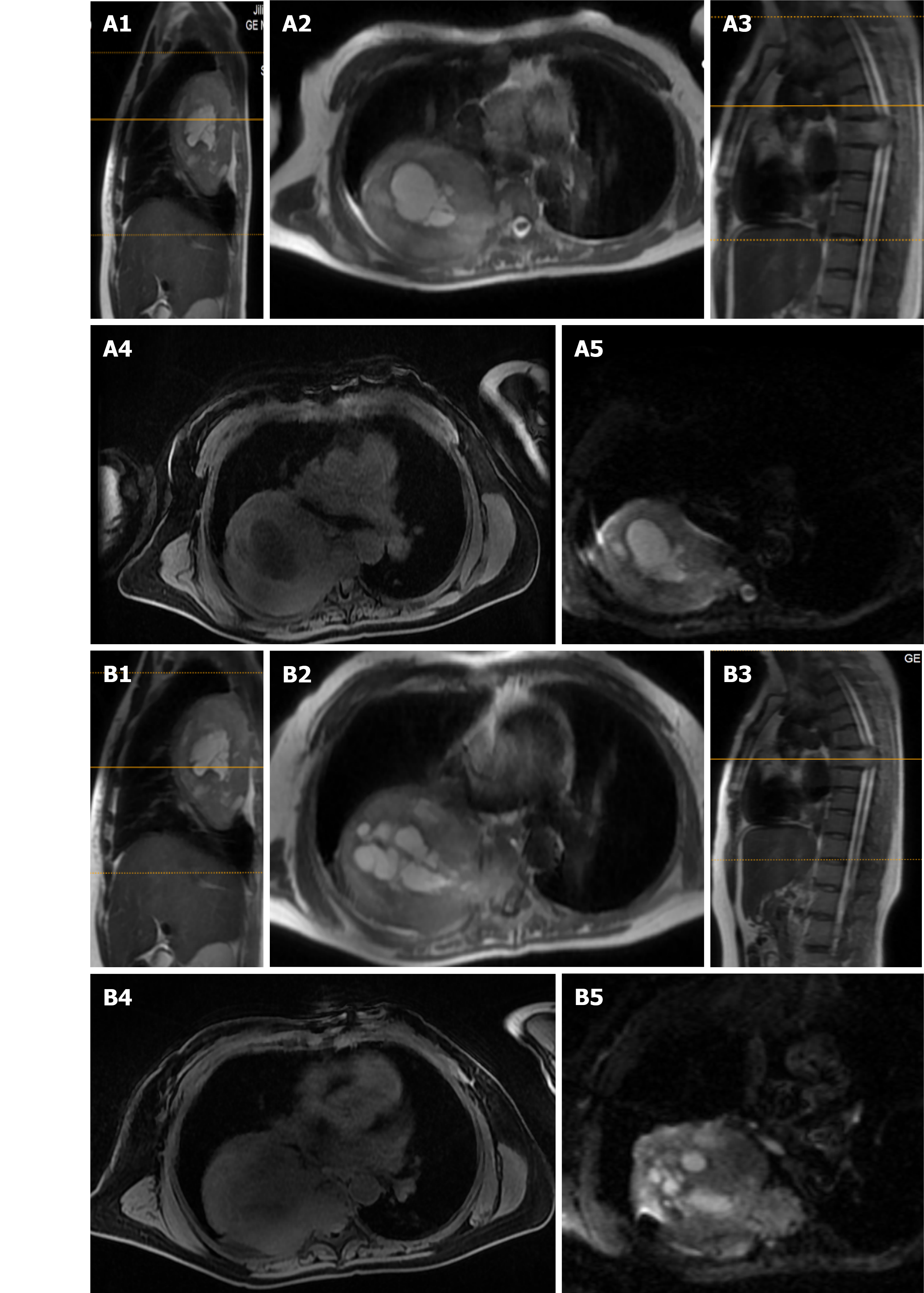
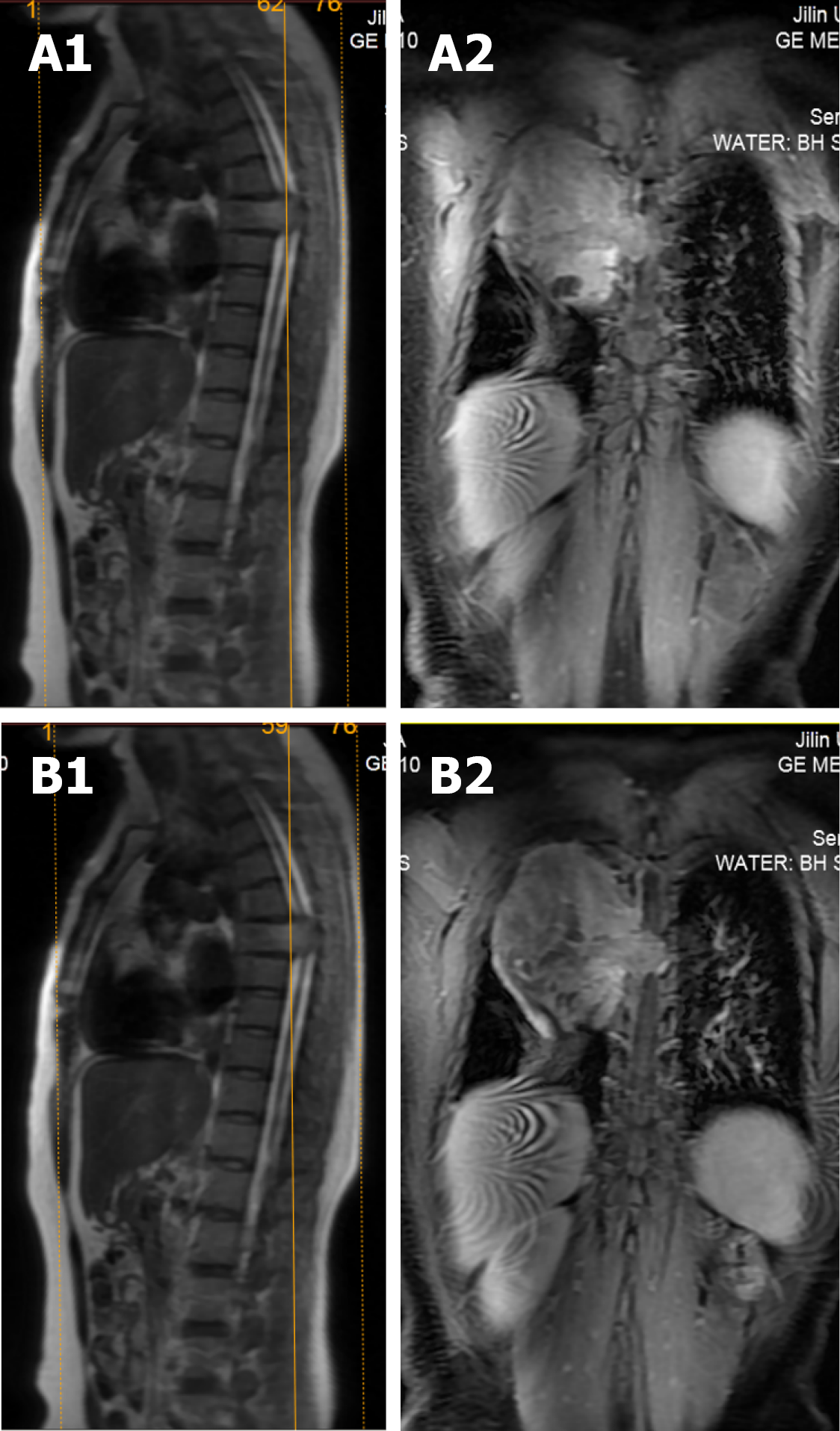
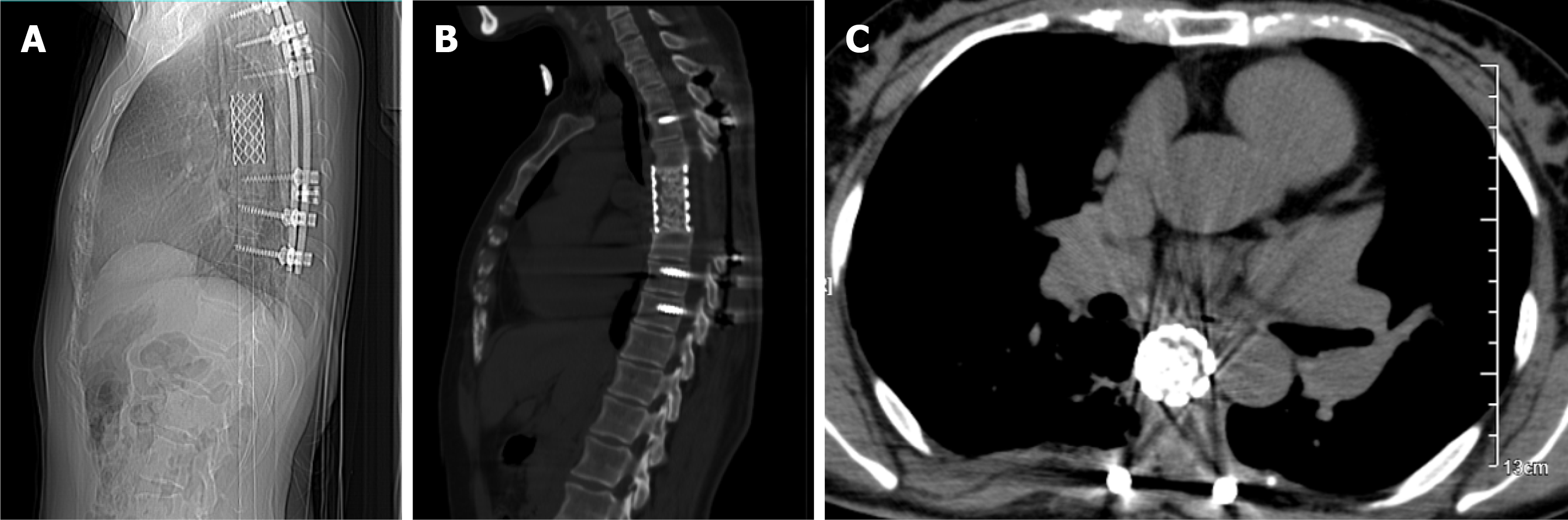
X-ray and computed tomography (CT) scans revealed that the bone shapes of T5 and T6 were irregular, with an irregular soft tissue density shadow inside, protruding into the thoracic cavity on the right side, approximately 87 mm × 93 mm × 121 mm, with an uneven internal density, where the CT value was approximately 12-35 HU. Bone destruction was seen in the vertebral bodies and right adnexa as well as part of the right ribs (Figure 1). Magnetic resonance imaging (MRI) revealed that the spinal canal and paravertebral space were occupied at the level of T5-6 vertebral bodies. They were irregular and had slightly longer T1-weighted (T1W) and T2-weighted (T2W) signals in the vertebral bodies with unclear boundaries. The right intervertebral foramen in the corresponding area was enlarged, adjacent bone was absorbed, the lesion extended to the right intervertebral foramen, invading the right paravertebral area, and grew to the right thoracic cavity, with a size of 86 mm × 109 mm × 116 mm. There were multiple round long T1W and T2W signals in the lesion in the right thoracic region. Lesions can be seen as patchy hyperintensity in diffusion-weighted imaging. The enhanced scan showed inhomogeneous enhancement with unenhanced areas. The MRI in Figures 2-6 shows the right pleural fluid.
The final diagnosis is schwannoma.
Surgical treatment was performed by a combination of thoracic surgeons and spine surgeons. First, the thoracic surgeon removed the chest tumor as follows: the patient was placed in the right lateral position, a linear incision of approximately 20 cm long was made on the posterior and lateral sides of the 5th rib, the subcutaneous muscles were incised layer by layer, and the chest cavity was on one-lung ventilation. The surface of the tumor is rich in blood supply, and the lesion is extensive, with adhesions to the middle lobe, lower lobe and diaphragm. Therefore, gradual blunt separation of the lesion from the lower lobe and diaphragm was performed. Because part of the middle lobe tissue could not be separated from the lesion, a wedge-shaped piece of lung tissue was removed along with the lesion, and a disposable linear cutter was used to suture the surface of the lesion. Thus, the lesion was completely removed in the thoracic cavity except for the spinal side. The thoracic cavity was then flushed, and the anesthesiologist was instructed to aspirate and blow the lung. A chest drain was left in the 7th intercostal space in the mid-axillary line, and the chest was closed. Intraoperative bleeding was approximately 150 mL.
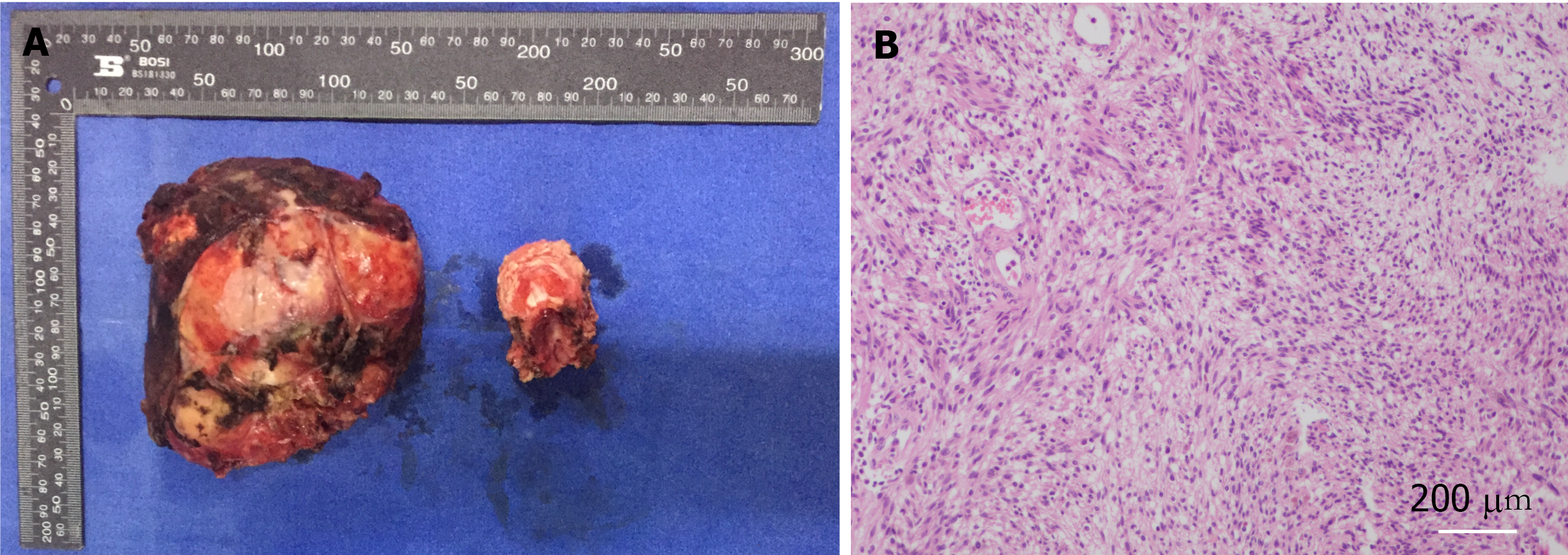
In the next step, the patient was placed in the prone position, and the spine surgeon continued the operation. A central posterior median incision was made at T5-6, approximately 30 cm in length, and the paravertebral muscles were separated layer by layer to reveal the T2-9 spinous process, bilateral laminae, upper and lower articular processes, and the outer edges of the joints. After drilling the arch channel at T2-9 (except for the left side T5-6 and right side T4-7) with good fluoroscopy, a vertebral arch screw was screwed in, the 5th-7th intercostal nerve and blood vessels were ligated, and the bilateral 5th, 6th and 7th ribs next to the spine were occluded, especially the bilateral 6th rib (occluded for nearly 6 cm). These steps allowed the pleural tissue to be separated from the thoracic tumor so that the tumor could be removed. A pre-curved suitable longitudinal connecting rod was then placed in the left arch screw and fixed. After this, the paravertebral tissues were separated after occluding the T5 and T6 spinous processes and the vertebral plates, the segmental vessels were ligated, the dural sac and nerve roots were exposed, the T4/5 and T6/7 intervertebral discs were removed, and the T5 and T6 vertebral bodies were completely removed after scraping out the cartilage endplates. The removed spinal tumor and vertebral bodies were sent for pathological examination. Next, titanium mesh reconstruction and a posterior pedicle nailing system were performed, and the transverse beam was connected between T3/4 and T7/8 bilaterally. Reinforced fixation was performed. After physiological saline irrigation, vancomycin was left in the incision to prevent infection, the closed pleural cavity and spinal area drainage were left in place, and the incision was closed layer by layer. Intraoperative bleeding was approximately 800 mL. Intraoperative blood transfusion was 1000 mL.
The pathological result reported the presence of a spindle mesenchymal tumor cell (right thoracic canal and T5-6 vertebral body). In combination with the immunohistochemical staining results, this supported cellular schwannomas with active cell proliferation and vertebral tissue destruction. Immunohistochemical staining results were as follows: S-100 (+), SOX10 (+), Ki67 (positive rate 10%), synaptophysin (partial +), Neurofilament (-), smooth muscle antibody (-), Desmin (-), CD34 (-), CD117 (-), and discovered on gastrointestinal stromal tumors 1 (-). In this case, after 2 years of follow-up, the patient was in good condition, and no recurrence was observed. Postoperatively, the patient was observed in the hospital for 2 wk without complications and was discharged with instructions to rest for 3 mo, wear a neck and chest brace and move around moderately to avoid trauma. Half a year after surgery, the Visual Analog Scale score decreased from 6 to 1, and the sf-36 score also significantly increased after surgery (Table 1).
| Preoperative | 6 mo after surgery | 2 yr after surgery | |
| Physical function | 45 | 60 | 75 |
| Physical restriction | 0 | 0 | 50 |
| Bodily pain | 0 | 40 | 40 |
| General health | 0 | 25 | 60 |
| Vitality | 20 | 50 | 65 |
| Social function | 25 | 62.5 | 87.5 |
| Emotional restriction | 0 | 33.3 | 66.6 |
| Mental health | 25 | 40 | 68 |
Schwannoma is the most common benign tumor of the spinal canal[4]. Although large schwannoma is unusual, it clinically often occupies two to three vertebral canal segments due to its slow tumor growth. In the early stages, it does not have an obvious symptom because the tumor biological characteristics develop over time alongside expansive tumor growth, after which the symptoms present as oppressive nerve, spinal cord and nerve root pain, paresthesia, movement disorders and other symptoms. CT, MRI and other auxiliary examinations are often used to diagnose schwannomas inside and outside the spinal canal. Surgical resection of the tumor should be performed at an early stage when pathological fracture and instability of the spine may occur or when compression of vital organs and severe symptoms may occur. Since schwannomas are insensitive to radiotherapy and chemotherapy, surgical resection is also the main treatment for intraspinal extramedullary schwannomas, and radical complete resection of schwannomas is the gold standard for the treatment of this disease[2,5,6]. Surgical treatment can be performed to remove the tumor, relieve compression and restore the stability of the spine to improve the symptoms of patients, improve the quality of life and prevent recurrence[4].
In the study there was a heated discussion regarding the surgical approach of complete as well as incomplete resection, which is mainly due to the consideration of spinal stability[1], preservation of neurological function and controversy over the recurrence of nerve sheath tumors[7]. For locally invasive benign tumors, complete resection is recommended because there is a risk of recurrence without complete removal of the tumor. In contrast, incomplete resection has been adopted by some scholars when the tumor grows in the lumbar spine[8,9], due to the consideration that removal of the tumor may sacrifice the nerve roots of its corresponding segment. They believe that not all nerve roots are invaded by the tumor and many exist in the "adherent" cases[10], so they do not remove the nerve root when removing tumors to avoid causing neurological deficits and believe that the few patients with neurological deficits mostly belong to other conditions such as nerve contusions. Some scholars think that tumors are not completely removed to avoid damage to the nerves and spinal cord[2], and only a very small percentage of patients require secondary surgery for tumor growth during long-term follow-up. Even in the literature[11,12], it was found that the recurrence rate is low, which does not mean that it does not occur. Therefore, doctors should fully inform patients and families to be prepared for all aspects of multiple surgeries when they are facing this type of disease. For example, some surgeries are too difficult and too challenging to try to remove the entire tumor completely within a single surgery, and incomplete resection cases tend to increase the chances of recurrence[13].
For the cases of giant schwannoma of the thoracic vertebra with bone destruction, due to the tumor invasion T5 and T6 that can be seen from imaging, the tumor destruction of vertebral bodies was extremely serious because it considerably affected the stability of the spine. In this case, it was not possible to remove completely the tumor via surgery with a single posterior approach. Thus, we used the joint operation method to treat this disease for the radical removal of the tumor and the invaded vertebral tissue. Vertebrae T5 and T6 were excised, and posterior fixation with a long segment pedicle screw system was performed by titanium mesh filling and artificial vertebral body implantation.
Since the destruction of spinal stability by this type of surgery is often devastating, the requirement for reconstruction is significantly higher than that of patients with degeneration and trauma. To prevent recurrence of the disease and to avoid the risk of physical injury and economic burden to the patients and their families, the tumor should be removed as thoroughly as possible through radical resection, while long segment posterior fixation should be performed to try to restore the stability of the spine[14]. Since the blood supply of the tumor surface capsule is relatively rich and the blood supply of the tumor is mainly concentrated at the input and output of the nerve bundle[15], special attention should be given to avoid destroying the capsule during surgery; otherwise, intractable bleeding may occur.
We suggest for these giant schwannomas that we can perform tumor angioembolization before surgery to reduce and avoid large intraoperative bleeding. Postoperative patients should rest for 3 mo, wear neck and chest braces, and perform moderate ground activities to avoid fatigue and other conditions.
Giant invasive spinal schwannomas are rare lesions, but their local aggressiveness and expansion in all directions can lead to bone destruction and tissue adhesion. Therefore, surgical planning is particularly important. This case proves that the removal of giant thoracic schwannomas is feasible through one-stage surgery through excision of the diseased tissue as standard and complete as possible. The surgical treatment of schwannomas is likely to have a good outcome, with a low rate of recurrence, a good prognosis and few malignant changes.
We would like to thank the patients and their families for their participation in this study.
| 1. | Sridhar K, Ramamurthi R, Vasudevan MC, Ramamurthi B. Giant invasive spinal schwannomas: definition and surgical management. J Neurosurg. 2001;94:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Altaş M, Cerçi A, Silav G, Sari R, Coşkun K, Balak N, Işik N, Elmaci I. Microsurgical management of non-neurofibromatosis spinal schwannoma. Neurocirugia (Astur). 2013;24:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Seppälä MT, Haltia MJ, Sankila RJ, Jääskeläinen JE, Heiskanen O. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 181] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Lee SE, Chung CK, Kim HJ. Intramedullary schwannomas: long-term outcomes of ten operated cases. J Neurooncol. 2013;113:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Conti P, Pansini G, Mouchaty H, Capuano C, Conti R. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, Zhang W, McCutcheon IE, Slopis JM, Lazar AJ, Pollock RE, Lev D. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Naganawa T, Miyamoto K, Hosoe H, Suzuki N, Shimizu K. Hemilaminectomy for removal of extramedullary or extradural spinal cord tumors: medium to long-term clinical outcomes. Yonsei Med J. 2011;52:121-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Celli P. Treatment of relevant nerve roots involved in nerve sheath tumors: removal or preservation? Neurosurgery. 2002;51:684-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Karekezi C, Egu K, Djoubairou BO, Boutarbouch M, Ouahabi AE. Unusual cause of non-discogenic sciatica: Foraminal lumbar root schwannoma. Surg Neurol Int. 2014;5:S208-S210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Celli P, Trillò G, Ferrante L. Spinal extradural schwannoma. J Neurosurg Spine. 2005;2:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Lu DC, Dhall SS, Mummaneni PV. Mini-open removal of extradural foraminal tumors of the lumbar spine. J Neurosurg Spine. 2009;10:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Kagaya H, Abe E, Sato K, Shimada Y, Kimura A. Giant cauda equina schwannoma. A case report. Spine (Phila Pa 1976). 2000;25:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Vadivelu S, Prasad P, Adesina AM, Kim E, Luerssen TG, Jea A. Giant invasive spinal schwannoma in children: a case report and review of the literature. J Med Case Rep. 2013;7:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Disch AC, Schaser KD, Melcher I, Luzzati A, Feraboli F, Schmoelz W. En bloc spondylectomy reconstructions in a biomechanical in-vitro study. Eur Spine J. 2008;17:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007;61:113-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dasgupta R, Haddadi S S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL